Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rares Ilie Orzan | -- | 1299 | 2023-04-10 07:57:40 | | | |
| 2 | Conner Chen | Meta information modification | 1299 | 2023-04-12 10:20:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Orzan, R.I.; Pojoga, C.; Agoston, R.; Seicean, R.; Seicean, A. Cholangiocarcinoma Detection and Staging of Extrahepatic Cholangiocarcinom. Encyclopedia. Available online: https://encyclopedia.pub/entry/42893 (accessed on 07 February 2026).
Orzan RI, Pojoga C, Agoston R, Seicean R, Seicean A. Cholangiocarcinoma Detection and Staging of Extrahepatic Cholangiocarcinom. Encyclopedia. Available at: https://encyclopedia.pub/entry/42893. Accessed February 07, 2026.
Orzan, Rares Ilie, Cristina Pojoga, Renata Agoston, Radu Seicean, Andrada Seicean. "Cholangiocarcinoma Detection and Staging of Extrahepatic Cholangiocarcinom" Encyclopedia, https://encyclopedia.pub/entry/42893 (accessed February 07, 2026).
Orzan, R.I., Pojoga, C., Agoston, R., Seicean, R., & Seicean, A. (2023, April 10). Cholangiocarcinoma Detection and Staging of Extrahepatic Cholangiocarcinom. In Encyclopedia. https://encyclopedia.pub/entry/42893
Orzan, Rares Ilie, et al. "Cholangiocarcinoma Detection and Staging of Extrahepatic Cholangiocarcinom." Encyclopedia. Web. 10 April, 2023.
Copy Citation
Extrahepatic cholangiocarcinoma (CCA) is a rare and aggressive type of cancer, presenting as a mass or as a biliary stricture. Depending on their localization, CCAs are classified into intrahepatic, perihilar, and distal. The EUS detection rate for distal CCAs is higher than that for the proximal CCAs. The accuracy of T staging varies between 60 and 80%, and vascular involvement is correctly assessed by conventional EUS.
extrahepatic cholangiocarcinoma
endoscopic ultrasound
1. Introduction
Biliary tract cancer comprises a variety of invasive adenocarcinomas, including extrahepatic and intrahepatic cholangiocarcinoma (CCA), gallbladder carcinoma, and ampullary carcinoma [1]. Although CCA is a rare type of cancer, it is the second-most frequent primary tumor of the liver and the most frequent biliary malignancy, accounting for about 3% of all gastro-intestinal neoplasia [2]. CCAs are associated with a 10–40% 5-year survival rate and show a high recurrence after surgery [3].
2. Pathology
Depending on their localization, CCAs are classified into intrahepatic, perihilar, and distal [4]. Intrahepatic CCAs are located in the segmental and smaller bile ducts of the liver and account for 20% of all cholangiocarcinomas [5][6][7]. Perihilar CCAs or Klatskin tumors account for 50–60% of all cholangiocarcinomas and occur between the segmental bile ducts and at the junction between the cystic and the main hepatic duct [8][9]. Distal CCAs account for 20–30% of cases and involve the common bile duct [10].
Macroscopically, intrahepatic CCA exhibits different growth patterns; the most frequent one is mass-formation (65%), followed by periductal infiltration and intraductal growth [11][12][13]. Perihilar and distal CCAs occur most frequently as nodular plus periductal infiltrating patterns (>80%) [11][14][15]. While periductal infiltration has a longitudinally growth pattern leading to biliary strictures, intraductal tumors tend to grow towards the duct lumina [14][16].
Microscopically, the vast majority of perihilar and distal CCA are mucinous adenocarcinomas (conventional type) or papillary tumors [10][16][17], originating from the columnar mucous cholangiocytes and peribiliary glands [14][17][18][19][20][21].
