
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Reinhard Werth | -- | 4679 | 2023-04-11 11:54:31 | | | |
| 2 | Lindsay Dong | Meta information modification | 4679 | 2023-04-13 08:03:19 | | |
Video Upload Options
Theories have been presented to explain the nature of dyslexia, but the causes of dyslexia remained unclear. Although the investigation of the causes of dyslexia presupposes a clear understanding of the concept of cause, such an understanding is missing. The causes of impaired reading include: an incorrect fixation location, too short a fixation time, the attempt to recognize too many letters simultaneously, too large saccade amplitudes, and too short verbal reaction times. It is assumed that a longer required fixation time in dyslexic readers results from a functional impairment of areas V1, V2, and V3 that require more time to complete temporal summation. These areas and areas that receive input from them, such as the fusiform gyrus, are assumed to be impaired in their ability to simultaneously process a string of letters. When these impairments are compensated by a new reading strategy, reading ability improves immediately.
1. Introduction
2. Causes of Dyslexia
2.1. Necessary Conditions, Sufficient Conditions, and Causes
2.2. Dyslexia Is Not Always Due to an Impaired Visual Attention Span, to Lateral Masking, or a Phononological Impairment
2.3. The Role of Inappropriate Reading Eye Movements in Dyslexia
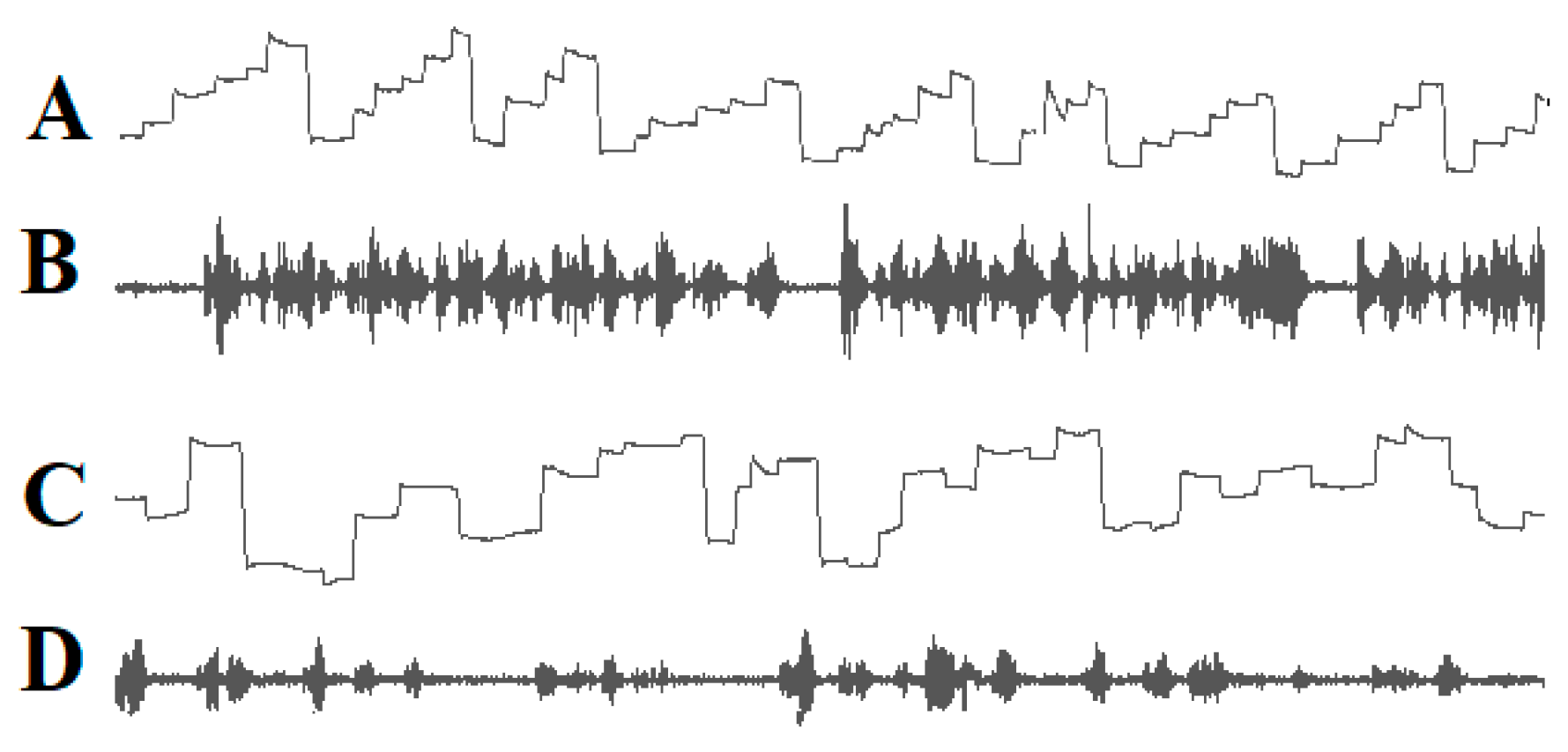
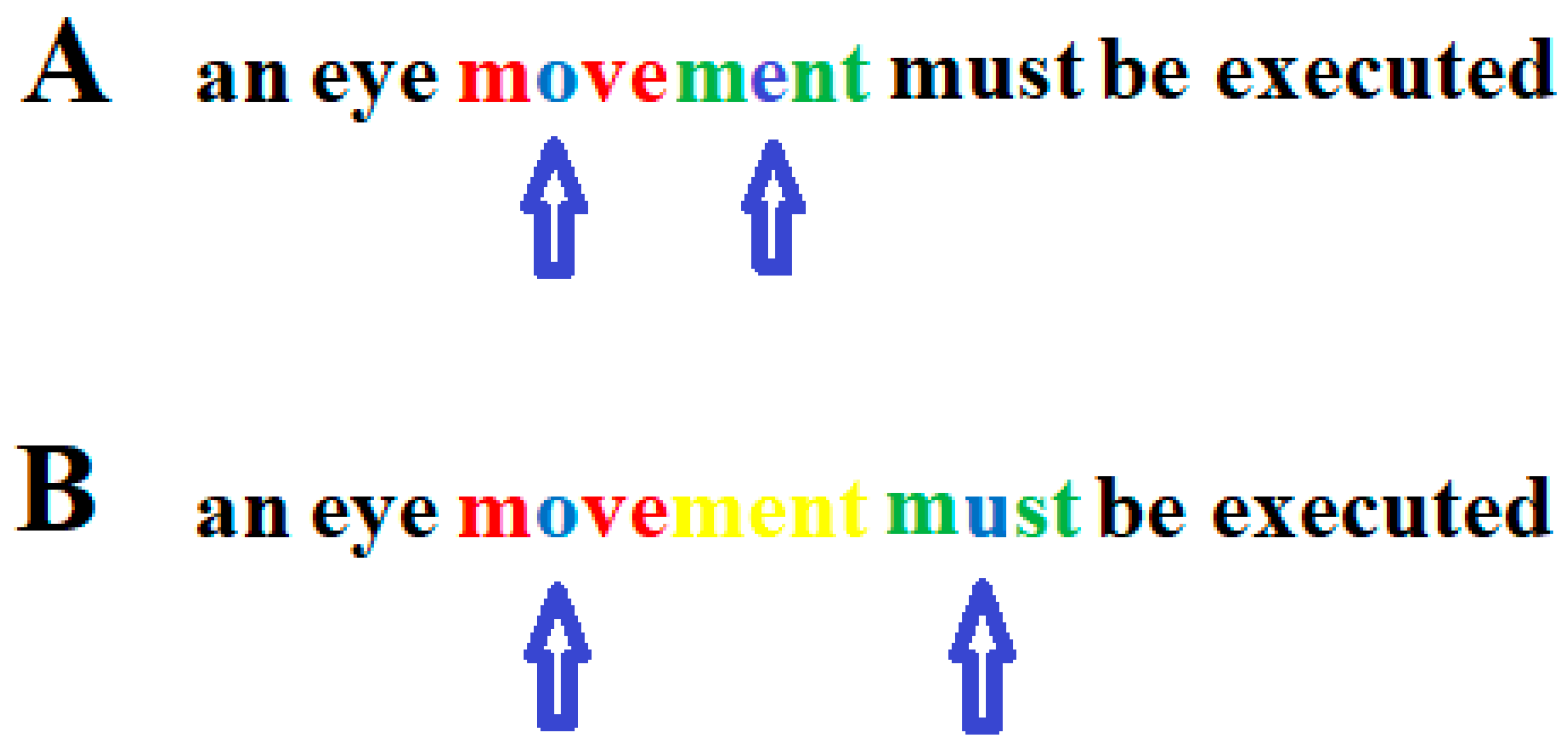
2.4. DD Is Not Caused by a Phonological Impairment
References
- Berkhan, O. Über Störungen der Sprache und der Schriftsprache: Für Ärzte und Lehrer Dargestellt; August Hirschwald: Berlin, Germany, 1889.
