Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lu Han | -- | 3260 | 2023-04-12 06:30:33 | | | |
| 2 | Conner Chen | + 2 word(s) | 3262 | 2023-04-14 02:32:17 | | | | |
| 3 | Queenie Zhao | -2 word(s) | 3260 | 2023-04-14 08:41:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ma, H.; Qiao, X.; Han, L. Mussel-Inspired Zero-Dimensional Nanomaterials-Loaded Hydrogels. Encyclopedia. Available online: https://encyclopedia.pub/entry/42967 (accessed on 07 February 2026).
Ma H, Qiao X, Han L. Mussel-Inspired Zero-Dimensional Nanomaterials-Loaded Hydrogels. Encyclopedia. Available at: https://encyclopedia.pub/entry/42967. Accessed February 07, 2026.
Ma, Haohua, Xin Qiao, Lu Han. "Mussel-Inspired Zero-Dimensional Nanomaterials-Loaded Hydrogels" Encyclopedia, https://encyclopedia.pub/entry/42967 (accessed February 07, 2026).
Ma, H., Qiao, X., & Han, L. (2023, April 12). Mussel-Inspired Zero-Dimensional Nanomaterials-Loaded Hydrogels. In Encyclopedia. https://encyclopedia.pub/entry/42967
Ma, Haohua, et al. "Mussel-Inspired Zero-Dimensional Nanomaterials-Loaded Hydrogels." Encyclopedia. Web. 12 April, 2023.
Copy Citation
Hydrogels, with 3D hydrophilic polymer networks and excellent biocompatibilities, have emerged as promising biomaterial candidates to mimic the structure and properties of biological tissues. Nanomaterials can be classified into three main types based on their dimensionality (size and morphology): zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D). The 0D nanomaterials are solid, porous, and hollow structures, such as mesoporous silica nanoparticles (NPs), metal-organic frameworks, hydroxyapatite NPs, iron oxide magnetic NPs, silver NPs, and conductive polymer-based NPs.
mussel-inspired hydrogel
nanocomposite hydrogel
nanomaterials
1. Hydroxyapatite Nanoparticles (HA NPs)
Hydroxyapatite (HA) is a natural bioceramic material that is an inorganic mineral component of bone tissue. Nanometre HA refers to the nanometre-sized (1–100 nm) HA NPs. HA NPs can be used as nanoreinforcing agents to enhance osteoconductivity of the polymer hydrogel. In order to achieve a uniform distribution of HA NPs inside the polymer network, a mussel-inspired strategy has been widely used to modify the HA NPs prior to being incorporated into the hydrogel. For instance, Gan et al. [1] developed a bilayer hydrogel to repair osteochondral defects (Figure 1a). The upper layer consisted of a GelMA-PDA hydrogel, which served as the cartilage repair layer, and the lower layer consisted of a GelMA-PDA/calcium phosphate (GelMA-PDA/HA) hydrogel, which served as the subchondral bone repair layer. Consequently, the bilayer hydrogel simultaneously promoted bone and cartilage tissue regeneration after being implanted into a full-layer cartilage defect of a rabbit knee joint. Liu et al. [2] also prepared an injectable hydrogel for bone repair by introducing PDA-modified nHA NPs (PHA) into a sodium alginate (OSA)/gelatin (Gel) hybrid network (Figure 1b). The addition of PHA increased the ultimate compressive strength of the hydrogel. In addition, the OSA-Gel-PHA hydrogel significantly promoted the adhesion, proliferation, and differentiation of bone marrow mesenchymal stem cells in vitro, and also repaired bone tissue in a rabbit bone defect model. In another study, Wang et al. [3] designed an injectable mussel-inspired nanocomposite hydrogel based on HA NPs, bisphosphonated poly(L-glutamic acid), and aldehyde-catechol bis-functionalized dextronic anhydride for bone tissue engineering (Figure 1c). The catechol groups, bisphosphate ligands (BPs), and aldehyde groups endowed the hydrogels with excellent tissue adhesion, while the BP and nHA effectively promoted proliferation, migration, and osteogenesis differentiation. The results of a rat cranial defect proved the bone regeneration ability of the injectable hydrogels. Based on these studies, it can be said that mussel-inspired addition of HA NPs not only facilitates the distribution of HA NPs, to enhance the mechanical properties of the hydrogel, but also improves cell/tissue affinity to stimulate osteogenesis for the nanocomposite hydrogel. Therefore, the HA NPs-loaded hydrogels could be used for hard tissue engineering for bone grafts.
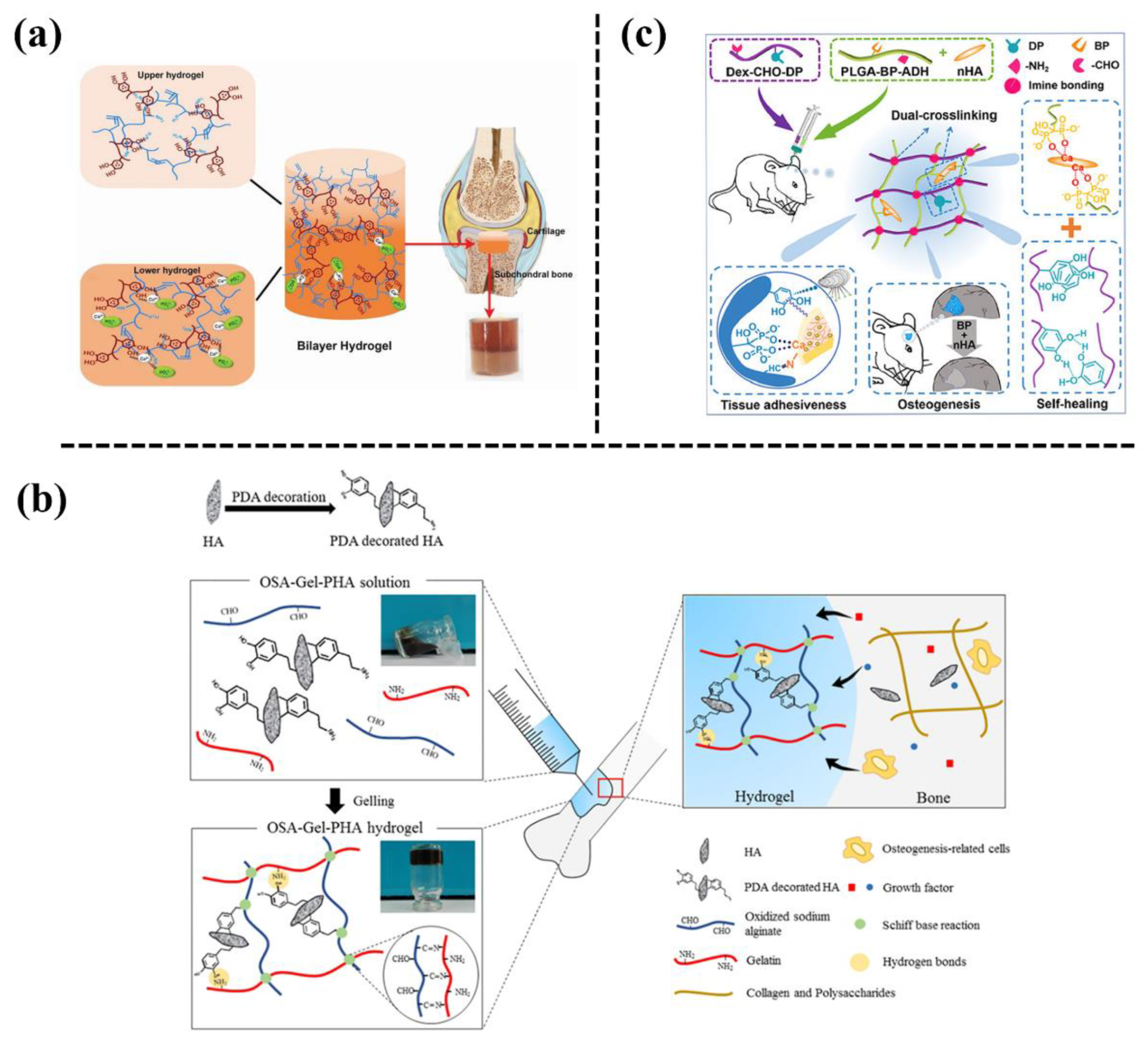
Figure 1. (a) Illustrative diagram for the preparation of a mussel-inspired GelMA hydrogel with in situ mineralization of nano-hydroxyapatite to repair osteochondral defects [1]. (b) Incorporation of PDA-modified hydroxyapatite nanoparticles (PHA NPs) into an oxidized sodium alginate/gelatin hybrid hydrogel for bone regeneration [2]. (c) Preparation of mussel-inspired bisphosphonated injectable nanocomposite hydrogels with adhesive, self-healing, and osteogenic properties [3].