Precancerous lesions include the following:
-
Intraductal papillary neoplasm (IPNBs) can develop within intrahepatic or extrahepatic bile ducts following the classical adenoma-carcinoma sequence, have fine fibrovascular stalks, often yellow and friable, and may have a clinical and biochemical impact. In most cases, there is a high degree of dysplasia, and the epithelium from which the IPNB arises exhibits flat dysplasia [25][26]. It is possible to identify invasive CCAs in approximately half of IPNB cases, and the pancreaticobiliary subtype is more likely to be associated with invasive CCA than any other subtype [27]. In the case of IPNBs with atypia or stromal invasion, the outcomes are better compared to concurrent invasive carcinoma [28].
-
Intraductal tubulopapillary neoplasm (ITPN) presents as a nodular mass up to 15 mm in size, with the same intraductal growth and tubular pattern as the pancreatic ITPNs [29], but with low mucin production and absent MUC5AC expression. The risk of invasive carcinoma is present in 70–80% of cases (typically tubular carcinoma [26][30]) but with a much better prognosis compared to IPNBs [26].
-
Mucinous cystic neoplasms usually present as multilocular cysts with septation, or show a cyst-in-cyst appearance on preoperative imaging. They have a higher incidence in females and are usually diagnosed at a younger age than IPNBs, with an excellent prognosis when resected [31].
3. Cholangiocarcinoma Detection and Staging
EUS may be helpful in the setting of bile duct dilation if no mass is seen on CT or MRI [32], and unnecessary ERCP can be avoided in about one-third of the patients [33]. EUS evaluation of the biliary tree is performed from the level of the duodenal bulb and the distal part of the gastric antrum, for both biliary strictures and CCAs. Using EUS, they can be visualized either as a mass or as a biliary stricture.
3.1. Biliary Mass Detection