- Berlin, R. Über Dyslexie. Arch. Psychiat. Nervenkrankh. 1884, 15, 276–278.
- Helland, T. Trends in dyslexia research during the period 1950 to 2020—Theories, definitions, and publications. Brain Sci. 2022, 12, 1323.
- Stein, J. Theories about developmental dyslexia. Brain Sci. 2023, 13, 208.
- Livingstone, M.S.; Rosen, G.D.; Drislane, F.W.; Galaburda, A.M. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc. Natl. Acad. Sci. USA 1991, 88, 7943–7947.
- Stein, J.; Walsh, V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997, 20, 147–152.
- Stein, J.; Walsh, V. Impaired neural timing in developmental dyslexia—The magnocellular hypothesis. Dyslexia 1999, 5, 59–77.
- Stein, J. The magnocellular theory of dyslexia. In Developmental Dyslexia, Early Precursors, Neurobehavioral Markers, and Biological Substrates; Benasich, A.A., Fitch, R.H., Eds.; Brooks: Baltimore, MD, USA; London, UK; Sydney, Australia, 2012.
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychologia 2019, 130, 66–77.
- Geiger, G.; Lettvin, J.Y. Peripheral vision in persons with dyslexia. N. Engl. J. Med. 1987, 316, 1238–1243.
- Geiger, G.; Lettvin, J.Y.; Fahle, M. Dyslexic children learn a new visual strategy for reading: A controlled experiment. Vis. Res. 1993, 34, 1223–1233.
- Spinelli, D.; De Luca, M.; Judica, A.; Zoccolotti, P. Crowding effects on word identification in developmental dyslexia. Cortex 2002, 38, 179–200.
- Lorusso, M.L.; Facoetti, A.; Pesenti, S.; Cattaneo, C.; Molteni, M.; Geiger, G. Wider recognition in peripheral vision common to different subtypes of dyslexia. Vis. Res. 2004, 44, 2413–2424.
- Whitney, D.; Levi, D.M. Visual crowding: A fundamental limit on conscious perception and object recognition. Trends. Cogn. Sci. 2011, 15, 160–168.
- Zorzi, M.; Barbiero, C.; Facoetti, A.; Lonciari, I.; Carrozzi, M.; Montico, M.; Bravar, L.; George, F.; Pech-Georgel, C.; Ziegler, J.C. Extra-large letter spacing improves reading in dyslexia. Proc. Natl. Acad. Sci. USA 2012, 109, 11455–11459.
- Gori, S.; Facoetti, A. How the visual aspects can be crucial in reading acquisition? The intriguing case of crowding and developmental dyslexia. J. Vis. 2015, 14, 15.1.8.
- Strasburger, H. Seven Myths on Crowding and Peripheral Vision. Iperception 2020, 11, 2041669520913052.
- Kirkby, J.A.; Barrington, R.S.; Drieghe, D.; Liversedge, S.P. Parafoveal processing and transposed-letter effects in dyslexic reading. Dyslexia 2022, 28, 359–374.
- Kewan-Khalayly, B.; Migó, M.; Yashar, A. Transient attention equally reduces visual crowding in radial and tangential axes. J. Vis. 2022, 22, 3.
- Werth, R. Rapid improvement of reading performance in children with dyslexia by altering the reading strategy: A novel approach to diagnoses und therapy of reading deficiencies. Restor. Neurol. Neurosci. 2018, 36, 679–691.
- Werth, R. What causes Dyslexia? Identifying the causes and effective compensatory therapy. Restor. Neurol. Neurosci. 2019, 37, 591–608.
- Werth, R. Dyslexic readers improve without training when using a computer-guided reading strategy. Brain Sci. 2021, 11, 526.
- Werth, R. Is developmental dyslexia due to a visual and not a phonological impairment? Brain Sci. 2021, 11, 1313.
- Tallal, P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980, 9, 182–198.