2. Iron Oxide Magnetic Nanoparticles (MNPs)
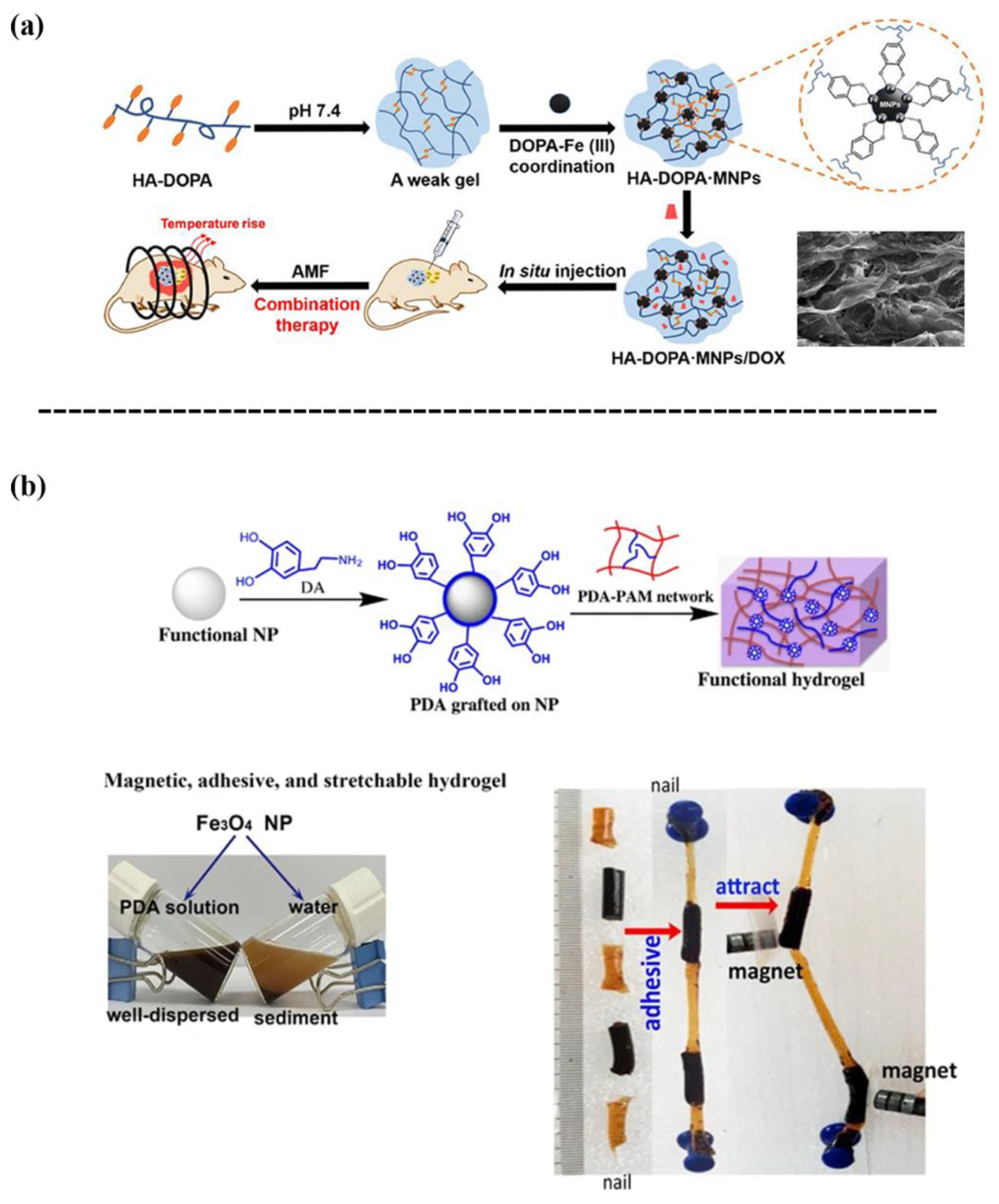
Iron oxide MNPs are classified as magnetic materials, and are generally embedded within a polymer network to achieve magnetic-based nanocomposite hydrogels that can remotely respond to external magnetic fields and can be used for biosensing, diagnostic, and actuators applications [4][5][6][7]. However, iron oxide MNPs have limited interactions with polymer chains, which requires surface modification prior to fabricating the hydrogel. The surface chemistry based on the coordination of catechol-Fe(III) is one crucial strategy for the synthesis of magnetic-based nanocomposite hydrogels. For instance, Dai et al. [8] reported a catechol-Fe(III) coordination hydrogel composed of dopamine-conjugated hyaluronic acid (HA-DOPA) and iron oxide MNPs (Figure 2a). The MNPs in the hydrogel not only served as a structural crosslinking agent to enhance the hydrogel, but also a magnetothermal conversion agent for on demand release of doxorubicin (DOX). Consequently, the HA-DOPA-MNPs/DOX hydrogel exhibited anticancer effects through the combined release of DOX and induction of hyperthermia in the tumor cells. One previous study [9] designed a magnetic hydrogel with a high flexibility, self-healing ability, and tissue adhesiveness by incorporating PDA-grafted-Fe3O4 NPs into a PAM hydrogel (Figure 2b). In short, the iron oxide MNPs-loaded hydrogels will generate magnetic responses and heat for drug release when they are exposed to an external magnetic field.
3. Mesoporous Silica Nanoparticles
Mesoporous silica nanoparticles (silica NPs) are widely used as drug delivery systems owing to their unique properties, such as high specific surface area, large pore volume, controllable morphology, and particle size [10][11][12]. Thus, various nanocomposite hydrogels containing silica NPs have been developed for adhesive and drug delivery. For example, Huang et al. [13] developed an adhesive comprised of polyvinyl alcohol (PVA) and PDA-hybrid mesoporous silica NPs (MS-PDA-NPs), which was formed based on the hydrogen bonding between the functional groups on MS-PDA-NPs and abundant hydroxyl groups on the PVA chain (Figure 3a). Thus, such a mesoporous silica NP-reinforced PVA hydrogel can be used for wound closure. A biocompatible bioadhesive was also fabricated by embedding extra-large pore mesoporous silica NPs into polyacrylamide/polydopamine (PAM/PDA) hydrogels [14] (Figure 3b). The incorporation of silica NPs enhanced the mechanical strength and tissue adhesiveness to skin due to molecular interactions between silica NPs and polymer chains. It was proved that a silica NPs-PAM/PDA hydrogel could be used as an adhesive patch for transdermal drug delivery. Another tissue adhesive was reported that used porous silica NPs to reinforce catechol-functionalized polyethylene glycol [15] (Figure 3c).
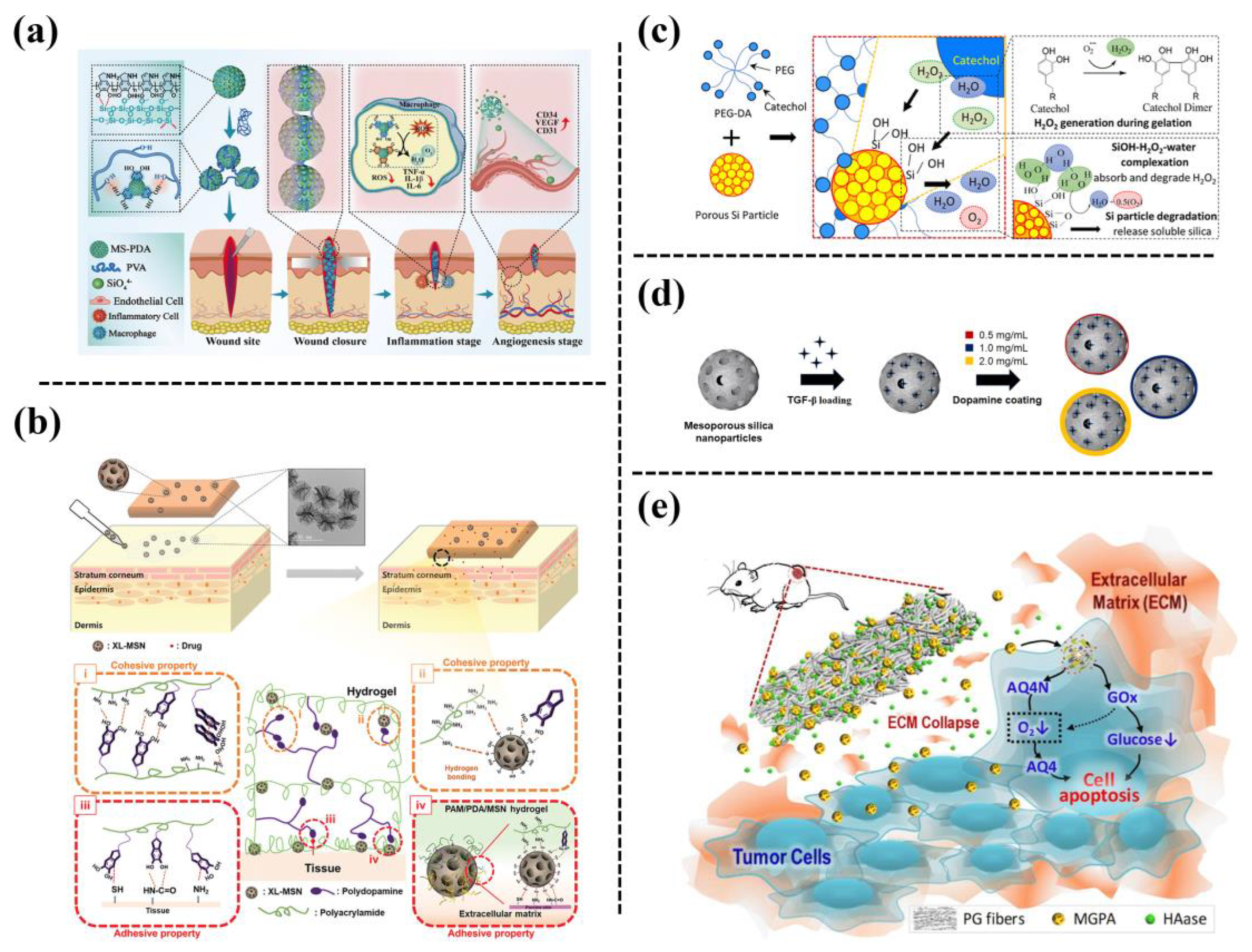
Figure 3. (a) Schematic of preparing a PVA entangled porous nanoadhesive system of PDA/silica with an ROS scavenging/angiogenesis ability for efficient skin wound closure [13]. (b) Schematic of adhesive hydrogel patch consisting of PAM/PDA hydrogels embedded with extra-large pore mesoporous silica nanoparticles (XL-MSNs) [14]. (c) Fabrication of a catechol-containing polyethylene glycol-based adhesive (PEG-DA) formulated with silica NPs to control the released amount of H2O2 [15]. (d) Fabrication of PDA-coated mesoporous silica NPs that can be stably incorporated within macroporous μRB scaffolds to allow the tunable release of transforming growth factor (TGF-β3) [16]. (e) Schematic illustration of combination therapy based on hierarchical PGH@MGPA composite fibers [17].