The EUS aspect suggestive of CCA is a mass extending beyond the bile duct wall or periductal infiltration, with a wall thickness of more than 3 mm, or an intraductal mass-growing lesion [34][35] (Figure 1). In previous research, distal tumors which were closer to the EUS transducer were diagnosed in 100% of the cases, while tumors located further from the transducers were only diagnosed in 83% of the cases. Overall, EUS performed better in identifying tumors in comparison to CT or MRI (94, 30, 42%) [36]. Extrahepatic CCAs were diagnosed at an early stage when MRCP was followed by EUS (sensitivity 90% and specificity 98%) [37]. EUS is also useful when assessing common bile duct dilatation associated with normal hepatic tests and inconclusive imaging [38].
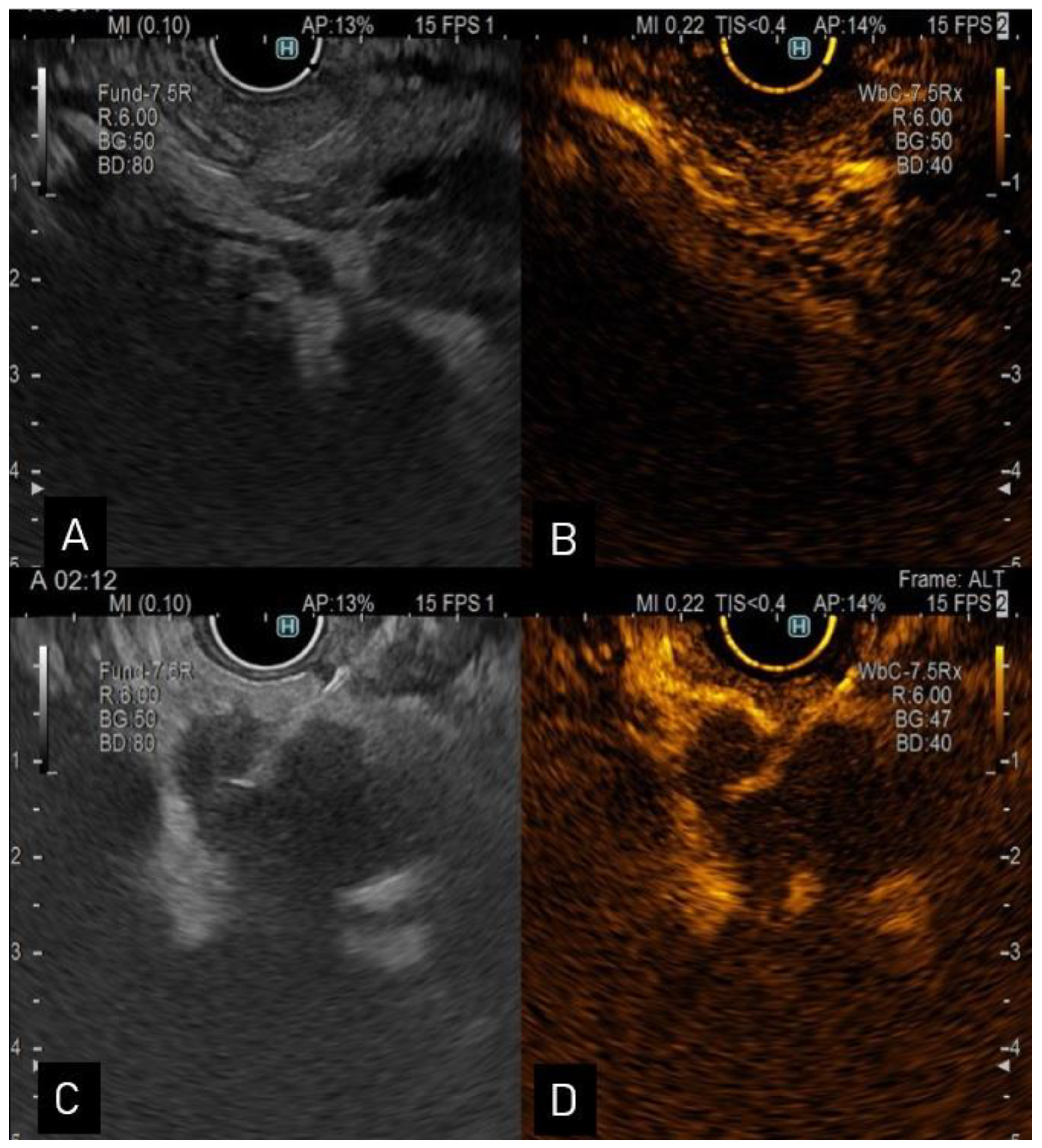
Figure 1. (A). Endoscopic ultrasound view of a distal CCA; (B). contrast enhanced-ultrasound showing hyperenhancement in the arterial phase; (C). endoscopic ultrasound view of a proximal hypoechoic bile duct lesion undergoing aspiration via a 22-G needle; (D). contrast enhanced-ultrasound guided aspiration.
3.2. T Staging
EUS proved a T staging accuracy of 60–81% (Table 1), while intraductal ultrasound (IDUS) can assess the T staging with 68% accuracy [39]. No comparative data exists for T staging between proximal or distal CCAs. The growth pattern of perihilar CCAs is an axial extension along and into the bile ducts, while distal CCAs also have an axial growth pattern, extending into the pancreatic parenchyma. Invasion into the liver parenchyma is isoechoic and can easily be missed on conventional B-mode ultrasound.
Table 1. Accuracy of endoscopic ultrasound staging in extrahepatic cholangiocarcinoma.
| Author, Year | No. of Patients (n) | Type of Study | Accuracy of T Staging (%) | Accuracy of N Staging (%) | Accuracy of Portal Vein Invasion (%) | Accuracy of Hepatic Artery Invasion (%) |
|---|---|---|---|---|---|---|
| Otsuka 2022, [40] | 38 (22 were T1 and T2 cases) | R 1 | CH-EUS 3 73.7 EUS 4 60.5 |
- | CH-EUS 100 EUS 100 |
CH-EUS 100 EUS 100 |
| Malikowski 2020, [41] | 133 | R | - | 86 | - | - |
| Sugiyama 1999, [42] | 19 | P 2 | - | - | 100 | - |
| Tio 1993, [43] | 46 | R | 66 | 64 | - | - |
| Mukai 1992, [44] | 16 | R | 81 | 81 | 88 | - |
1 R—retrospective; 2 P—prospective; 3 CH-EUS—contrast-enhanced harmonic endoscopic ultrasonography; 4 EUS—endoscopic ultrasound.