- Tallal, P.; Miller, S.; Fitch, R.H. Neurobiological basis of speech: A case for the pre-eminence of temporal processing. Ann. N. Y. Acad. Sci. 1993, 682, 27–47.
- Merzenich, M.M.; Jenkins, W.M.; Johnston, P.; Schreiner, C.; Miller, S.L.; Tallal, P. Temporal processing deficits of language learning impaired children ameliorated by training. Science 1996, 271, 77–81.
- Nagarajan, S.; Mahnke, H.; Salz, T.; Tallal, P.; Roberts, T.; Merzenich, M.M. Cortical auditory signal processing in poor readers. Proc. Natl. Acad. Sci. USA 1999, 96, 6483–6488.
- Pavlidis, G.T. Do eye movements hold the key to dyslexia? Neuropsychologia 1981, 19, 57–64.
- Rayner, K.; Pollatsek, A. Eye movement control during reading: Evidence for direct control. Q. J. Exp. Psychol. Sect. A 1982, 33, 351–373.
- Pavlidis, G.T. Eye movements in dyslexia: Their diagnostic significance. J. Learn. Disabil. 1985, 18, 42–50.
- Rayner, K. Do faulty eye movements cause dyslexia? Dev. Neuropsychol. 1985, 1, 3–15.
- Rainer, K. Eye movements and the perceptual span in beginning and skilled readers. J. Exp. Child Psychol. 1986, 41, 211–236.
- Fischer, B.; Biscaldi, M.; Otto, P. Saccadic eye movements of dyslexic adult subjects. Neuropsychologia 1993, 31, 887–906.
- Eden, G.F.F.; Stein, J.F.F.; Wood, H.M.M.; Wood, F.B.B. Differences in eye movements and reading problems in dyslexic and normal children. Vis. Res. 1994, 34, 1345–1358.
- Hyönä, J.; Olson, R.K. Eye fixation patterns among dyslexic and normal readers: Effect of word length and word frequency. J. Exp. Psychol. Learn. Mem. Cogn. 1995, 21, 1430–1440.
- Biscaldi, M.; Gezeck, S.; Stuhr, V. Poor saccadic control correlates with dyslexia. Neuropsychologia 1998, 36, 1189–1202.
- De Luca, M.; Di Pace, E.; Judica, A.; Spinelli, D.; Zoccolotti, P. Eye movement patterns in linguistic and non-linguistic tasks in developmental surface dyslexia. Neuropsychologia 1999, 37, 1407–1420.
- Biscaldi, M.; Fischer, B.; Hartnegg, K. Voluntary saccadic control in dyslexia. Perception 2000, 29, 509–521.
- Rayner, K.; Slattery, T.J.; Bélanger, N.N. Eye movements, the percepzual span, and reading speed. Bull. Rev. 2010, 17, 834–839.
- Ward, L.M.; Kapoula, Z. Dyslexics’ Fragile Oculomotor Control Is Further Destabilized by Increased Text Difficulty. Brain Sci. 2021, 11, 990.
- Temelturk, R.D.; Ozer, E. Binocular coordination of children with dyslexia and typically developing children in linguistic and non-linguistic tasks: Evidence from eye movements. Ann. Dyslexia 2022, 72, 426–444.
- Premeti, A.; Bucci, M.P.; Isel, F. Evidence from ERP and eye movements as markers of language dysfunction in dyslexia. Brain Sci. 2022, 12, 73.
- Caldani, S.; Acquaviva, E.; Moscoso, A.; Peyre, H.; Delorme, R.; Bucci, M.P. Reading performance in children with ADHD: An eye-tracking study. Ann. Dyslexia 2022, 72, 552–565.
- Wagner, R.K.; Torgesen, J.K. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol. Bull. 1987, 101, 192–212.
- Bruce, D. An analysis of word sounds by young children. Br. J. Educ. Psychol. 1964, 34, 158–170.
- Bryant, P.E.; MacLean, M.; Bradley, L.L.; Crossland, J. Rhyme and alliteration, phoneme detection, and learning to read. Dev. Psychol. 1990, 26, 429–438.
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A. Longitudinal studies of phonological processing and reading. J. Learn. Disabil. 1994, 27, 276–286.
- McBride-Chang, C. What is phonological awareness? J. Educ. Psychol. 1995, 87, 179–192.
- Yopp, H.K. A test for assessing phonemic awareness in young children. Read. Teach. 1995, 49, 20–29.