Mesoporous silica NPs are also promising drug delivery carriers. The drugs can be loaded into the pores of the silica NPs through diffusion with minimal interference with the biological activity of the drugs. For example, Barati et al. [16] employed PDA-coated mesoporous silica NPs for the encapsulation of transforming growth factor (TGF-β3), and then incorporated the TGF β3-loaded silica NPs into gelatin scaffolds to accelerate cartilage regeneration (Figure 3d). Their results showed that the PDA coating on the surface of the mesoporous silica NPs prevented the burst release of TGF-β3, and the sustained release of TGF-β3 could enhance MSC-based cartilage formation in vivo. The PDA-coated mesoporous silica NPs were also used for dual drug encapsulation, in which glucose oxidase was loaded inside the NPs while anoxic-activated prodrug (AQ4N) was adsorbed onto the surface [17]. Then the drug-loaded NPs were incorporated into polycaprolactone/gelatin to prepare a biodegradable fiber scaffold (PGH@MGPA) as an implantable drug delivery system for synergistic cancer therapy (Figure 3e). The porous silica NPs have a high specific surface area and strong interactions with catechol groups, thus improving the curing rate, mechanical properties, and bonding strength of the adhesive. In addition, the porous silica NPs degraded into soluble Si, which promoted cell proliferation.
4. Metal-Organic-Framework
Nanocarriers based on MOFs have received significant interest owing to their large surface area, high porosity, and possibility of designing organic ligands for various applications. Zeolite imidazole MOF (ZIF-8), synthesized using zinc ions and 2-methylimidazole, is one of the most widely used and promising MOFs, which can be used as a biocompatible, degradable, and flexible drug carrier. For example, Han et al. [18] reported producing PDA-hybridized nanosized ZIF-8 (pZIF-8 nanoMOFs), through catechol-controlled chemistry, which possessed versatile adhesiveness, a porous structure, high stability under physiological conditions, and pH-/oxidative dual-responsiveness. Thus, pZIF-8 nanoMOFs were highly efficient for encapsulation of both bone morphogenetic protein-2 (BMP-2) and cisplatin. Then the drug-loaded pZIF-8 nano-MOFs were assembled with PDA-modified HA NPs on a three-dimensional (3D)-printed gelatin hydrogel scaffold, which achieved on demand release of the cisplatin, responding to the local tumor microenvironment to inhibit tumor growth, which enabled the sustained release of BMP-2, to maintain effective long-term osteogenic effects (Figure 4a). Liu et al. [19] loaded curcumin into ZIF-8 MOFs and obtained nanocomposite hydrogels with pH responsiveness and NIR-photosensitive drug release ability, by incorporating curcumin-ZIF-8 MOFs into PDA-modified cellulose nanofiber hydrogels (Figure 4b). By being co-incubated with normal buffalo rat liver cells (BRL) and liver cancer cells (HepG2), the hydrogel had anticancer activity against HepG2 cells, but was not toxic to normal BRL cells. In order to improve the applicability of ZIF-8 in the powder crystallization state, and to increase the drug loading of PDA NPs, Liu et al. obtained PDA@ZIF-8 by in situ growth of ZIF-8 on the surface of PDA NPs, and then mixing PDA@ZIF-8 into a cellulose nanofibril (CNFs)-based hydrogel (Figure 4c). The PDA@ZIF-8 exhibited a photothermal effect from the PDA NPs, and pH-responsiveness from ZIF-8, and therefore, the resulting PDA@ZIF-8/CNFs nanocomposite hydrogel achieved pH/NIR radiation-dependent release of tetracycline hydrochloride [20].
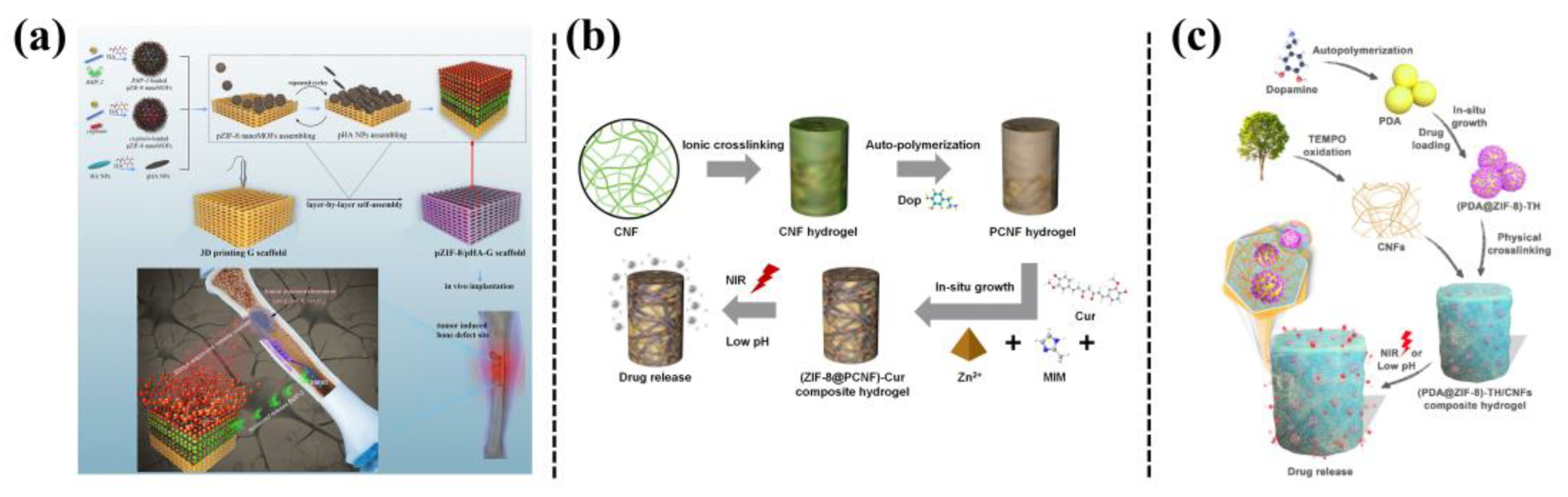
Figure 4. (a) Schematics of assembling polydopamine (PDA)-hybridized ZIF-8 (pZIF-8 nanoMOFs) with PDA-modified hydroxyapatite NPs (pHA NPs) on a 3D printed gelatin scaffold, with dual functions of anticancer and bone formation capabilities [18]. (b) Preparation of a ZIF-8@PCNF composite hydrogel for on-demand drug release by responding to pH and NIR light irradiation [19]. (c) Fabrication of a PDA@ZIF-8/CNFs composite hydrogel and its drug delivery behavior upon NIR light irradiation or under pH variation [20].
