1. Introduction
Brain and other nervous system cancers are among the most fatal cancers in several countries around the world
[1][2][3]. In 2019, there were 347,992 global cases of brain and central nervous system (CNS) cancers, which showed a significant increase in its incidence (94.35%) from the period between 1990 to 2019
[4]. An estimated 251,329 people passed away from primary cancerous brain and central nervous system (CNS) tumors in 2020
[5]. Among brain tumors, malignant brain tumor incidence rates are slightly decreasing over the last decade; however, mortality rates increased in the same period of time
[1]. Specifically, in the malignant brain tumor group, 5-year glioblastoma (GBM) survival only increased from 4% to 7% during the last years
[1]. However, survival rates vary widely and depend on several factors, including the degree of malignancy and cellular and molecular distinctive features.
Over the years, the identification of distinct genetic and epigenetic profiles in various brain tumors has improved the classification of more than 100 cancerous diseases that can appear in this preferential location and allows the discovery of new diagnostic, prognostic, and predictive molecular biomarkers to improve the prediction of response to treatment and therapeutic outcome
[6]. The classification of brain tumors has experienced numerous changes over the past half century. The World Health Organization (WHO) has played a key role in the effort to split malignancies according to clinical and histological profiles from the first classification launched in 1979
[7]. This increased complexity as reflected in the last classification in 2021 summarizes the current understanding of the clinical, histologic, and molecular features of CNS tumors and paves the way for further precision in tumor classification and a shift towards increased use of targeted therapeutics
[8].
Among malignant gliomas, GBM is one of the most aggressive malignancies, accounting for 14.5% of all central nervous system tumors and 48.6% of malignant central nervous system tumors
[9]. The median overall survival (OS) of GBM patients is only 15 months, which highlights the failure with conventional treatments applied so far
[10]. The ongoing effort to identify potential new molecular or cellular targets for the development of effective clinical therapies has not yet led to significant improvements in survival rate, with most patients surviving not more than a few years. In this sense, the understanding of the molecular interactions among not only tumor cells but also other types of non-tumor cells that reside into tumors has made it possible to improve therapeutic targeting
[11]. Nevertheless, the majority of studies related to GBM treatments over the last decades has focused on eradication of tumor cells, whereas more recent efforts have been placed on understanding the microenvironment surrounding tumor cells, the interaction between these cellular and acellular components in different preformed tumor niches, and how to design new treatment options that target these components in a multi-attack approach
[12][13]. Tumor-associated macrophages (TAMs) play an essential role in the GBM microenvironment since this non-tumoral cell population represents up to 50% of tumor mass and specific treatments to eliminate these cells have been proposed in the past
[14][15].
2. Classification, Biological Features, and Tumor Niches of GBMs
Tumors generated from different glial cells in the CNS are known as gliomas. To unify the diagnostic criteria, WHO proposed a CNS tumor classification and nomenclature guide based on the combination of parameters such as tumor mass extension into the brain tissue, the proliferation of the microvasculature, genetic alterations, presence of necrotic areas, and cell proliferation index
[16]. Low-grade gliomas (LGG) (grades 1 and 2) are less invasive while high-grade gliomas (3 and 4) represent the most challenging brain tumors. WHO Classification of Tumors of the CNS (WHO CNS5), revised recently, has suffered substantial changes by moving further to advance the role of molecular and genetic biomarkers’ identification in the diagnostics of CNS tumor classification but remaining rooted in other established approaches to tumor characterization, including histology and immunohistochemistry
[8]. In addition, the number denoted in the gradation is now Arabic instead of a Roman numeral. This classification would have an impact in the correct diagnosis, treatment definition, and prognosis of the disease. For example, the identification of mutations in isocitrate dehydrogenase (IDH) defines gliomas with the best prognosis independently of their tumor grade
[17]. IDH mutation in GBM is frequently associated with TP53 mutation, and it has a generally better prognosis than IDH-wildtype glioblastoma.
Among malignant gliomas, grade 4 tumors or GBM are the most aggressive, and they possess high levels of intratumoral and intertumoral heterogeneity. Apart from containing different genetic signatures, GBMs present different transcriptomic profiles, which have recently originated a new classification: classical, mesenchymal, neural, and proneural tumors
[18]. However, this classification does not impose a different therapeutic approach, so it is not routinely performed in the clinic
[11]. For this reason, the WHO classification includes GBM as part of the diffuse astrocytic and oligodendroglial tumors group and they are divided into three subgroups based on IDH mutations: (1) glioblastoma, IDH-wildtype, clinically identified as primary GBM and predominant in patients over 55 years of age, (2) glioblastoma, IDH-mutant, clinically identified as secondary GBM and more common in younger patients, and (3) glioblastoma NOS (not otherwise specified), which does not fit into the other categories and is not well defined
[9].
