Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lea Juretić | -- | 2651 | 2023-04-05 15:10:32 | | | |
| 2 | Dean Liu | Meta information modification | 2651 | 2023-04-06 04:55:11 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pilipović, K.; Jurišić Grubešić, R.; Dolenec, P.; Kučić, N.; Juretić, L.; Mršić-Pelčić, J. Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/42822 (accessed on 07 February 2026).
Pilipović K, Jurišić Grubešić R, Dolenec P, Kučić N, Juretić L, Mršić-Pelčić J. Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/42822. Accessed February 07, 2026.
Pilipović, Kristina, Renata Jurišić Grubešić, Petra Dolenec, Natalia Kučić, Lea Juretić, Jasenka Mršić-Pelčić. "Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases" Encyclopedia, https://encyclopedia.pub/entry/42822 (accessed February 07, 2026).
Pilipović, K., Jurišić Grubešić, R., Dolenec, P., Kučić, N., Juretić, L., & Mršić-Pelčić, J. (2023, April 05). Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases. In Encyclopedia. https://encyclopedia.pub/entry/42822
Pilipović, Kristina, et al. "Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases." Encyclopedia. Web. 05 April, 2023.
Copy Citation
Oxidative stress was proposed to be involved in neurodegenerative processes and to play an important role in the morbidity and progression of various neurodegenerative disorders. Accordingly, a number of studies discovered the potential of natural plant constituents to have significant antioxidant activity.
neurodegenerative diseases
oxidative stress
neuroinflammation
1. Introduction
Neurodegenerative diseases (NDDs) are disorders characterized by the progressive loss of neuronal cell structure and function. The incidence of NDDs increases over the years due to increased life expectancy [1]. NDDs are generally considered age-related diseases, but can also affect the younger population [2]. Despite decades of preclinical and clinical research aimed at uncovering the processes underlying the development of NDDs, the pathogenesis remains largely unknown. Evidence suggests that both genetic predisposition and environmental factors play an important role [3].
Depending on which group of neurons degenerates, specific diseases and symptoms occur. The most common NDDs are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), multiple sclerosis, and other less common diseases [2]. AD is characterized by memory loss, cognitive impairment, confusion, aggression, and mood swings, leading to the progressive deterioration of behavior and functionality. Key pathological features of AD include extracellular deposits of insoluble beta-amyloid (Aβ) plaques and intracellular accumulations of neurofibrillary tangles composed of hyperphosphorylated tau protein [4][5][6]. Regional neuron loss is found in the medial temporal lobe, hippocampus, and cerebral cortex, with cholinergic pathways particularly affected [7]. PD is characterized by selective loss of dopaminergic neurons in the supstantia nigra pars compacta, resulting in decreased dopamine levels in the nigrostriatal pathway. Consequently, motor disturbances such as resting tremor, muscle rigidity, bradykinesia, and postural instability are typical clinical manifestations of the disease. The pathological feature of PD is the presence of Lewy bodies, which are aggregates of various proteins such as α-synuclein, ubiquitin, parkin, and neurofilaments. HD is a genetic disease that leads to the progressive degeneration of neurons in the striatum, cerebral cortex, and thalamus, resulting in a steady progression of motor dysfunction. ALS is characterized by the progressive degeneration of motor neurons in the spinal cord, brainstem, and cortex, resulting in spasticity, muscle weakness, progressive paralysis, dysphagia, and respiratory insufficiency [7]. The main pathological feature of ALS is the mislocalization of proteins and the formation of ubiquitinated cytoplasmic aggregates in degenerating neurons and their surrounding oligodendrocytes. Although certain NDDs have their own specific features, they are all irreversible, progressive, and without effective therapeutic options.
Currently available treatments can help alleviate symptoms and slow disease progression, but there is no therapeutic option to prevent or cure NDDs.
The molecular pathogenesis of NDDs is still controversial, but evidence suggests that it involves a complex interplay between genetic disorders, environmental factors, misfolding and aggregation of various proteins, disturbances in metal ion homeostasis, mitochondrial dysfunction, excitotoxicity, neuroinflammation, oxidative stress (OS), and the activation of programmed cell death [2][4][7][8][9]. There is increasing evidence that OS plays a central role in the development of NDDs [2][10][11][12]. Moreover, it may be a very important factor in secondary neurodegenerative changes observed after CNS injuries such as ischemia or traumatic brain injury [13].