5. Silver Nanoparticles (Ag NPs)
Silver nanoparticles (Ag NPs) exhibit a high thermal stability and a broad spectrum of antibacterial activities, which has attracted much attention in the preparation of nanocomposite hydrogels [21]. However, the aggregation of Ag NPs results in their uneven dispersion in a hydrogel network. Mussel-inspired PDA, with catechol and amine groups, is considered as a reducing or stabilizing agent for preparing Ag NPs in a simple and environmentally friendly manner, and the as-prepared Ag NPs serve as nanofillers to endow the hydrogels with multiple functions. For example, Zhao et al. [22] prepared a hydrogel (PDA@Ag NPs-CPHs) by incorporating polydopamine-modified Ag NPs into polyaniline-PVA hydrogel (Figure 5a). The PDA@Ag NPs-CPHs hydrogel exhibited adhesiveness, conductivity, and antibacterial properties, and can be used in wearable and implantable biomedical devices. The PDA@Ag NPs-CPHs retained the antibacterial and electroactive properties and demonstrated significant therapeutic effects on diabetic-foot wounds by promoting angiogenesis, accelerating collagen deposition, and inhibiting wound infection. The Ag NPs can also be formed in situ in catechol-groups-modified hydrogels. For example, a gelatin-tannic acid (Gel-TA) hydrogel was formed through a Michael addition reaction, and the silver nitrate was reduced in situ by TA to Ag NPs as crosslinking agents [23] (Figure 5b). The hydrogel thus exhibited excellent wet tissue adhesion and cytocompatibility, as well as antibacterial and antifungal properties.
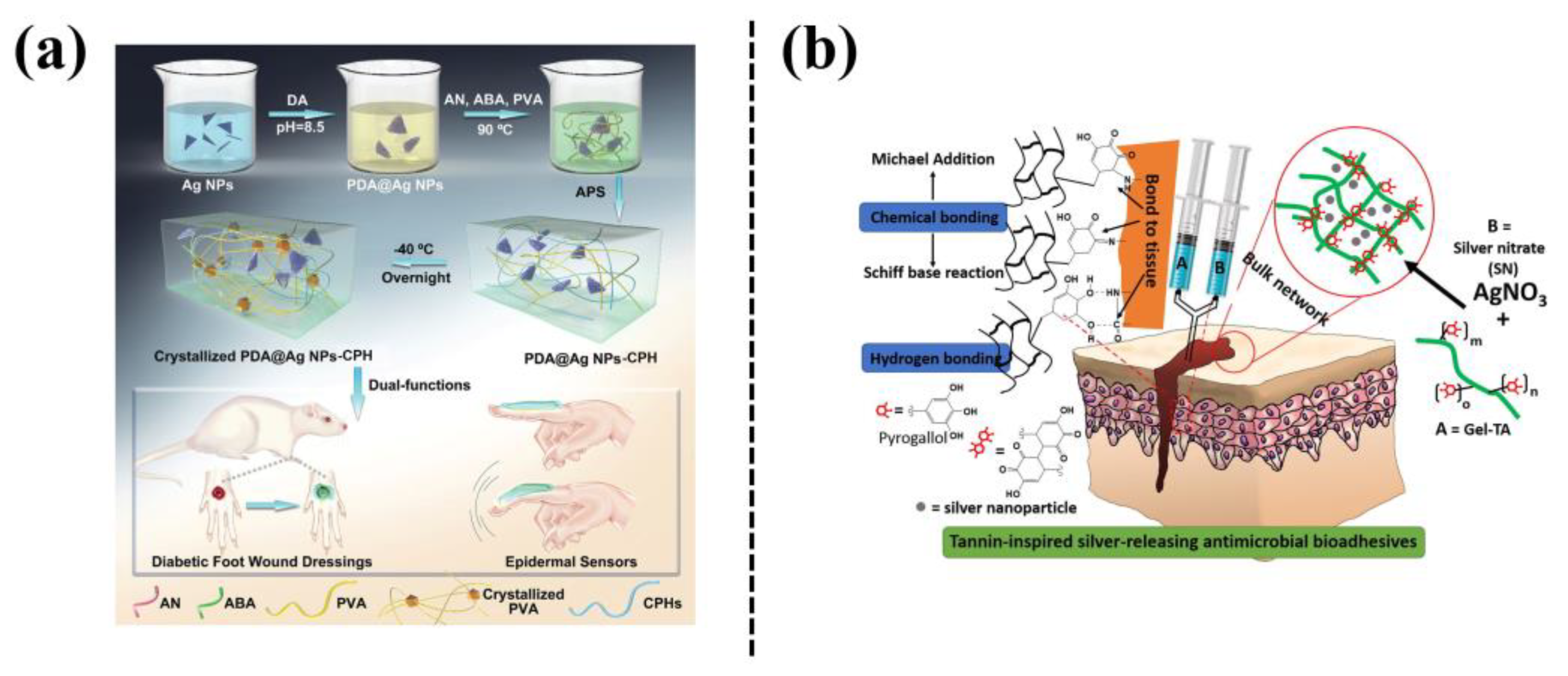
In addition, phenol/catechol groups-chelated Ag NPs can form a dynamic redox system, which can not only trigger hydrogel gelation but also endow the hydrogel with long-term tissue adhesiveness. For example, a plant-inspired adhesive hydrogel was fabricated based on a dynamic catechol redox system created by catechol-containing lignin-chelated Ag (Ag-Lignin) NPs [24] (Figure 6a). The Ag-Lignin NPs catalyzed ammonium persulfate to generate free radicals and initiated the polymerization of the hydrogel. The hydrogel showed long-term repeatable adhesion because Ag-lignin NPs maintained a dynamic balance of the catechol groups. In another study, Jia et al. [25] reported mussel-inspired TA-Ag nanozymes by in situ reduction of ultrasmall Ag NPs with tannic acid (TA) (Figure 6b). The TA-Ag nanozymes exhibited peroxidase activity, which triggered self-setting of the hydrogel without external stimuli. The as-prepared hydrogel could be used as an adhesive and antibacterial bioelectrode to detect bio-signals, and also as a wound dressing to promote the regeneration of skin tissue, with a healing rate of up to 90% in a rat model of full-thickness skin defect. In mussel-inspired nanocomposite hydrogels based on Ag NPs, the catechol-containing agents not only serve as reducing agents to obtain Ag NPs for achieving antibacterial ability, but also can be used to create a dynamic redox system inside the hydrogel for maintaining long-term adhesion.
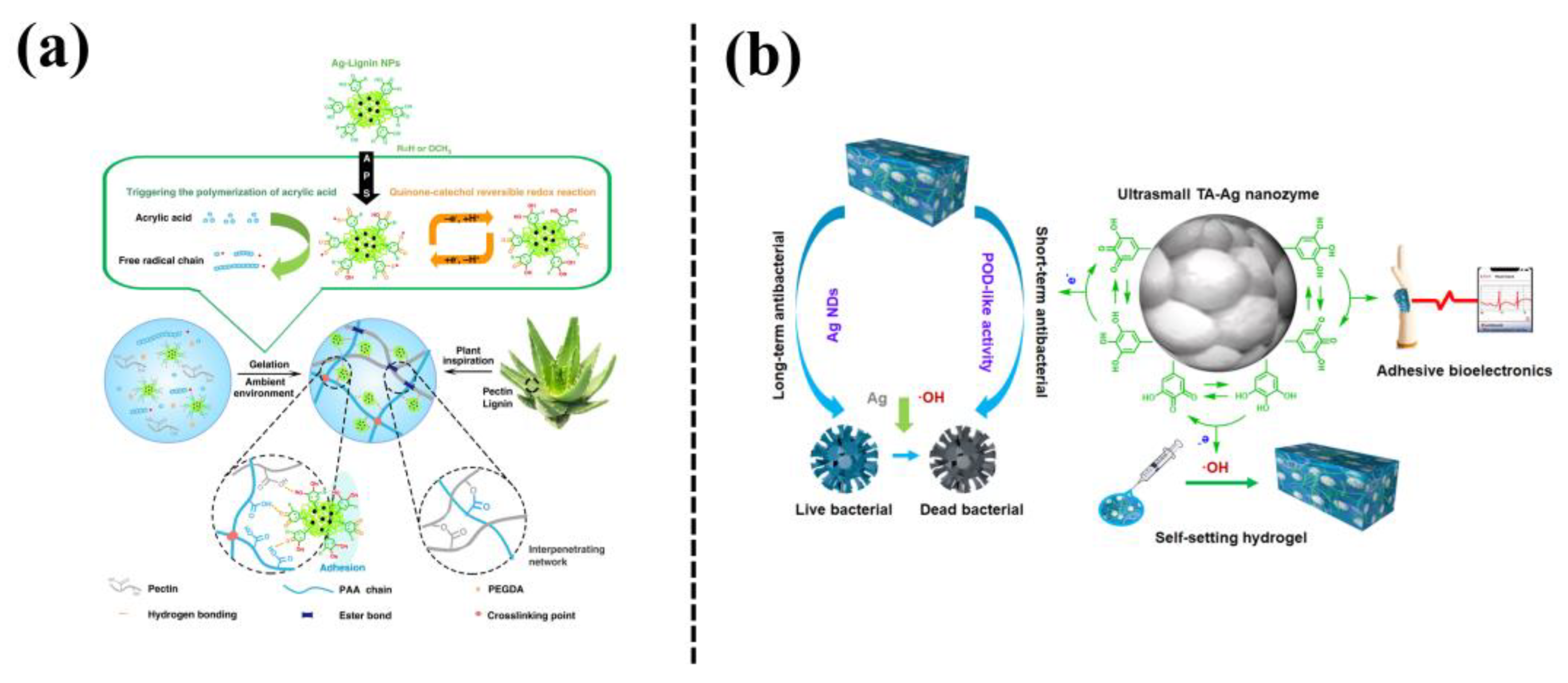
Figure 6. (a) Fabrication process of plant-inspired catechol-chemistry-based hydrogels by incorporation of the silver-lignin NPs to achieve self-adhesive, tough, and antibacterial properties [24]. (b) The TA-Ag nanozyme-loaded polyacrylic acid hydrogel (TA-Ag-PAA) with conductive and self-setting properties [25].