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A. Prevention and remediation of severe reading disabilities: Keeping the end in mind. Sci. Stud. Read. 1997, 1, 217–234.
- Muter, V.; Hulme, C.; Snowling, M.J.; Taylor, S. Segmentation, not rhyming, predicts early progress in learning to read. J. Exp. Child. Psychol. 1998, 71, 3–27.
- Ehri, L.C. Research on learning to read and spell: A personal historical perspective. Sci. Stud. Read. 1998, 2, 97–114.
- Snowling, M. From language to reading and dyslexia. Dyslexia. Dyslexia 2001, 7, 37–46.
- Goswami, U. Phonology, reading development, and dyslexia: A cross-linguistic perspective. Ann. Dyslexia 2002, 52, 139–163.
- Vellutino, F.R.; Fletcher, J.M.; Snowling, M.J. Scanlon, D.M. Specific reading disability (dyslexia): What have we learned in the past four decades. J. Child Psychol. Psychiat. 2004, 45, 2–40.
- Goswami, U. Phonology, reading and reading difficulties. In Interdisciplinary Perspectives on Learning to Read. Culture, Cognition and Pedagogy; Hall, K., Goswami, U., Harrison, C., Ellis, S., Soler, Eds.; Routledge: London, UK, 2010; pp. 103–116.
- Ligges, C.; Lehmann, T. Multiple case study in German children with dyslexia: Characterization of phonological, auditory, visual, and cerebellar processing on the group and individual level. Brain Sci. 2022, 12, 1292.
- Mackie, J.L. Causes and conditions. Am. Philos. Q. 1965, 2, 245–264.
- Spirtes, P.; Glymour, C.; Scheines, R. Causation, Prediction, and Search; MIT Press: Cambridge, UK, 1992.
- Lewis, D. Causation as influence. J. Philos. 2000, 97, 182–197.
- Spohn, W. Causation: An alternative. Brit. J. Philos. Sci. 2006, 57, 93–119.
- Pearl, J.; Glymour, M.; Jewell, N.P. Causal Inference in Statistics; Wiley: Chichester, UK, 2016.
- Pearl, J. Causality—Models, Reasoning and Inference; Cambridge University Press: Cambridge, UK; New York, NY, USA; Port Melbourne, Australia, 2018; pp. 316–320.
- Bressler, S.L.; Seth, A.K. Wiener-Granger causality: A well established methodology. Neuroimage 2011, 58, 323–329.
- Stokes, P.A.; Purdon, P.L. A study of problems encountered in Granger causality analysis from a neuroscience perspective. Proc. Natl. Acad. Sci. USA 2017, 114, E7063–E7072.
- Farah, M.; Stowe, R.M.; Levinson, K.L. Phonological dyslexia. Loss of a reading specific component of cognitive architecture? Cogn. Neuropsychol. 1996, 13, 849–868.
- Hari, R.; Renvall, H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn. Sci. 2001, 5, 525–532.
- Roach, N.W.; Hogben, J.H. Attentional modulation of visual processing in adult dyslexia: A spatial cuing deficit. Psychol. Sci. 2004, 15, 650–654.
- Buchholz, J.; Davis, A.A. Adults with dyslexia demonstrate space-based and object-based covert attention deficits: Shifting attention to the periphery and shifting attention between objects in the left visual field. Brain Cogn. 2005, 57, 30–34.
- Heim, S.; Tschierse, J.; Amunts, K.; Wilms, M.; Vossel, S.; Willmes, K.; Grabowska, A.; Huber, W. Cognitive subtypes of dyslexia. Acta Neurobiol. Exp. 2008, 68, 73–82.
- Daniel, A.; Abrams, D.A.; Nicol, T.; Zecker, S.; Kraus, N. Abnormal cortical processing of the syllable rate of speech in poor readers. J. Neurosci. 2009, 29, 7686–7693.
- Menghini, D.; Finzi, A.; Benassi, M.; Bolzani, R.; Facoetti, A.; Giovagnoli, S.; Ruffino, M.; Vicari, S. Different underlying neurocognitive deficits in developmental dyslexia: A comparative study. Neuropsychologia 2010, 48, 863–872.
- Hornickel, J.; Kraus, N. Unstable representation of sound: A biological marker of dyslexia. J. Neurosci. 2013, 33, 3500–3504.67.