Oxidative and nitrosative stress are the result of an imbalance between the generation of two types of reactive molecules, reactive oxygen species (ROS) and reactive nitrogen species (RNS), on the one hand, and the balancing antioxidant mechanisms on the other [14]. In general, ROS/RNS have some useful functions, but their accumulation may trigger the activation of pathophysiological pathways leading to the development of NDDs via different mechanisms [7][11]. Elevated levels of reactive species can damage cellular components such as lipids, proteins, and DNA, leading to neuronal dysfunction and death [11]. Therefore, the search for therapeutic agents that could influence this pathological cascade by modulating the production of reactive species and/or antioxidant mechanisms seems to be a promising strategy.
Throughout history, people around the world used medicinal plants and their preparations to improve health. Today, many plant species already have an established place in scientific medicine and are used in the treatment of a wide range of health conditions, including NDDs. However, the golden field of medicinal plants still hides many secrets and is a great challenge for modern scientific research in discovering new potential phytotherapeutics [15].
Both synthetic and natural plant products with antioxidant properties are being explored as potential treatments for NDDs [16][17][18]. Natural plant products recently gained attention, as they were shown to have neuroprotective effects and reduce OS and inflammation [1][19]. Natural antioxidants are found in fruits, vegetables, nuts, and herbs, and include compounds such as polyphenols, carotenoids, and vitamins C and E. Some examples of plant-based antioxidants that were extensively studied are polyphenols from green tea, resveratrol from grapes, and curcumin from turmeric, etc. [7][16][20][21][22]. Although promising, further research is needed to fully understand their role and possible therapeutic potential in NDDs. In addition, the best dosages and delivery methods need to be determined to ensure their bioavailability.
2. Oxidative Stress in the Pathophysiology of Neurodegenerative Diseases
Oxygen is essential to all aerobic organisms because it is the final electron acceptor in the electron transport chain during oxidative phosphorylation in mitochondria, the major energy-producing machinery. The oxidation of glucose is the main energy source of the brain because of its high ATP generation rate, which is required to maintain the high energy demand of neurons [6]. As a result, ROS and RNS are produced. Both groups of reactive species include free radicals, which are extremely reactive chemical species that possess one or more unpaired electrons [14]. Hydroxyl (OH•), superoxide (O2•−), and nitric oxide (NO•−) are free radicals, while hydrogen peroxide (H2O2), nitric oxide (NO), peroxynitrite (ONOO−), and hypochlorous acid (HOCl) are not free radicals, but they can generate them through various chemical reactions [4][9]. Although they are mainly produced in mitochondria, there are other sources such as cytochrome P450 enzymes, peroxisomes (by-product of the degradation of fatty acids and other lipid molecules), and phagocytosis of bacteria or viruses [9].
Cells have various safety mechanisms to keep the concentration of reactive species below the level at which they become toxic, as they can cause significant damage to the cell if this threshold is exceeded. These antioxidant systems are responsible for removing and scavenging free radicals and their precursors, inhibiting their formation, and binding metal ions needed to catalyze free radical formation [9]. They can be enzymatic or non-enzymatic. The most important antioxidant enzymes are superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione reductase, and peroxiredoxins [14]. Non-enzymatic antioxidants are mainly exogenous substances derived from food, such as vitamins C and E, carotenoids, polyphenols (e.g., flavonoids, anthocyanins, phenolic acids, quinones, coumarins, tannins), etc. [19][23]. Endogenous non-enzymatic antioxidants include glutathione and coenzyme Q10 [19][24].
ROS/RNS play an important role in physiological processes such as signal transduction, gene transcription, immune response, response to stressors, synaptic plasticity, learning, and memory [6][9][12]. However, when free radical production and detoxification are out of balance, OS results. This may be due to either the excessive production of reactive species or the dysfunctionality of antioxidant systems. Oxygen overload causes oxidative damage to biomolecules (lipids, proteins, DNA) and alters their structure and function, thus initiating a pathophysiological cascade that leads to cell damage and eventual cell death. Persistent OS can lead to the development of various chronic diseases, including aging and degenerative diseases [9]. Indeed, high levels of OS are often observed in many patients diagnosed with NDDs [10][12][19], but it is currently unknown whether OS is a driving force or a consequence that further exacerbates the disease [25].
The brain is particularly susceptible to OS damage because of high oxygen consumption, inadequate antioxidant defense systems, abundant redox-active metals (such as iron and copper), and high levels of polyunsaturated fatty acids (PUFAs), which are susceptible to peroxidation and oxidative changes [6][10]. In addition, glial cells and neurons are postmitotic, highly differentiated cells and are, therefore, particularly sensitive to ROS [9]. Lipids are highly susceptible to free radical attacks and undergo lipid peroxidation, proteins undergo carbonylation and nitration, and nucleic acids are oxidized [6][19]. These processes have complex effects on mitochondria, metal metabolism, and glial cells, causing neuroinflammation and triggering various forms of programmed cell death such as apoptosis, parthanatos, and ferroptosis [19][25].