6. Polydopamine Nanoparticles (PDA-NPs)
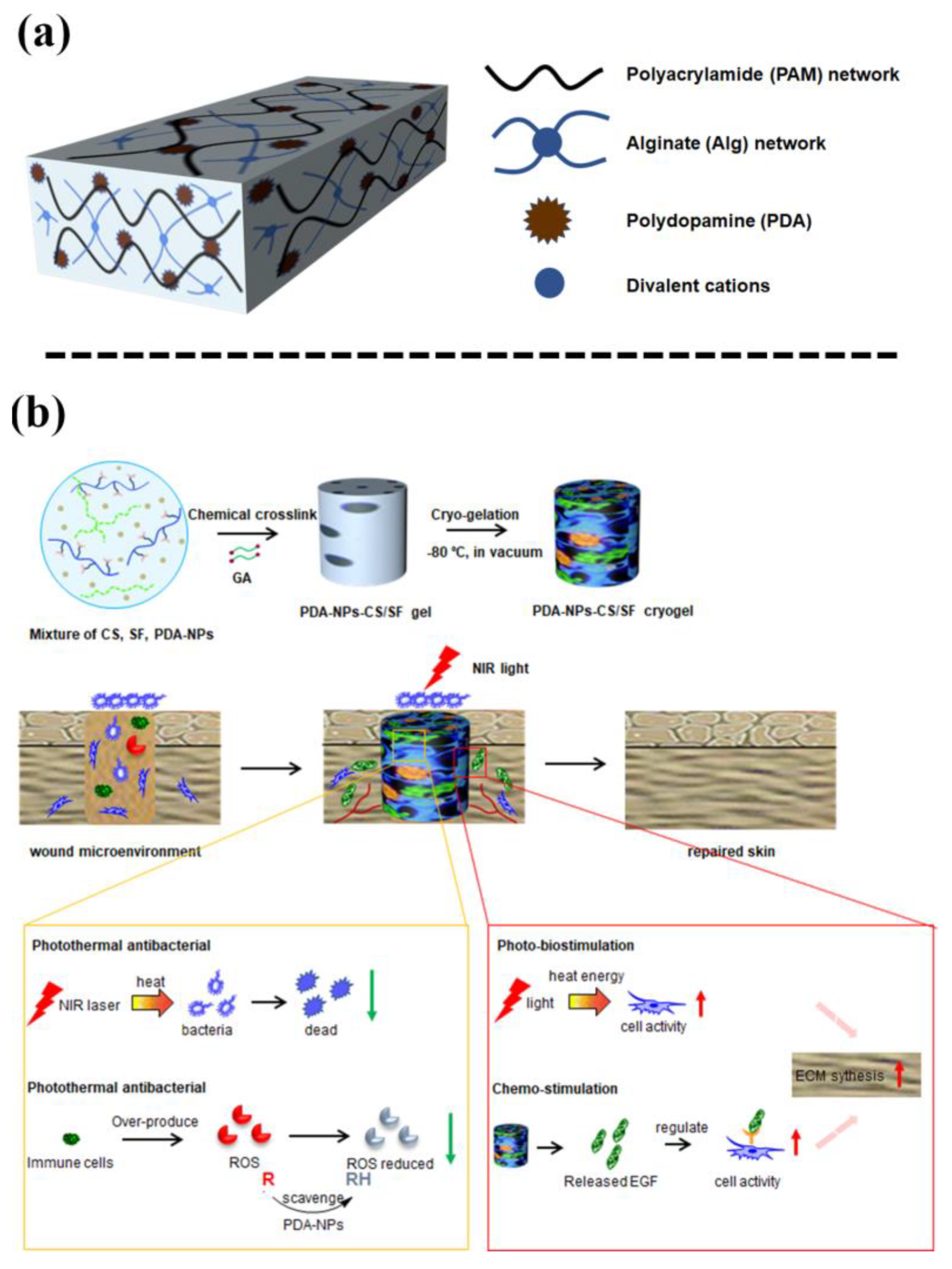
PDA-NPs, formed by self-polymerization of PDA, have been widely used in diagnosing and treating diseases owing to their excellent properties, such as good adhesiveness, anti-oxidative ability, photothermal conversion ability, drug loading ability, and biocompatibility [26]. PDA-NPs with highly reactive catechol groups can improve the adhesive and mechanical properties of hydrogels. For example, Liang et al. [27] incorporated PDA-NPs into a dual-network of polyacrylamide and alginate, thereby enhancing the adhesive ability of the hydrogel in a seawater environment (Figure 7a). The optimal adhesive strength of the hydrogel in seawater can be as high as 146.84 ± 7.78 kPa. In addition, one study also prepared a cryogel for wound dressing by incorporating PDA-NPs into a chitosan (CS) and silk fibroin (SF) hybrid network [28] (Figure 7b). The PDA-NPs endowed the cryogel with high elasticity and flexibility, cell affinity, and strong antibacterial activity under NIR irradiation. Consequently, the PDA-NPs-CS-SF cryogel accelerated wound healing under NIR irradiation.
Owing to the excellent photothermal conversion ability of PDA-NPs, thermal responsive polymers such as poly(n-isopropyl acrylamide) (PNIPAM) are generally used to develop NIR responsive nanocomposite hydrogels. For a successful example, one study designed a NIR responsive hydrogel by introducing PDA-NPs into a PNIPAM/PAM hybrid network [29] (Figure 8a). The photothermal effect of the NIR-responsive PDA-NPs endowed the PNIPAM-based hydrogel with pulsed drug release, NIR drive, and NIR response-self-healing capabilities, in addition to an improved cell affinity and tissue adhesion. Di et al. [30] also synthesized a nanocomposite hydrogel with excellent durability and repeatable adhesion based on PDA-NPs, clay, and PNIPAM (Figure 8b). The hydrogel achieved thermal-responsiveness and local controllable deformation under remote NIR irradiation, owing to the phase transition and volume change of the PNIPAM network.