During the gliomagenesis process, different genetic abnormalities signatures lead to GBM malignant cell transformation; however, tumors masses formed need a great amount of genetic, epigenetic, and metabolic changes in order to continue proliferation and expansion to the surrounding healthy brain tissue, including changes in energetic metabolism, invasive capacity, remodeling of the extracellular matrix (ECM), cell migration and promotion of angiogenesis
[9]. The detachment of invading tumor cells from the primary tumor mass accompanied by decreased expression of Cx43 and increased CD44 expression, followed by the anchored and degradation of ECM by overexpressed MMP-9 and MMP-2, allow the colonization of tumor cells into normal brain tissues such as brain parenchyma, leptomeningeal space, white matter tracts of corpus callosum, and perivascular space
[19][20]. GBM cells also attract non-tumoral cells such as microglia, astroytes, and endothelial cells that secrete proteases to enhance migration
[14]. In this migration movement, tumor cells in immediate proximity of pre-existing and degenerated vessels begin to die, forming foci of necrosis. These foci become surrounded by tumor cells, which eventually form pseudopalisade and upregulate the expression of vascular endothelial growth factor (VEGF), leading to vascular hyperplasia, distinguishing glomeruloid vascular proliferation areas. Different niches within the tumor mass will be created, which contemplate the coexistence of tumor cells and non-tumor cells in different areas such as the hypoxic/necrotic niche, invasive front, and perivascular zones that not only define different cell constituents
[21], but are also characterized by cell plasticity, heterogeneity, and resistance to radiotherapy and chemotherapy
[12].
3. Tumor Microenvironment (TME) in GBM Niches
TME plays an essential role in cancer development. Various non-tumor cells participate in the TME, collaborating in growth, survival, invasion, and metastasis of tumor cells
[22]. Tumor cells structure the tumor parenchyma and non-tumor cells, which are part of the stroma, have a cellular heterogeneity. Normal and reactive astrocytes, fibroblasts, immune cells, microglia, macrophages, endothelial cells, and vascular pericytes are part of the microenvironment of the GBM. Furthermore, proteins and non-protein biomolecules (polysaccharides, hormones, nitric oxide, etc.) are produced by all the cell types to promote neoplastic growth, and they are also main components of the TME
[23]. More importantly, glioma stem cells (GSCs) have the capacity to generate new tumor cells and support cancer growth and regrowth even after the majority of treatments employed
[22]. The location of GSCs into the tumor has been discussed, but they can be found in different niches of GBM close to central necrosis
[22].
Perivascular niches are composed of blood vessels such as capillaries or arterioles, and GSCs have close contact with them
[24]. Furthermore, reactive astrocytes presented in these areas generate angiopoietins 1 and 2 (Ang-1 or Ang-2) and VEGF, which are important cytokines for tumor cells that use the perivascular space for invasion and co-opt existing vessels as satellite tumors
[25]. VEGF induced Ang-1 pericytes’ recruitment to improve vascular stability. Moreover, these molecules also participate in the recruitment of myeloid cell populations into GBM
[26][27]. Around necrotic zone, Ang-1 is absent because hypoxia down-regulates Ang-1 expression; nevertheless, Ang-1 is more perceived in the tumor periphery
[28].
The main molecular inductor of angiogenesis in perinecrotic areas is hypoxia-inducible factor 1 (HIF-1), which intensifies VEGF expression after translocation to nuclei
[28]. On the other hand, perinecrotic niches are considered zones of high tumor cell proliferation and low endothelial cell development. An important feature in necrotic foci is the appearance of GSC around them
[28].
Moreover, other non-cellular components belonging to ECM are upregulated into TME, such as hyaluronan, vitronectin, osteopontin, tenascin-C, SPARC, and BEHAB with an impact on the GBM progression. Their overexpression is correlated with poor prognosis
[29]. This is of particular interest because hyaluronan helps in the progression of malignant gliomas by facilitating primary brain tumor invasion in and migration through its two cellular receptors, CD44 and RHAMM
[29]. CD44 is the major receptor for hyaluronan and it contributes to cell–matrix interactions, cell migration, and regulation of tumor growth
[29]. Tight junctions between ECM components and integrins of neoplastic cells lead to an increment in apoptotic resistance, proliferation, and migration
[30]. Other overexpressed proteins such as fibronectin, which has the ability to regulate cell adhesion and migration, have been proposed as promoters of tumor invasion
[31]. The overexpression of TGF-β, TGF-α, EGF, VEGF, and TNF-α promote both survival and tumor proliferation of GBM
[32]. Many GBMs present EGFR amplification and/or mutation, and to a lesser extent they overexpress PDGF receptors. Those EGFR-dependent tumors would develop drug treatment resistance
[33][34].