- Lorusso, M.L.; Cantiani, C.; Molteni, M. Age, dyslexia subtype and comorbidity modulate rapid auditory processing in developmental dyslexia. Front. Hum. Neurosci. 2014, 8, 313.
- van Bergen, E.; de Jong, P.F.; Maassen, B.; Krikhaar, E.; Plakas, A.; van der Leij, A. IQ of four-year-olds who go on to develop dyslexia. J. Learn. Disabil. 2014, 47, 475–484.
- Hendren, R.L.; Haft, S.L.; Black, J.M.; White, N.C.; Hoeft, F. Recognizing Psychiatric Comorbidity With Reading Disorders. Front. Psychiatry 2018, 9, 101.
- Peters, L.; Bulthé, J.; Daniels, N.; Op de Beeck, H.; De Smedt, B. Dyscalculia and dyslexia: Different behavioral, yet similar brain activity profiles during arithmetic. Neuroimage Clin. 2018, 18, 663–674.
- Moll, K.; Landerl, K.; Snowling, M.J.; Schulte-Körne, G. Understanding comorbidity of learning disorders: Task-dependent estimates of prevalence. J. Child. Psychol. Psychiat. 2019, 60, 286–294.
- Maziero, S.; Tallet, J.; Bellocchi, S.; Jover, M.; Chaix, Y.; Jucla, M. Influence of comorbidity on working memory profile in dyslexia and developmental coordination disorder. J. Clin. Exp. Neuropsychol. 2020, 42, 660–674.
- Facoetti, A.; Paganoni, P.; Turatto, M.; Marzola, V.; Mascetti, G.G. Visual spatial attention in developmental dyslexia. Cortex 2000, 36, 109–123.
- Carrasco, M.; Williams, P.E.; Yeshurun, Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. J. Vision 2002, 2, 467–479.
- Bosse, M.L.; Tainturier, M.J.; Valdois, S. Developmental dyslexia: The visual attention span deficit hypothesis. Cognition 2007, 104, 198–230.
- Franceschini, S.; Gori, M.; Ruffino, K.; Pedrolli, A.; Facoetti, A. A causal link between visual spatial attention and reading acquisition. Curr. Biol. 2012, 22, 814–819.
- Ruffino, M.; Gori, S.; Boccardi, D.; Molteni, M.; Facoetti, A. Spatial and temporal attention in developmental dyslexia. Front. in Human Neurosci. 2014, 8, 8–331.
- Chen, C.; Schneps, M.H.; Masyn, K.E.; Thomson, J.M. The effects of visual attention span and phonological decoding in reading comprehension in dyslexia: A path analysis. Dyslexia 2016, 22, 322–344.
- Facoetti, A.; Turatto, M.; Lorusso, M.L.; Mascetti, G.G. Orienting of visual attention in dyslexia: Evidence for asymmetric hemispheric control of attention. 2001, 138, 46–53. Exp. Brain Res. 2001, 138, 46–53.
- Facoetti, A.; Lorusso, M.L.; Paganoni, P.; Cattaneo, C.; Galli, R.; Umiltà, C.; Mascetti, G.G. Auditory and visual automatic attention deficits in developmental dyslexia. Brain Res. Cogn. Brain Res. 2003, 16, 185–191.
- Roach, N.W.; Hogben, J.H. Spatial cueing deficits in dyslexia reflect generalised difficulties with attentional selection. Vision Res. 2008, 48, 193–207.
- Facoetti, A.; Zorzi, M.; Cestnick, L.; Lorusso, M.L.; Molteni, L.M.; Paganoni, P.; Umilta, C.; Mascetti, G.G. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cogn. Neuropsych. 2006, 23, 841–855.
- Pina Rodrigues, A.; Castelo-Branco, M.; van Asselen, M. Disrupted spatial organization of cued exogenous attention persists into adulthood in developmental dyslexia. Front. Psychol. 2021, 12, 769237.
- Skottun, B.C.; Skoyles, J. Dyslexia: Sensory deficits or inattention? Perception 2007, 36, 1084–1088.
- Skottun, B.C. On the use of cues to assess attention in dyslexia. Front. Hum. Neurosci. 2014, 8, 983.
- De Luca, M.; Burani, C.; Paizi, D.; Spinelli, D.; Zoccolotti, P. Letter and letter-string processing in developmental dyslexia. Cortex 2010, 46, 1272–1283.