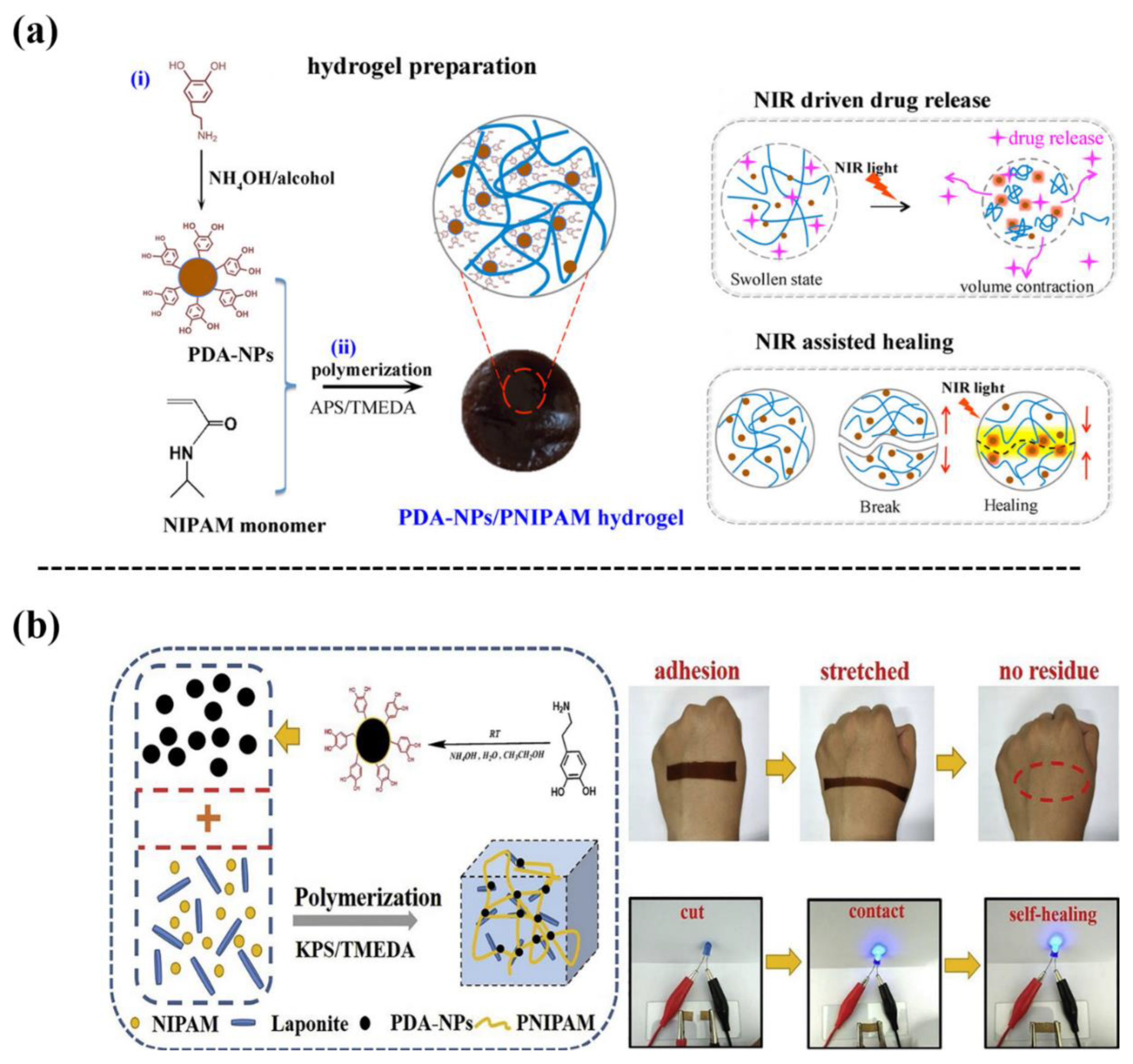
Figure 8. (a) Incorporating PDA-NPs into a PNIPAM hydrogel to fabricate a PDA-NPs/PNIPAM hydrogel with NIR responsiveness, self-healing, on-demand drug releasing, and adhesive properties [29]. (b) Fabrication of a PDA-NPs and laponite-embedded nanocomposite hydrogel with stretchability, conductivity, dual light- and thermo-responsive, and adhesive properties [30].
In addition, PDA-NPs have a large number of phenolic hydroxyl groups on their surfaces, which can be smart nanocarriers for drug loading by combining their photothermal conversion ability. In a recent report, PDA-NPs were used to load bortezomib (BTZ) and doxorubicin (DOXO), and then the drug-loaded PDA-NPs were incorporated into a PNIPAM-co-PAAM hydrogel (Figure 9a). The PDA-NPs served as photothermal agents, facilitating the controlled release of DOXO to kill the tumor cells under NIR irradiation [31]. Wang et al. [32] also used PDA-NPs as crosslinking agents for mercaptoylated four-arms PEG (4-arms-PEG-SH) to form an injectable PDA/PEG hydrogel for on-demand administration and chemotherapy-photothermal combination therapy (Figure 9b). The anticancer drug 7-ethyl-10-hydroxycamptothecin (SN38) was loaded onto PDA-NPs via π-π interactions and hydrogen bonding. Under NIR irradiation, the PDA/PEG hydrogel achieved the controlled release of SN38 to ablate solid tumors. Using the same method, ciprofloxacin (Cip) was loaded on PDA-NPs and then the Cip-loaded PDA-NPs were mixed with ethylene glycol chitosan to form an injectable hydrogel (Gel-Cip) [33] (Figure 9c). Under NIR irradiation, the Gel-Cip achieved a controlled release of Cip. Simultaneously, NIR irradiation activated the photothermal PDA-NPs, resulting in local high temperatures to damage the bacterial integrity. In a mouse model of skin injury caused by Staphylococcus aureus infection, the Gel-Cip hydrogel promoted the regeneration of blood vessels and hair follicles, along with epidermal thickening and fibroblast proliferation, with the assistance of NIR irradiation. In short, the photothermal conversion ability of PDA-NPs has been widely employed for designing thermal-responsive hydrogel to realize antibacterial, drug responsive release, and tumor killing under NIR irradiation.
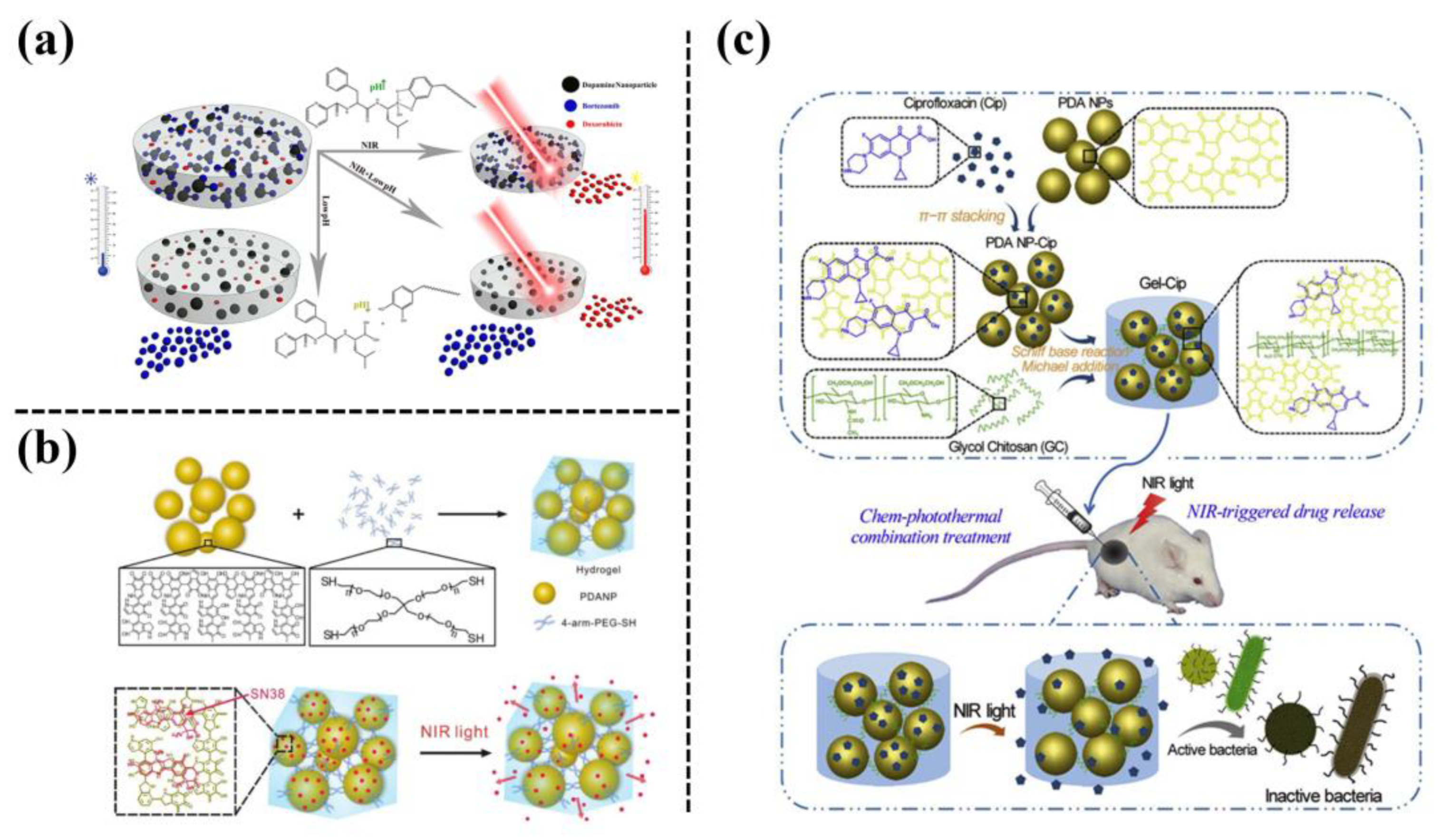
Figure 9. (a) Schematic of a multi-stimulus responsive mussel-inspired hybrid hydrogel as a single platform for synergistic anticancer treatment by combining PTT and multidrug chemotherapy [31]. (b) Preparation of an NIR-responsive hydrogel by using PDA-NPs as a crosslinking agent to crosslink 4-arm-PEG-SH to realize NIR-triggered drug release [32]. (c) Preparation of an NIR light-triggerable thermo-sensitive hydrogel by mixing ciprofloxacin (Cip)-loaded PDA-NPs with glycol chitosan (GC) [33].