For this reason, the contrast-enhanced harmonic endoscopic ultrasonography (CH-EUS) can identify the tumor as grape-like clusters that invade the biliary ducts and expand into the liver and portal vein, features useful for assessing its resectability [45]. The suggestive contrast enhanced value of CCAs is that of a hyperenhanced lesion with rapid wash-out. In a single-center retrospective study, Otsuka et al. pointed out the importance of CH-EUS for T staging in patients with perihilar CCA and distal CCA compared to surgical assessment, with a better accuracy of CH-EUS for T staging compared to conventional EUS or contrast enhanced CT (73.7% vs. 60.5 vs. 39.5%) [46]. The detection of invasion into other organs did not differ significantly between the two EUS methods (e.g., for diagnosing invasion beyond the biliary wall, the accuracy was 92.1% for CH-EUS vs. 78.9% for EUS vs. 45.9% for contrast-enhanced CT, respectively) [46]. However, CH-EUS is not recommended by the authors to be used for N or M staging, since the contrast agent is washed out rapidly [46].
In recent studies, EUS had an accuracy of 100% for identifying major vascular invasion [46], while in studies performed 10 years ago, the sensitivity for detecting unresectable tumors was only 53% [36].
No data exist about elastography during EUS assessment in extrahepatic CCAs due to the fact that these are small tumors, surrounded by many vessels, and this impedes an appropriate elastographic examination.
3.3. N Staging
The regional lymph nodes of the bile duct are located along the portal vein and the hepatic artery, anteriorly and posteriorly to the pancreatic head, and along the superior mesenteric artery [47]. The distant lymph nodes are located in the aortocaval, celiac, and periesophageal regions [48]. The most frequently affected lymph nodes are those in the periportal region, followed by the regional gastrohepatic sites and by the distant lymph nodes [48]. The presence of at least one malignant lymph node shortens the median survival from 1050 days to 353 [48].
The N staging accuracy varies between 66 and 81% (Table 1). It is important to keep in mind that a round, hypoechoic lesion more than 10 mm in diameter is not specific for malignancy [40]. EUS can identify nodal involvement with a higher accuracy than cross-sectional imaging (86 vs. 47%) [48]. The presence of malignant regional lymph nodes precludes curative oncological resection or liver transplant for CCA [49], and is associated with a four-fold higher risk of death [48].
References
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280.
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527.
- Krampitz, G.W.; Aloia, T.A. Staging of Biliary and Primary Liver Tumors. Surg. Oncol. Clin. N. Am. 2019, 28, 663–683.
- Sarcognato, S.; Sacchi, D.; Fassan, M.; Fabris, L.; Cadamuro, M.; Zanus, G.; Cataldo, I.; Capelli, P.; Baciorri, F.; Cacciatore, M.; et al. Cholangiocarcinoma. Pathologica 2021, 113, 158–169.
- Krasinskas, A.M. Cholangiocarcinoma. Surg. Pathol. Clin. 2018, 11, 403–429.
- Soares, K.C.; Jarnagin, W.R. The Landmark Series: Hilar Cholangiocarcinoma. Ann. Surg. Oncol. 2021, 28, 4158–4170.
- Saleh, M.; Virarkar, M.; Bura, V.; Valenzuela, R.; Javadi, S.; Szklaruk, J.; Bhosale, P. Intrahepatic cholangiocarcinoma: Pathogenesis, current staging, and radiological findings. Abdom. Radiol. 2020, 45, 3662–3680.
- Zhang, X.-F.; Beal, E.W.; Chakedis, J.; Chen, Q.; Lv, Y.; Ethun, C.G.; Salem, A.; Weber, S.M.; Tran, T.; Poultsides, G.; et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J. Surg. 2018, 42, 2919–2929.