- Zoccolotti, P.; De Luca, M.; Spinelli, D. Discrete versus multiple word displays: A re-analysis of studies comparing dyslexic and typically developing children. Front. Psychol. 2015, 6, 1530.
- Burani, C.; Marcolini, S.; Traficante, D.; Zoccolotti, P. Reading derived words by Italian children with and without dyslexia: The effect of root length. Front. Psychol. 2018, 9, 647.
- Franceschini, S.; Bertoni, S.; Puccio, G.; Gori, S.; Termine, C.; Facoetti, A. Visuo-spatial attention deficit in children with reading difficulties. Sci. Rep. 2022, 12, 13930.
- Valdois, S. The visual-attention span deficit in developmental dyslexia: Review of evidence for a visual-attention-based deficit. Dyslexia 2022, 28, 397–415.
- Perry, C.; Long, H. What is going on with visual attention in reading and dyslexia? A critical review of recent studies. Brain Sci. 2022, 12, 87.
- Stenneken, P.; Egetemeir, J.; Schulte-Körne, G.; Müller, H.J.; Schneider, W.X.; Finke, K. Slow perceptual processing at the core of developmental dyslexia: A parameter-based assessment of visual attention. Neuropsychologia 2011, 49, 3454–3465.
- Pammer, K.; Lavis, R.; Hansen, P.; Cornelissen, P.L. Symbol-string sensitivity and children’s reading. Brain Lang. 2004, 89, 601–610.
- Graham, C.H.; Cook, C. Visual acuity as a function of intensity and exposure-time. Am. J. Psychol. 1937, 49, 654–661.
- Barlow, H.B. Temporal and spatial summation in human vision at different background intensities. J. Physiol. Lond. 1958, 141, 337–350.
- Niwa, K.; Tokoro, T. Measurement of temporal summation of visual acuity with use of modified tachistoscope. Jpn. J. Ophthalmol. 1997, 41, 403–408.
- Sandberg, K.; Bibby, B.M.; Timmermans, B.; Cleeremans, A.; Overgaard, M. Measuring consciousness: Task accuracy and awareness as sigmoid functions of stimulus duration. Conscious. Cogn. 2011, 20, 1659–1675.
- McAnany, J.J. The effect of exposure duration on visual acuity for letter optotypes and gratings. Vision Res. 2014, 105, 86–91.
- Windey, B.; Vermeiren, A.; Atas, A.; Cleeremans, A. The graded and dichotomous nature of visual awareness. Philos. Trans. R.Soc. B. Biol. Sci. 2014, 369, 20130282.
- Mulholland, P.J.; Redmond, T.; Garway-Heath, D.F.; Zlatkova, M.B.; Anderson, R.S. The Effect of Age on the Temporal Summation of Achromatic Perimetric Stimuli. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6467–6472.
- Holmes, R.; Victora, M.; Wang, R.F.; Kwiat, P.G. Measuring temporal summation in visual detection with a single-photon source. Vis. Res. 2017, 140, 33–43.
- Stigliani, A.; Jeska, B.; Grill-Spector, K. Encoding model of temporal processing in human visual cortex. Proc. Natl. Acad. Sc.i USA 2017, 114, E11047–E11056.
- Zhou, J.; Benson, N.C.; Kay, K.N. Winawer, J. Compressive temporal summation in human visual cortex. J. Neurosci. 2018, 38, 691–709.
- Beauny, A.; de Heering, A.; Muñoz Moldes, S.; Martin, J.-R.; de Beir, A.; Cleeremans, A. Unconscious categorization of sub-millisecond complex images. PLoS One 2020, 15, e0236467.
- Heinrich, S.P.; Blechenberg, T.; Reichel, C.; Bach, M. The "speed" of acuity in scotopic vs. photopic vision. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2791–2798.
- Harvey, B.M.; Dumoulin, S.O.; Fracasso, A.; Paul, J.M. A Network of Topographic Maps in Human Association Cortex Hierarchically Transforms Visual Timing-Selective Responses. Curr. Biol. 2020, 30, 1424–1434.e6.
- Hendrikx, E.; Paul, J.M.; van Ackooij, M.; van der Stoep, N.; Harvey, B.M. Visual timing-tuned responses in human association cortices and response dynamics in early visual cortex. Nat. Commun. 2022, 8, 3952.