7. Conductive-Polymer Nanoparticles
Conductive polymers (CPs) exhibit unique electronic/ionic conductivity and biocompatibility. These mainly include polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), polythiophene (PTH), and polyaniline (PANI) [34]. The CPs are designed in the form of nanoparticles that are then incorporated into a polymer network to form conductive hydrogels. However, it is challenging to prepare CP nanoparticle-based hydrogels because the poor water solubility and hydrophobic nature of CPs has limited their integration with hydrophilic hydrogel networks. One group reported a mussel-inspired strategy by using highly hydrophilic and active catechol groups to dope the CPs during processing, which shed light on how to construct hydrophilic CPs nanoparticles and prepare adhesive and conductive hydrogels. For example, a transparent, conductive, stretchable, and self-adhesive hydrogel was designed by the in situ formation of PDA-doped-PPy nanofibrils in a polyacrylamide (PAM) network [35] (Figure 10a). The obtained PDA-PPy-PAM hydrogel can be used in self-adhesive biosensors, to be directly adhered to the human body to detect biosignals about human health. Inspired by the same mechanism, Chen et al. [36] synthesized a PPy-PDA/polyacrylic acid (PAA) hydrogel, which possessed excellent tissue adhesiveness, electrical conductivity, and antioxidant ability (Figure 10b). The hydrogel was used as a wound dressing, as demonstrated in a rabbit wound model. In another study, plant-derived sulfonated lignin was employed for doping a variety of CPs to prepare hydrophilic conductive nanoparticles (CP/LS NPs), and then the CP/LS NPs were incorporated into hydrogels [37] (Figure 10c). In addition, the CP/LS NPs created a dynamic redox environment to maintain the balance of catechol and quinone groups, similar to the behavior of mussels, which endowed the hydrogel with a high and repeatable adhesiveness. This conductive and adhesive hydrogel has the potential to be used for tissue regeneration and implantable bioelectronics owing to its good electrical activity and biocompatibility. In one recent study, conductive and hydrophilic dPEDOT NPs were prepared by confining the polymerization of 3,4-ethylenedioxythiophene (EDOT) with the assistance of dopamine methacrylate (DMA) oligomer templates. The dPEDOT NPs were then incorporated into a PAM hydrogel, which endowed the hydrogel with high conductivity, brain-level modulus, robust adhesiveness, and an immune-evasive ability [38]. Consequently, the hydrogel can be integrated with metallic microcircuits to form nondestructive, and conformal brain-machine interfaces, enabling long-term and accurate electro-encephalographic signal acquisition and communication with brain tissue (Figure 10d). In short, the introduction of mussel inspired strategies to fabricate conductive polymer NPs can not only ensure their conductivity but also improve their integration with polymer hydrogels, which can be widely applied in biomedical applications.
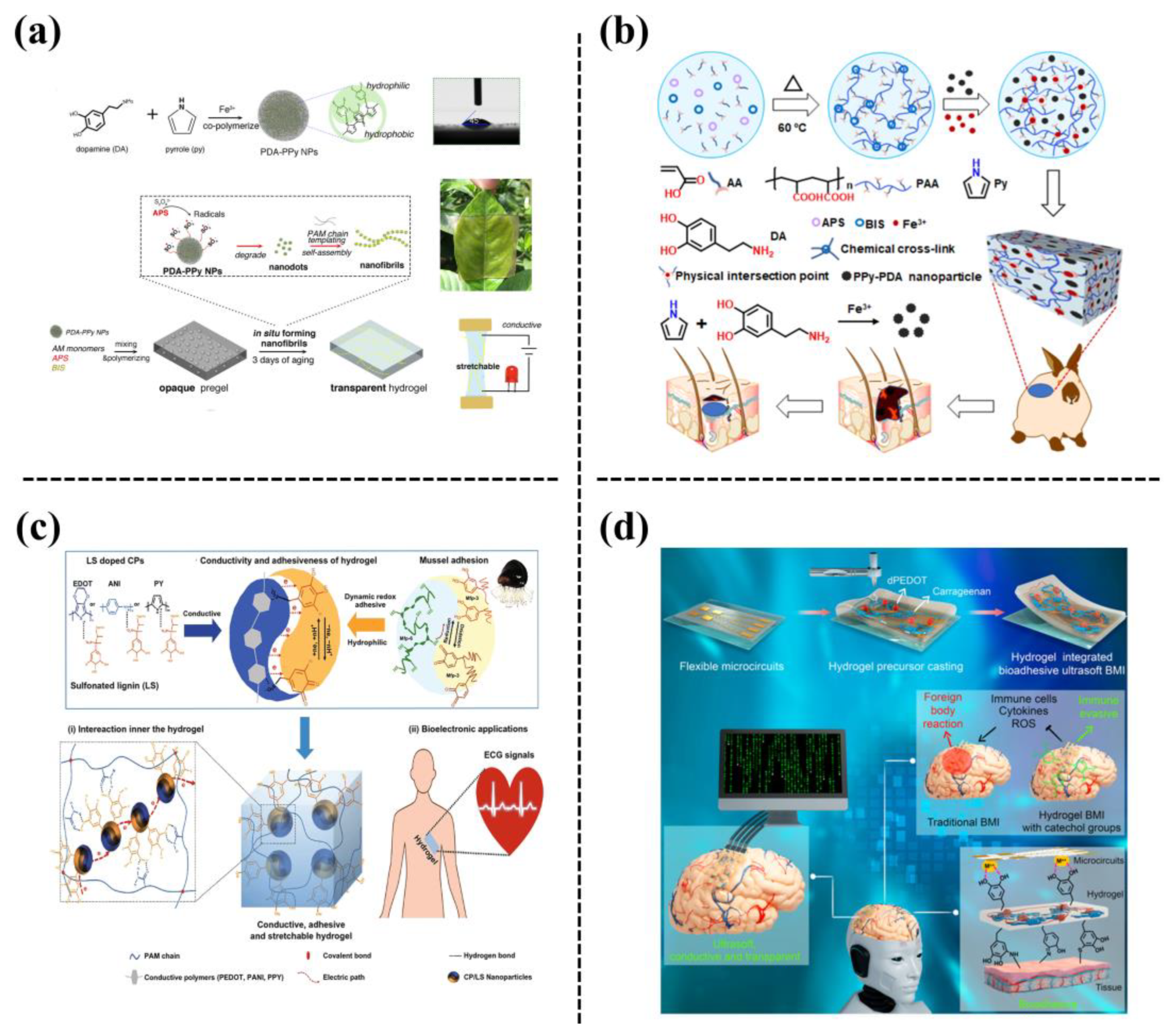
Figure 10. (a) A transparent, conductive, stretchable, and adhesive hydrogel was formed by in-situ formation of PDA-PPy nanofibrils in a PAM hydrogel [35]. (b) Schematic of the fabrication processes and application of the PPy-PDA/PAA hydrogels [36]. (c) Preparation of conductive and adhesive hydrogel based on hydrophilic and redox-active conductive-polymer/sulfonated lignin (CP/LS) NPs [37]. (d) A bioadhesive ultra-soft brain-machine interface (BMI) was fabricated based on integration of metallic microcircuits with a dopamine methacrylate-hybridized poly(3,4-ethylenedioxythiophene) nanoparticle (dPEDOT NP)-loaded hydrogel [38].
References
- Gan, D.; Wang, Z.; Xie, C.; Wang, X.; Xing, W.; Ge, X.; Yuan, H.; Wang, K.; Tan, H.; Lu, X. Mussel-Inspired Tough Hydrogel with in Situ Nanohydroxyapatite Mineralization for Osteochondral Defect Repair. Adv. Healthc. Mater. 2019, 8, 1901103.
- Liu, C.; Wu, J.; Gan, D.; Li, Z.; Shen, J.; Tang, P.; Luo, S.; Li, P.; Lu, X.; Zheng, W. The Characteristics of Mussel-Inspired nHA/OSA Injectable Hydrogel and Repaired Bone Defect in Rabbit. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 1814–1825.
- Wang, B.; Liu, J.; Niu, D.; Wu, N.; Yun, W.; Wang, W.; Zhang, K.; Li, G.; Yan, S.; Xu, G.; et al. Mussel-Inspired Bisphosphonated Injectable Nanocomposite Hydrogels with Adhesive, Self-Healing, and Osteogenic Properties for Bone Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 32673–32689.
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent Advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front. Chem. 2020, 8, 124.
- Fragal, E.H.; Fragal, V.H.; Silva, E.P.; Paulino, A.T.; da Silva Filho, E.C.; Maurício, M.R.; Silva, R.; Rubira, A.F.; Muniz, E.C. Magnetic-Responsive Polysaccharide Hydrogels as Smart Biomaterials: Synthesis, Properties, and Biomedical Applications. Carbohydr. Polym. 2022, 292, 119665.
- Mertz, D.; Harlepp, S.; Goetz, J.; Bégin, D.; Schlatter, G.; Bégin-Colin, S.; Hébraud, A. Nanocomposite Polymer Scaffolds Responding under External Stimuli for Drug Delivery and Tissue Engineering Applications. Adv. Therap. 2020, 3, 1900143.
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.; Xu, F. Magnetic Hydrogels and Their Potential Biomedical Applications. Adv. Funct. Mater. 2013, 23, 660–672.