- Oliveira, I.S.; Kilcoyne, A.; Everett, J.M.; Mino-Kenudson, M.; Harisinghani, M.G.; Ganesan, K. Cholangiocarcinoma: Classification, diagnosis, staging, imaging features, and management. Abdom. Radiol. 2017, 42, 1637–1649.
- Kendall, T.; Verheij, J.; Gaudio, E.; Evert, M.; Guido, M.; Goeppert, B.; Carpino, G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019, 39 (Suppl. S1), 7–18.
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522.
- Yamasaki, S. Intrahepatic cholangiocarcinoma: Macroscopic type and stage classification. J. Hepatobiliary Pancreat. Surg. 2003, 10, 288–291.
- Vijgen, S.; Terris, B.; Rubbia-Brandt, L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 22–34.
- Nakanuma, Y.; Sato, Y.; Harada, K.; Sasaki, M.; Xu, J.; Ikeda, H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J. Hepatol. 2010, 2, 419–427.
- De Rose, A.M.; Cucchetti, A.; Clemente, G.; Ardito, F.; Giovannini, I.; Ercolani, G.; Giuliante, F.; Pinna, A.D.; Nuzzo, G. Prognostic significance of tumor doubling time in mass-forming type cholangiocarcinoma. J. Gastrointest. Surg. 2013, 17, 739–747.
- Aishima, S.; Oda, Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: Different characters of perihilar large duct type versus peripheral small duct type. J. Hepatobiliary Pancreat. Sci. 2015, 22, 94–100.
- Komuta, M.; Govaere, O.; Vandecaveye, V.; Akiba, J.; Van Steenbergen, W.; Verslype, C.; Laleman, W.; Pirenne, J.; Aerts, R.; Yano, H.; et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012, 55, 1876–1888.
- Liau, J.-Y.; Tsai, J.-H.; Yuan, R.-H.; Chang, C.-N.; Lee, H.-J.; Jeng, Y.-M. Morphological subclassification of intrahepatic cholangiocarcinoma: Etiological, clinicopathological, and molecular features. Mod. Pathol. 2014, 27, 1163–1173.
- Cardinale, V.; Wang, Y.; Carpino, G.; Reid, L.M.; Gaudio, E.; Alvaro, D. Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology 2012, 55, 2041–2042.
- Carpino, G.; Cardinale, V.; Folseraas, T.; Overi, D.; Grzyb, K.; Costantini, D.; Berloco, P.B.; Di Matteo, S.; Karlsen, T.H.; Alvaro, D.; et al. Neoplastic Transformation of the Peribiliary Stem Cell Niche in Cholangiocarcinoma Arisen in Primary Sclerosing Cholangitis. Hepatology 2019, 69, 622–638.
- Komuta, M.; Spee, B.; Borght, S.V.; De Vos, R.; Verslype, C.; Aerts, R.; Yano, H.; Suzuki, T.; Matsuda, M.; Fujii, H.; et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008, 47, 1544–1556.
- Sato, Y.; Sasaki, M.; Harada, K.; Aishima, S.; Fukusato, T.; Ojima, H.; Kanai, Y.; Kage, M.; Nakanuma, Y.; Tsubouchi, H. Pathological diagnosis of flat epithelial lesions of the biliary tract with emphasis on biliary intraepithelial neoplasia. J. Gastroenterol. 2014, 49, 64–72.
- Hughes, N.R.; Pairojkul, C.; Royce, S.G.; Clouston, A.; Bhathal, P.S. Liver fluke-associated and sporadic cholangiocarcinoma: An immunohistochemical study of bile duct, peribiliary gland and tumor cell phenotypes. J. Clin. Pathol. 2006, 59, 1073–1078.
- Wu, T.-T.; Levy, M.; Correa, A.M.; Rosen, C.B.; Abraham, S.C. Biliary intraepithelial neoplasia in patients without chronic biliary disease: Analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer 2009, 115, 4564–4575.