Lipid peroxidation is the result of cell membrane damage due to the attack of reactive species on lipids containing carbon-carbon double bonds, especially PUFAs, which are abundant in the brain [6][26]. As a result, a heterogeneous group of relatively stable end products is formed that can bind proteins and DNA, leading to a change in their conformation and function [27][28]. The membrane becomes permeable to substances that are normally unable to cross the barrier, and as a result, these substances cause damage to membrane proteins, enzymes, receptors, and other cell components [19]. Lipoxygenase as well as cyclooxygenase (COX) cascades, which can further impair PUFA metabolism, are upregulated in chronic and age-related brain pathologies [10]. COX-1, COX-2 are responsible for the formation of many eicosanoids, and arachidonic acid and other PUFAs play a critical role in the formation of bioactive lipids, that significantly affect the course of neurodegeneration [10]. The nucleic acids are highly susceptible to oxidative damage. Guanine in particular is more susceptible to attack by ROS, leading to the formation of 8-hydroxyguanine and 8-hydroxy-2-deoxyguanosine. The increased levels of these modified bases were observed in PD brains, for example. In addition, protein carbonylation and nitration were observed in neurodegenerative brains [19]. Oxidative damage results in a variety of oxidative modifications to proteins, as multiple amino acid residues are amenable to oxidation. These modifications can alter cellular function by regulating the stability, activity, subcellular localization, or protein–protein interaction of oxidized proteins [25]. The most important result of protein oxidation is the formation of large protein aggregates, the accumulation of which in cells leads to toxicity. Insoluble aggregates can result from covalent cross-links between peptide chains, as in the case of mutant SOD1 in ALS, α-synuclein in PD, and neurofibrillary tangles and Aβ in AD [10]. The relationship between OS and protein aggregation appears to be bidirectional, as aggregation-prone conformers enhance ROS production, while OS, in turn, promotes protein aggregation [25] (Figure 1).
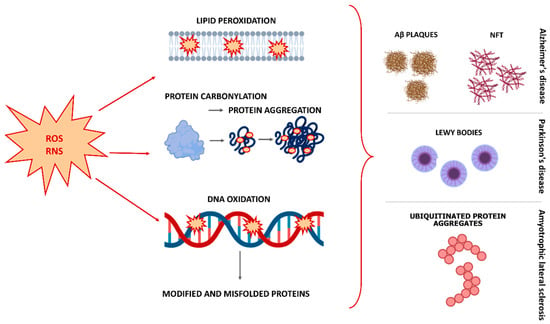
Figure 1. Effects of oxidative stress on lipids, proteins, and DNA, and generation of key pathological features in Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species; Aβ, beta-amyloid; NFT, neurofibrillary tangles.
Several cellular events such as mitochondrial dysfunction, Ca2+ overload, excitotoxicity, and induction in intracellular signaling mechanisms were shown to be major contributors to the molecular and cellular processes underlying OS-stimulated cell death (Figure 2). Some of the most important functions of mitochondria include the production of ATP, regulation of Ca2+ homeostasis, control of cell division, and control of cell death by apoptosis [29][30]. Mitochondria are the first to be affected by OS as they are the main source of production of ROS. OS in mitochondria can lead to oxidative damage to mitochondrial membranes, mitochondrial DNA, and mitochondrial proteins, ultimately resulting in mitochondrial damage [10]. Oxidative damage to mitochondrial DNA disrupts the synthesis of respiratory complexes and proteins involved in electron transport, leading to impaired mitochondrial function [24]. This may contribute to a further increase in the production of ROS and the development of OS. Mitochondrial damage may also lead to uncontrolled Ca2+ overload because mitochondria play a role in Ca2+ homeostasis by secreting excess cytosolic Ca2+ in their matrix. In addition, oxidative damage to mitochondria plays a critical role in the release of cytochrome c into the cytosol, where it can activate caspase-dependent apoptosis [24]. Mitochondrial dysfunction is one of the main features of the aging process, especially in organs that require a high energy source, such as the brain [6][18]. Moreover, many of the genes associated with NDDs development are linked to mitochondria [2], and all aggregated misfolded proteins (Aβ, tau, and α-synuclein) are known to inhibit mitochondrial function and induce OS [6][31]. In addition, mitophagy can cause the removal of damaged mitochondria, and recent studies showed that it is involved in the pathological mechanisms of many diseases, especially NDDs and cerebral ischemic diseases [32].