- Paul, J.M.; van Ackooij, M.; Ten Cate, T.C.; Harvey, B.M. Numerosity tuning in human association cortices and local image contrast representations in early visual cortex. Nat. Commun. 2022, 13, 1340.
- Weiner, K.S.; Sayres, R.; Vinberg, J.; Grill-Spector, K. fMRI-adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J. Neurophysiol. 2010, 103, 3349–3365.
- Fornaciai, M.; Brannon, E.M.; Woldorff, M.G.; Park, J. Numerosity processing in early visual cortex. Neuroimage 2017, 157, 429–438.
- DeWind, N.K.; Park, J.; Woldorff, M.G.; Brannon, E.M. Numerical encoding in early visual cortex. Cortex 2019, 114, 76–89.
- Mechelli, A.; Humphreys, G.W.; Mayall, K.; Olson, A.; Price, C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc. Royal Soc. Lond. Series B. 2000, 267, 1909–1913.
- Schurz, M.; Sturm, D.; Richlan, F.; Kronbichler, M.; Ladurner, G.; Wimmer, H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA). Neuroimage 2010, 49, 2649–2661.
- Bálint, D. Seelenlähmung des “Schauens”, optische Ataxie, räumliche Störung der Aufmerksamkeit. Mschr. Psychiat. Neurol. 1909, 25, 51–81.
- Poppelreuter, W. Die Psychischen Schädigungen durch Kopfschuß im Kriege 1914–1918. Bd. I. Die Störungen der Niederen und Höheren Sehleistungen durch Verletzungen des Okzipitalhirns; L. Voss: Leipzig, Germany, 1917.
- Luria, A.R. Disorders of “simultaneous perception” in a case of bilateral occipito-parietal brain injury. Brain 1959, 82, 437–449.
- Baylis, G.C.; Driver, J.; Baylis, L.L.; Rafal, R.D. Reading of letters and words in a patient with Balint’s syndrome. Neuropsychologia 1994, 32, 1273–1286.
- Rizzo., M.; Vecera, S.P. Psychoanatomical substrates of Bálint’s syndrome. J. Neurol. Neurosurg. Psychiatry 2002, 72, 162–178.
- Moreaud, O. Balint syndrome. Arch. Neurol. 2003, 60, 1329–1331.
- Michel, F.; Henaff, M.A. Seeing without the occipito-parietal cortex: Simultagnosia as a shrinkage of the attentional visual field. Behav. Neurol. 2004, 15, 3–13.
- Farah, M.J. Visual Agnosia, 2nd ed.; MIT Press: Cambridge, MA, USA; London, UK, 2004; pp. 43–58.
- Chechlacz, M.; Rotshtein, P.; Hansen, P.C.; Riddoch, J.M.; Deb, S.; Humphreys, G.W. The neural underpinings of simultanagnosia: Disconnecting the visuospatial attention network. J. Cogn. Neurosci. 2012, 247, 18–35.
- Chechlacz, M.; Humphreys, G.W. The enigma of Balint’s syndrome: Neural substrates and cognitive deficits. Front. Hum. Neurosci. 2014, 8, 123.
- Chechlacz, M. Bilateral parietal dysfunctions and disconnections in simultanagnosia and Bálint syndrome. Handb. Clin. Neurol. 2018, 151, 249–267.
- Webster, R.G.; Haslerud, G.M. Influence on extreme peripheral vision of attention to a visual or auditory task. J. Exp. Psychol. 1964, 68, 269–272.
- Engel, F.L. Visual conspicuity, directed attention and retinal locus. Vision Res. 1971, 11, 563–576.
- Ikeda, M.; Takeuchi, T. Influence of foveal load on the functional visual field. Perc. Psychophys. 1975, 18, 225–260.
- Henderson, J.M.; Ferreira, F. Effects of foveal processing difficulty on the perceptual span in reading: Implication for attention and eye movement control. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 417–429.
- Handy, T.C.; Kingstone, A.; Mangun, G.R. Spatial distribution of visual attention: Perceptual sensitivity and response latency. Percept. Psychophys. 1996, 58, 613–627.
- Werth, R. Therapie von Lesestörungen durch Erkennen und Beheben der Ursachen. Ergother. Rehabil. 2006, 9, 6–11.
- Klische, A. Leseschwächen gezielt beheben. Individuelle Diagnose und Therapie mit dem Programm Celeco; Tectum: Marburg, Germany, 2007.