- Zen, Y.; Fujii, T.; Itatsu, K.; Nakamura, K.; Minato, H.; Kasashima, S.; Kurumaya, H.; Katayanagi, K.; Kawashima, A.; Masuda, S.; et al. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology 2006, 44, 1333–1343.
- Schlitter, A.M.; Jang, K.-T.; Klöppel, G.; Saka, B.; Hong, S.-M.; Choi, H.; Offerhaus, G.J.; Hruban, R.H.; Zen, Y.; Konukiewitz, B.; et al. Intraductal tubulopapillary neoplasms of the bile ducts: Clinicopathologic, immunohistochemical, and molecular analysis of 20 cases. Mod. Pathol. 2016, 29, 93.
- Schlitter, A.M.; Born, D.; Bettstetter, M.; Specht, K.; Kim-Fuchs, C.; Riener, M.-O.; Jeliazkova, P.; Sipos, B.; Siveke, J.T.; Terris, B.; et al. IIntraductal papillary neoplasms of the bile duct: Stepwise progression to carcinoma involves common molecular pathways. Mod. Pathol. 2014, 27, 73–86.
- Jung, G.; Park, K.-M.; Lee, S.S.; Yu, E.; Hong, S.-M.; Kim, J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J. Hepatol. 2012, 57, 787–793.
- Park, H.J.; Jang, K.-T.; Heo, J.S.; Choi, Y.-L.; Han, J.; Kim, S.H. A potential case of intraductal tubulopapillary neoplasms of the bile duct. Pathol. Int. 2010, 60, 630–635.
- Nakagawa, T.; Arisaka, Y.; Ajiki, T.; Fujikura, K.; Masuda, A.; Takenaka, M.; Shiomi, H.; Okabe, Y.; Fukumoto, T.; Ku, Y.; et al. Intraductal tubulopapillary neoplasm of the bile duct: A case report and review of the published work. Hepatol. Res. 2016, 46, 713–718.
- Zen, Y.; Pedica, F.; Patcha, V.R.; Capelli, P.; Zamboni, G.; Casaril, A.; Quaglia, A.; Nakanuma, Y.; Heaton, N.; Portmann, B. Mucinous cystic neoplasms of the liver: A clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod. Pathol. 2011, 24, 1079–1089.
- Lurie, R.H.; Cancer, C.; Abbott, D.E. Hepatobiliary Cancers; NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®); Version 5.2022—13 January 2023. Available online: https://www.nccn.org (accessed on 11 February 2023).
- Zaheer, A.; Anwar, M.M.; Donohoe, C.; O’Keeffe, S.; Mushtaq, H.; Kelleher, B.; Clarke, E.; Kirca, M.; McKiernan, S.; Mahmud, N.; et al. The diagnostic accuracy of endoscopic ultrasound in suspected biliary obstruction and its impact on endoscopic retrograde cholangiopancreatography burden in real clinical practice: A consecutive analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 850–857.
- Seicean, A.; Gincul, R. Mistakes in endoscopic ultrasonography and how to avoid them. UEG Educ. 2021, 21, 1–9.
- Lee, J.H.; Salem, R.; Aslanian, H.; Chacho, M.; Topazian, M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am. J. Gastroenterol. 2004, 99, 1069–1073.
- Mohamadnejad, M.; DeWitt, J.M.; Sherman, S.; LeBlanc, J.K.; Pitt, H.A.; House, M.G.; Jones, K.J.; Fogel, E.L.; McHenry, L.; Watkins, J.L.; et al. Role of EUS for preoperative evaluation of cholangiocarcinoma: A large single-center experience. Gastrointest. Endosc. 2011, 73, 71–78.
- Sai, J.K.; Suyama, M.; Kubokawa, Y.; Watanabe, S.; Maehara, T. Early detection of extrahepatic bile-duct carcinomas in the nonicteric stage by using MRCP followed by EUS. Gastrointest. Endosc. 2009, 70, 29–36.