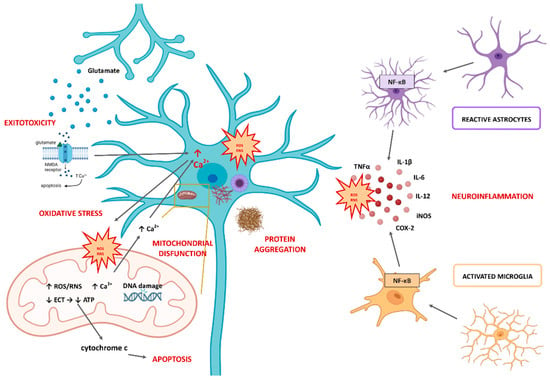
Figure 2. Molecular and cellular mechanisms underlying oxidative stress-stimulated neurodegeneration. Abbreviations: ROS, reactive oxygen species; RNS, reactive nitrogen species; NMDA, N-methyl-D-aspartate receptor; ETC, electron transport chain; NF-κB, nuclear factor-κB; IL-, interleukin-; TNFα, tumor necrosis factor alpha; iNOS, inducible form of nitric oxide; COX-2, cyclooxygenase-2.
The maintenance of Ca2+ homeostasis is a crucial component for preventing neuronal cell death triggered by excitotoxicity. In the brain and neuronal tissue, excitatory amino acids and neurotransmitters are factories for free radicals and ROS [9]. Excessive excitatory stimuli, i.e., excitotoxicity, cause massive calcium influx, membrane depolarization, mitochondrial dysfunction, and subsequent death in neurons. This occurs through the production of reactive species and activation of calcium-dependent pro-death factors, such as calpains.
There is much evidence to suggest that neuroinflammation may be a major factor in the progression of NDDs in addition to OS [5][33] (Figure 2). Inflammatory responses caused by various disorders in the central nervous system (CNS) environment are mediated by glial cells, with microglia playing the central role in neuroinflammation. Microglia promote healthy overall brain physiology by participating in many important processes, such as neuroplasticity and brain remodeling, the release of specific neurotrophic factors, clearance of damaged neurons, response to damage and pathogen-induced stimuli, etc. When glia are activated by certain stimuli, they respond in a pro-inflammatory manner. This response is beneficial for a certain period, but if it is prolonged or becomes abnormal, it becomes maladaptive, and as a result, functional deficits may occur. Mitochondrial dysfunction and OS are thought to cause microglia and astrocytes to become pro-inflammatory and release various cytokines, cytotoxic mediators, and reactive species that increase inflammatory responses in the brain and lead to neuroinflammation. Interleukin (IL)-12, IL-1β, IL-6, nitric oxide (NO), tumor necrosis factor alpha (TNFα) are just some of them. This constant activation of glial cells creates a vicious cycle that promotes chronic neurodegenerative processes [2]. Moreover, nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) are two crucial signaling pathways that regulate the expression of pro-inflammatory cytokines during the inflammatory process in glial cells [33]. As a result, the inducible form of nitric oxide (iNOS), COX-2, and subunits of nicotinamide adenine dinucleotide phosphate (H) (NADPH) oxidase (NOX) are produced, leading to further production of ROS. Altogether, this interplay of OS and neuroinflammation forms a loop of chronic neuronal damage, possibly leading to the development of NDDs. The involvement of the immune system in NDDs is supported by the findings that proinflammatory microglia are closely associated with protein aggregate pathologies characteristic of most dementias [34]. Moreover, microglia spatially correlate with microtubule-associated protein tau pathology and are able to recognize and eliminate tau. They can also phagocytose α-synuclein, the constituent protein of Lewy bodies, becoming proinflammatory by expressing cytokines, ROS, and COX-2 [34].
It is also important to mention nuclear factor erythroid 2-related factor 2 (Nrf2), as its role was reported to be altered in many NDDs [19]. Nrf2 can bind with high affinity to the antioxidant-responsive element (ARE) and contribute to the upregulation of genes involved in modulating cellular redox status and protecting cells from OS [10]. Numerous data support the protective role of the Nrf2 ARE pathway in NDDs by reducing OS and neuroinflammation [19].
In response to stressors such as OS, inflammation, heat stress, and hypoxia, heat shock protein (HSP) expression is upregulated in cells to promote their survival [35]. HSPs are a family of different chaperones named after their molecular mass. They play a role in the proper folding of newly synthesized proteins and proteins subjected to stress-induced denaturation, and some of them also directly interfere with apoptosis [35][36]. These homeostatic functions are particularly important in proteinopathic NDDs, in which certain proteins are misfolded and aggregated. The protective nature of many HSPs was demonstrated in many experimental models of neurodegeneration [36].