TAMs play an essential role in the GBM microenvironment. These cells can come from two different tissue origins. Microglia cells are derived from primitive hematopoiesis in the fetal yolk sac and take up residence in the brain during early fetal development
[35]. Microglia differentiation and proliferation requires colony-stimulating factor 1 (CSF1), CD34, and the transcription factor PU.1
[35]. Under normal physiological conditions, the brain is only occupied by resident microglia, and the presence of other bone-marrow-derived macrophages (BMDM) are associated with the diseased brain. Microglia are long-lived and have self-renewal capacity compared with BMDM
[36]. In addition, peripheral macrophages driven by inflammatory factors from GBM tumor cells and other TME cell populations promote the infiltration of circulating BMDM derived from hematopoietic stem cells that can migrate to tumor tissue; they penetrate the blood–brain tumor barrier (BBTB), and probably the intact blood–brain barrier (BBB), where they differentiate into monocyte-derived macrophages and promote tumor progression
[14][37]. The BBB provides both a physical and a physiological barrier between the brain parenchyma and the bloodstream restricting the entry of various components such as peptides and proteins, due to tight junctions
[38] and also limits the permeability of immune cells from blood
[39]. Upon brain injury produced by GBM tumorigenesis, the BBB becomes compromised (forming the BTBB) leading to significant influx of circulating BMDM and other immunological cells
[40]. Moreover, Wang L.J. reported through immune landscape analysis that the risk score was significantly related to TME, specifically taking into account the macrophage cell population in malignant gliomas. Authors demonstrated the value of TAMs-related signature in predicting the prognosis of glioma, and they provided potential targeted therapy for glioma by in silico analysis
[41]. Pinto L. et al. analyzed and characterized myeloid and lymphoid infiltrate in grade 2, 3, and 4 gliomas human samples by multicolor flow cytometry, along with the composition of the cell subsets of circulating myeloid cells
[40]. They described that the infiltration by BMDM reached the highest percentages in GBM, and it increased from the periphery to the center of the lesion, where it exerted a strong immunosuppression that was absent in marginal areas instead. Chen et al. in 2017 agreed that BMDMs predominate within the GBM parenchyma, while microglia reside at the tumor periphery, so TAMs are represented by ~85% of infiltrating BMDM and ~15% of microglia