- Bruno, M.; Brizzi, R.F.; Mezzabotta, L.; Carucci, P.; Elia, C.; Gaia, S.; Mengozzi, G.; Romito, A.V.; Eloubeidi, M.A.; Rizzetto, M.; et al. Unexplained common bile duct dilatation with normal serum liver enzymes: Diagnostic yield of endoscopic ultrasound and follow-up of this condition. J. Clin. Gastroenterol. 2014, 48, e67–e70.
- Tamada, K.; Ido, K.; Ueno, N.; Kimura, K.; Ichiyama, M.; Tomiyama, T. Preoperative staging of extrahepatic bile duct cancer with intraductal ultrasonography. Am. J. Gastroenterol. 1995, 90, 239–246.
- Gleeson, F.C.; Rajan, E.; Levy, M.J.; Clain, J.E.; Topazian, M.D.; Harewood, G.C.; Papachristou, G.I.; Takahashi, N.; Rosen, C.B.; Gores, G.J. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest. Endosc. 2008, 67, 438–443.
- Nguyen, N.Q.; Schoeman, M.N.; Ruszkiewicz, A. Clinical utility of EUS before cholangioscopy in the evaluation of difficult biliary strictures. Gastrointest. Endosc. 2013, 78, 868–874.
- Brooks, C.; Gausman, V.; Kokoy-Mondragon, C.; Munot, K.; Amin, S.P.; Desai, A.; Kipp, C.; Poneros, J.; Sethi, A.; Gress, F.G.; et al. Role of Fluorescent In Situ Hybridization, Cholangioscopic Biopsies, and EUS-FNA in the Evaluation of Biliary Strictures. Dig. Dis. Sci. 2018, 63, 636–644.
- KHirata, K.; Kuwatani, M.; Suda, G.; Ishikawa, M.; Sugiura, R.; Kato, S.; Kawakubo, K.; Sakamoto, N. A Novel Approach for the Genetic Analysis of Biliary Tract Cancer Specimens Obtained Through Endoscopic Ultrasound-Guided Fine Needle Aspiration Using Targeted Amplicon Sequencing. Clin. Transl. Gastroenterol. 2019, 10, e00022.
- Rompianesi, G.; Di Martino, M.; Gordon-Weeks, A.; Montalti, R.; Troisi, R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J. Gastrointest. Oncol. 2021, 13, 332–350.
- Meacock, L.M.; Sellars, M.E.; Sidhu, P.S. Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. Br. J. Radiol. 2010, 83, 615–627.
- Otsuka, Y.; Kamata, K.; Hyodo, T.; Chikugo, T.; Hara, A.; Tanaka, H.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for T-staging of patients with extrahepatic bile duct cancer. Surg. Endosc. 2022, 36, 3254–3260.
- Miyazaki, M.; Ohtsuka, M.; Miyakawa, S.; Nagino, M.; Yamamoto, M.; Kokudo, N.; Sano, K.; Endo, I.; Unno, M.; Chijiiwa, K.; et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J. Hepatobiliary. Pancreat. Sci. 2015, 22, 181–196.
- Malikowski, T.; Levy, M.J.; Gleeson, F.C.; Storm, A.C.; Vargas, E.J.; Topazian, M.D.; Abu Dayyeh, B.K.; Iyer, P.G.; Rajan, E.; Gores, G.J.; et al. Endoscopic Ultrasound/Fine Needle Aspiration Is Effective for Lymph Node Staging in Patients with Cholangiocarcinoma. Hepatology 2020, 72, 940–948.
- Bowlus, C.L.; Lim, J.K.; Lindor, K.D. AGA Clinical Practice Update on Surveillance for Hepatobiliary Cancers in Patients with Primary Sclerosing Cholangitis: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 2416–2422.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
732
Revisions:
2 times
(View History)
Update Date:
12 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