The presence of various groups of secondary metabolites, especially phytochemical components such as phenolic compounds and essential oils, were related to health promoting features of medical plants. The most important and widely investigated feature of phenolics is their protective role in OS-induced damage. This protection can cause the delay and/or inhibition of various diseases and degenerative conditions, such as CNS disorders, cardiovascular diseases, atherosclerosis, cancer, diabetes, respiratory, and autoimmune diseases [15].
References
- Renaud, J.; Martinoli, M.-G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 1883.
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139.
- Boyd, R.J.; Avramopoulos, D.; Jantzie, L.L.; McCallion, A.S. Neuroinflammation represents a common theme amongst genetic and environmental risk factors for Alzheimer and Parkinson diseases. J. Neuroinflammation 2022, 19, 223.
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340.
- Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708.
- Cenini, G.; Lloret, A.; Cascella, R. Oxidative Stress in Neurodegenerative Diseases: From a Mitochondrial Point of View. Oxidative Med. Cell. Longev. 2019, 2019, e2105607.
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125.
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385.
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74.
- Uddin, S.; Al Mamun, A.; Kabir, T.; Ahmad, J.; Jeandet, P.; Sarwar, S.; Ashraf, G.M.; Aleya, L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020, 886, 173412.
- Yaribeygi, H.; Panahi, Y.; Javadi, B.; Sahebkar, A. The Underlying Role of Oxidative Stress in Neurodegeneration: A Mechanistic Review. CNS Neurol. Disord.-Drug Targets 2018, 17, 207–215.
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967.
- Merelli, A.; Repetto, M.; Lazarowski, A.; Auzmendi, J. Hypoxia, Oxidative Stress, and Inflammation: Three Faces of Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, S109–S126.
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78.
- Mekinić, I.G.; Skroza, D.; Ljubenkov, I.; Katalinić, V.; Šimat, V. Antioxidant and Antimicrobial Potential of Phenolic Metabolites from Traditionally Used Mediterranean Herbs and Spices. Foods 2019, 8, 579.
- Pohl, F.; Lin, P.K.T. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283.
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Kharazmi, M.S.; Jafari, S.M. Natural Bioactive Molecules as Neuromedicines for the Treatment/Prevention of Neurodegenerative Diseases. ACS Omega 2023, 8, 3667–3683.
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583.
- Zhao, B. Natural Antioxidants for Neurodegenerative Diseases. Mol. Neurobiol. 2005, 31, 283–293.
- Ikram, M.; Saeed, K.; Khan, A.; Muhammad, T.; Khan, M.S.; Jo, M.G.; Rehman, S.U.; Kim, M.O. Natural Dietary Supplementation of Curcumin Protects Mice Brains against Ethanol-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment via Nrf2/TLR4/RAGE Signaling. Nutrients 2019, 11, 1082.
- Ahmad, A.; Ali, T.; Rehman, S.U.; Kim, M.O. Phytomedicine-Based Potent Antioxidant, Fisetin Protects CNS-Insult LPS-Induced Oxidative Stress-Mediated Neurodegeneration and Memory Impairment. J. Clin. Med. 2019, 8, 850.
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975.
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333.
- Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438.
- Winczura, A.; Zdżalik, D.; Tudek, B. Damage of DNA and proteins by major lipid peroxidation products in genome stability. Free. Radic. Res. 2012, 46, 442–459.
- Reed, T.T. Lipid peroxidation and neurodegenerative disease. Free. Radic. Biol. Med. 2011, 51, 1302–1319.
- García-Beltrán, O.; Urrutia, P.J.; Núñez, M.T. On the Chemical and Biological Characteristics of Multifunctional Compounds for the Treatment of Parkinson’s Disease. Antioxidants 2023, 12, 214.
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; D’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132.
- Abramov, A.Y.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Dolgacheva, L.P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochem. Soc. Trans. 2017, 45, 1025–1033.
- Hou, W.; Hao, Y.; Sun, L.; Zhao, Y.; Zheng, X.; Song, L. The dual roles of autophagy and the GPCRs-mediating autophagy signaling pathway after cerebral ischemic stroke. Mol. Brain 2022, 15, 14.
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817.
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743.
- Namazi, F.; Bordbar, E.; Bakhshaei, F.; Nazifi, S. The effect of Urtica dioica extract on oxidative stress, heat shock proteins, and brain histopathology in multiple sclerosis model. Physiol. Rep. 2022, 10, e15404.
- Leak, R.K. Heat shock proteins in neurodegenerative disorders and aging. J. Cell Commun. Signal. 2014, 8, 293–310.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
598
Revisions:
2 times
(View History)
Update Date:
06 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