- Dai, G.; Sun, L.; Xu, J.; Zhao, G.; Tan, Z.; Wang, C.; Sun, X.; Xu, K.; Zhong, W. Catechol-Metal Coordination-Mediated Nanocomposite Hydrogels for on-Demand Drug Delivery and Efficacious Combination Therapy. Acta Biomater. 2021, 129, 84–95.
- Han, L.; Yan, L.; Wang, K.; Fang, L.; Zhang, H.; Tang, Y.; Ding, Y.; Weng, L.-T.; Xu, J.; Weng, J.; et al. Tough, Self-Healable and Tissue-Adhesive Hydrogel with Tunable Multifunctionality. NPG Asia Mater. 2017, 9, 372.
- Yang, B.; Chen, Y.; Shi, J. Mesoporous Silica/Organosilica Nanoparticles: Synthesis, Biological Effect and Biomedical Application. Mater. Sci. Eng. R. Rep. 2019, 137, 66–105.
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-Property Relationship for Different Mesoporous Silica Nanoparticles and its Drug Delivery Applications: A Review. Front. Chem. 2022, 10, 823785.
- Knežević, N.Ž.; Durand, J.O. Large Pore Mesoporous Silica Nanomaterials for Application in Delivery of Biomolecules. Nanoscale 2015, 7, 2199–2209.
- Huang, J.; Wang, S.; Wang, X.; Zhu, J.; Wang, Z.; Zhang, X.; Cai, K.; Zhang, J. Combination Wound Healing Using Polymer Entangled Porous Nanoadhesive Hybrids with Robust ROS Scavenging and Angiogenesis Properties. Acta Biomater. 2022, 152, 171–185.
- Jung, H.; Kim, M.K.; Lee, J.Y.; Choi, S.W.; Kim, J. Adhesive Hydrogel Patch with Enhanced Strength and Adhesiveness to Skin for Transdermal Drug Delivery. Adv. Funct. Mater. 2020, 30, 2004407.
- Pinnaratip, R.; Forooshani, P.K.; Li, M.; Hu, Y.H.; Rajachar, R.M.; Lee, B.P. Controlling the Release of Hydrogen Peroxide from Catechol-Based Adhesives Using Silica Nanoparticles. ACS Biomater. Sci. Eng. 2020, 6, 4502–4511.
- Barati, D.; Gegg, C.; Yang, F. Nanoparticle-Mediated TGF-β Release from Microribbon-Based Hydrogels Accelerates Stem Cell-Based Cartilage Formation in Vivo. Ann. Biomed. Eng. 2020, 48, 1971–1981.
- Fu, Y.; Cen, D.; Zhang, T.; Jiang, S.; Wang, Y.; Cai, X.; Li, X.; Han, G. Implantable Fibrous Scaffold with Hierarchical Microstructure for the ‘on-Site’ synergistic Cancer Therapy. Chem. Eng. J. 2020, 402, 126204.
- Jiang, Y.; Pan, X.; Yao, M.; Han, L.; Zhang, X.; Jia, Z.; Weng, J.; Chen, W.; Fang, L.; Wang, X.; et al. Bioinspired Adhesive and Tumor Microenvironment Responsive NanoMOFs Assembled 3D-Printed Scaffold for Anti-Tumor Therapy and Bone Regeneration. Nano Today 2021, 39, 101182.
- Liu, Y.; Huo, Y.; Li, M.; Qin, C.; Liu, H. Synthesis of Metal-Organic-Frameworks on Polydopamine Modified Cellulose Nanofibril Hydrogels: Constructing Versatile Vehicles for Hydrophobic Drug Delivery. Cellulose 2022, 29, 379–393.
- Liu, Y.; Huo, Y.; Fan, Q.; Li, M.; Liu, H.; Li, B.; Li, Y. Cellulose Nanofibrils Composite Hydrogel with Zeolitic Imidazolate Framework-8 Encapsulated in Used as Efficient Vehicles for Controlled Drug Release. J. Ind. Eng. Chem. 2021, 102, 343–350.
- Li, H.; Qu, J. Mussel-Inspired Synthesis of Silver Nanoparticles as Fillers for Preparing Waterborne Polyurethane/Ag Nanocomposites with Excellent Mechanical and Antibacterial Properties. Polym. Int. 2022, 71, 146–153.
- Zhao, Y.; Li, Z.; Song, S.; Yang, K.; Liu, H.; Yang, Z.; Wang, J.; Yang, B.; Lin, Q. Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv. Funct. Mater. 2019, 29, 1901474.
- Guo, J.; Sun, W.; Kim, J.P.; Lu, X.; Li, Q.; Lin, M.; Mrowczynski, O.; Rizk, E.B.; Cheng, J.; Qian, G.; et al. Development of Tannin-Inspired Antimicrobial Bioadhesives. Acta Biomater. 2018, 72, 35–44.
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-Inspired Adhesive and Tough Hydrogel Based on Ag-Lignin Nanoparticles-Triggered Dynamic Redox Catechol Chemistry. Nat. Commun. 2019, 10, 1487.
- Jia, Z.; Lv, X.; Hou, Y.; Wang, K.; Ren, F.; Xu, D.; Wang, Q.; Fan, K.; Xie, C.; Lu, X. Mussel-Inspired Nanozyme Catalyzed Conductive and Self-Setting Hydrogel for Adhesive and Antibacterial Bioelectronics. Bioact. Mater. 2021, 6, 2676–2687.
- Tang, Y.; Tan, Y.; Lin, K.; Zhu, M. Research Progress on Polydopamine Nanoparticles for Tissue Engineering. Front. Chem. 2021, 9, 727123.
- Liang, M.; He, C.; Dai, J.; Ren, P.; Fu, Y.; Wang, F.; Ge, X.; Zhang, T.; Lu, Z. A High-Strength Double Network Polydopamine Nanocomposite Hydrogel for Adhesion under Seawater. J. Mat. Chem. B 2020, 8, 8232–8241.
- Han, L.; Li, P.; Tang, P.; Wang, X.; Zhou, T.; Wang, K.; Ren, F.; Guo, T.; Lu, X. Mussel-Inspired Cryogels for Promoting Wound Regeneration through Photobiostimulation, Modulating Inflammatory Responses and Suppressing Bacterial Invasion. Nanoscale 2019, 11, 15846–15861.
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine Nanoparticles Modulating Stimuli-Responsive Pnipam Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100.
- Di, X.; Kang, Y.; Li, F.; Yao, R.; Chen, Q.; Hang, C.; Xu, Y.; Wang, Y.; Sun, P.; Wu, G. Poly(N-Isopropylacrylamide)/Polydopamine/Clay Nanocomposite Hydrogels with Stretchability, Conductivity, and Dual Light- and Thermo- Responsive Bending and Adhesive Properties. Colloid Surf. B Biointerfaces 2019, 177, 149–159.
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594.
- Wang, X.; Wang, C.; Wang, X.; Wang, Y.; Zhang, Q.; Cheng, Y. A Polydopamine Nanoparticle-Knotted Poly(Ethylene Glycol) Hydrogel for On-Demand Drug Delivery and Chemo-Photothermal Therapy. Chem. Mater. 2017, 29, 1370–1376.
- Gao, G.; Jiang, Y.W.; Jia, H.R.; Wu, F.G. Near-Infrared Light-Controllable on-Demand Antibiotics Release Using Thermo-Sensitive Hydrogel-Based Drug Reservoir for Combating Bacterial Infection. Biomaterials 2019, 188, 83–95.
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of Conductive Polymer Hydrogels and Their Application in Sensors. J. Polym. Sci. 2019, 57, 1606–1621.
- Han, L.; Yan, L.; Wang, M.; Wang, K.; Fang, L.; Zhou, J.; Fang, J.; Ren, F.; Lu, X. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018, 30, 5561–5572.
- Chen, Q.; Feng, L.; Cheng, H.; Wang, Y.; Wu, H.; Xu, T.; Zhao, W.; Zhao, C. Mussel-Inspired Ultra-Stretchable, Universally Sticky, and Highly Conductive Nanocomposite Hydrogels. J. Mat. Chem. B 2021, 9, 2221–2232.
- Gan, D.; Shuai, T.; Wang, X.; Huang, Z.; Ren, F.; Fang, L.; Wang, K.; Xie, C.; Lu, X. Mussel-Inspired Redox-Active and Hydrophilic Conductive Polymer Nanoparticles for Adhesive Hydrogel Bioelectronics. Nano Micro Lett. 2020, 12, 169.
- Wang, X.; Sun, X.; Gan, D.; Soubrier, M.; Chiang, H.-Y.; Yan, L.; Li, Y.; Li, J.; Yu, S.; Xia, Y.; et al. Bioadhesive and Conductive Hydrogel-Integrated Brain-Machine Interfaces for Conformal and Immune-Evasive Contact with Brain Tissue. Matter 2022, 5, 1204–1223.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
825
Revisions:
3 times
(View History)
Update Date:
14 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