[15].
Thus, the majority of immune cells in GBM includes a vast diversity of myeloid and lymphoid cells, which comprise BMDMs, myeloid-derived suppressor cells (MDSCs), DCs, lymphocytes, natural killer (NK), neutrophils, etc.
[42]. However, the complex cell–cell interactions provide a unique physiological advantage for glioma cells that establishes an immune-suppressive and tumor-development-permissive microenvironment that is featured with high resident and recruited myeloid cell substances, hyporesponsive, and exhausted tumor infiltrating lymphocyte (TIL), which makes malignant glioma known as an immunologically “cold” tumor
[43][44]. In addition, some studies indicated that reducing the number of MDSCs recruitment may slow the progression of glioma tumor cells
[45]. Lymphoid cells are presented in GBM, but they are infrequent and they represent less than 2% of the tumor mass
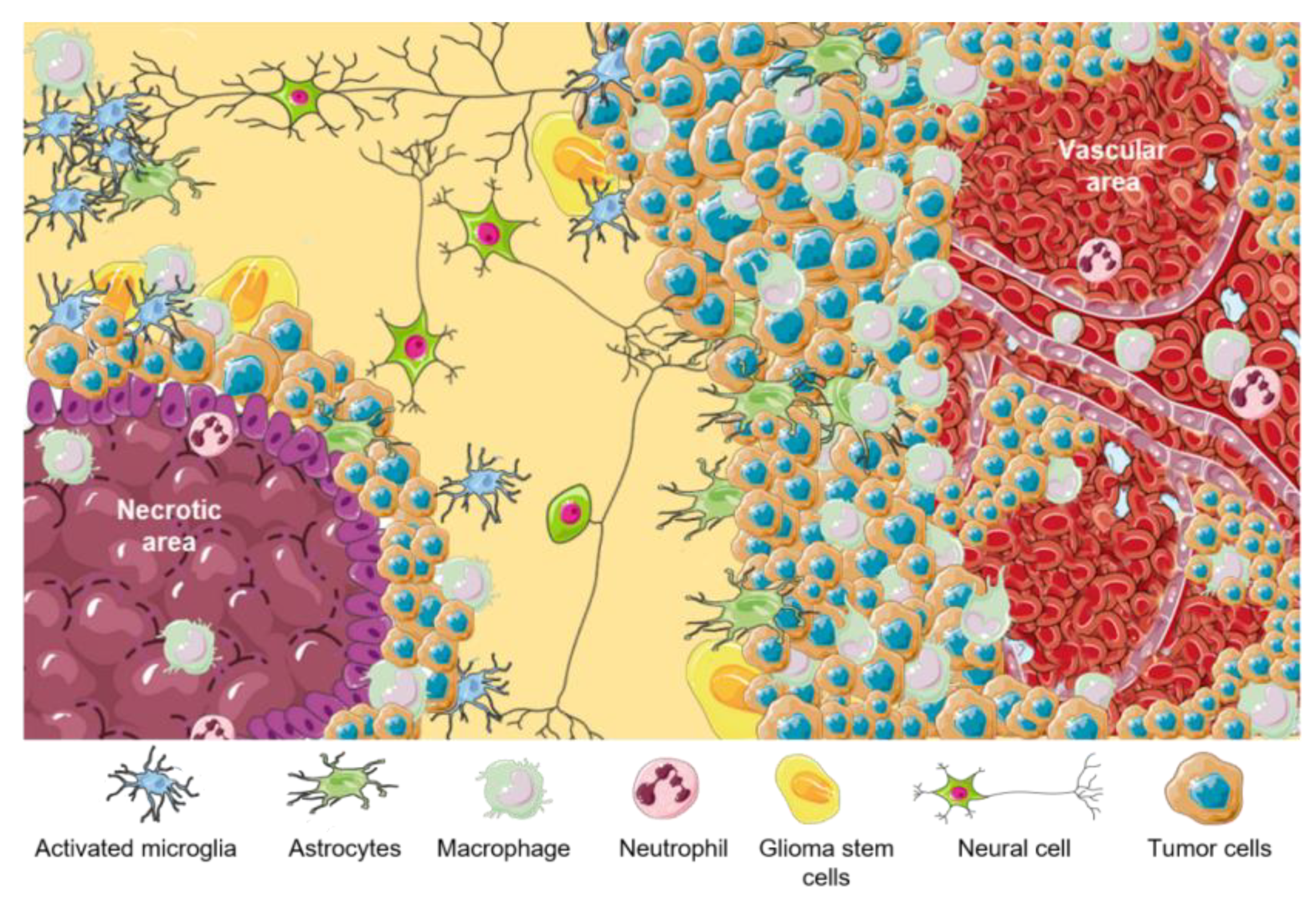
[46]. A representative scheme of different cell components of GBM TME is summarized in
Figure 1. Principal functions of GBM cellular components are listed in
Table 1.
Figure 1. TME in GBM. Representative scheme of different GBM tumor areas. TAMs are associated with perinecrotic core centers, perivascular areas, and tumor front invasion zones. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License;
https://smart.servier.com (accessed on 1 February 2023).
Table 1. Main functions of cellular components of TME of GBM.
As mentioned previously, the prominent genomic feature that mostly distinguishes LGG from malignant gliomas, such as GBM, is the mutational status of the two genes encoding the cytoplasmatic IDH1 and/or the mitochondrial IDH2, where ~80% of LGGs present IDH mutations, compared to only ~5% of GBMs. Interestingly, IDH mutations are an independent prognostic factor in gliomas and they are associated with increased survival in all types, including GBM
[17][47]. IDH status also denote TME cell components differences between tumors with the wild-type isoform and those with the mutated IDH
[48]. Unlike GBMs with IDH-wildtype, GBMs with the IDH mutation have been shown to have less M2 macrophage infiltration and fewer PD-1-expressing T cells
[49]. A study based on samples from patients with GBM showed that there is less infiltration of TAMs in GBM with IDH mutation, being more proinflammatory, which could reflect a better prognosis for these patients, and the fact that microglia in mutated IDH also have a proinflammatory role
[50].