Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bingnan Han | -- | 3469 | 2023-04-05 12:13:10 | | | |
| 2 | Sirius Huang | Meta information modification | 3469 | 2023-04-06 03:36:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, N.; Zhang, S.; Javeed, A.; Jian, C.; Liu, Y.; Sun, J.; Wu, S.; Fu, P.; Han, B. Anti-Allergic Activities of Natural Products from Marine Organisms. Encyclopedia. Available online: https://encyclopedia.pub/entry/42817 (accessed on 02 March 2026).
Chen N, Zhang S, Javeed A, Jian C, Liu Y, Sun J, et al. Anti-Allergic Activities of Natural Products from Marine Organisms. Encyclopedia. Available at: https://encyclopedia.pub/entry/42817. Accessed March 02, 2026.
Chen, Na, Shanshan Zhang, Ansar Javeed, Cuiqin Jian, Yi Liu, Jinlyu Sun, Shandong Wu, Peng Fu, Bingnan Han. "Anti-Allergic Activities of Natural Products from Marine Organisms" Encyclopedia, https://encyclopedia.pub/entry/42817 (accessed March 02, 2026).
Chen, N., Zhang, S., Javeed, A., Jian, C., Liu, Y., Sun, J., Wu, S., Fu, P., & Han, B. (2023, April 05). Anti-Allergic Activities of Natural Products from Marine Organisms. In Encyclopedia. https://encyclopedia.pub/entry/42817
Chen, Na, et al. "Anti-Allergic Activities of Natural Products from Marine Organisms." Encyclopedia. Web. 05 April, 2023.
Copy Citation
It is essential to find alternative anti-allergic agents from natural products. The special environment of high salt and pressure, low temperature, oligotrophicity, hypoxia, and limited light determines that the secondary metabolites of marine organisms have very unique traits compared with secondary metabolites of terrestrial organisms. Secondary metabolites of marine natural products possess many biological effects like anti-tumor, anti-inflammatory, anti-allergic, antiviral, antibacterial, etc.
anti-allergic
secondary metabolites
marine organisms
molecular docking
1. Marine Plants
1.1. Natural Products Derived from Marine Plants with Anti-Allergic Activity
In the study of anti-allergy, the most studied marine plants are algae, along with some mangrove plants. Major kinds of algae are red algae, green algae, and brown algae, which are mostly found in intertidal and subtidal zones [1]. Asian countries are rich in algal resources, especially China, Japan, and Korea. Since ancient times, humans have known of marine algae and are using it for different purposes. The secondary metabolites found in it are abundant, because they are rich in proteins, fatty acids, minerals and vitamins, and are often used as food. Some algal species also have great potential as cosmetics, drugs, and drug adjuvants [2], which play an important role to treat fever, cough, dermatitis, allergies, and other diseases [3]. Humans and other biological organisms consume a large amount of polyphenols, which is the largest compound group present in plants [4]. Polyphenols have a wide range of functions like antioxidant capacity, scavenging free radicals, and metal-chelating activity, and it is beneficial to human health, and can be used to treat and prevent cancer, cardiovascular disease, and other pathology [5]. In marine algae, the structure of most anti-allergic natural products is polyphenols. Three compounds were isolated from Ecklonia cava by Li et al. [6] which stimulated human basophilic KU812F cells with IgE and anti-IgE antibodies. At 100 μM, the relative levels of histamine released by three compounds 1, 2, and 3 (Figure 1) were 23.97, 44.26 and 34.54%, respectively. Then calcium ionophore A23187 was used to mediate the degranulation of KU812F cells and RBL-2H3 cells. Three compounds at 100 μM inhibited histamine release from both cells. After flow cytometry analysis, it was proved that three compounds play an anti-allergic role by inhibiting FcεRI and IgE binding activity, and the inhibition rates were 30.58, 47.60 and 34.23%, respectively. Compounds 1 and 3 had the strongest inhibitory effect on histamine release, with IC50 values of 31.65 μM (RBL-2H3), 44.20 μM (KU812F), 38.87 μM (RBL-2H3), and 65.81 μM (KU812F). These compounds inhibited allergic reactions in a dose-dependent manner. Han et al. [7] also studied the anti-allergic effect of eckol (compound 2) separated from Ecklonia cava (brown algae) through BMCMC (mouse bone marrow-derived mast cells) stimulated by bovine serum albumin (BSA)/immunoglobulin E (IgE) and allergic reaction models. The results showed for the first time that the compound 2 inhibited mast cell activation by inducing degranulation and cytokine production after IgE/BSA exposure. 100 μg/mL of compound 2 remarkably decreased the release of β-hexosaminidase by inhibiting the production of Th2 cytokines, i.e., IL-5, IL-4, IL-13. It reduced FcεRI expression on the cell surface, and compound 2 binds to the active site of IgE for blocking the IgE binding to FcεRI. Two bioactive phloroglucinol derivatives DHE (compound 4) and PFF-α (compound 5) were isolated from Ecklonia stolonifera (brown algae) by Shim et al. [8], as shown in Figure 1. They studied the effects of these two compounds on human basophilic KU812F cells and found that compounds 4 and 5 inhibited FcεRI expression on the surface of the cell by 16.9 and 15.4% at 50 μM, respectively. At the same time, these two compounds lessened the expression of total FcεRI α chain protein and mRNA in a dose-dependent manner, and inhibited the increase of intracellular Ca2+ stimulated by CRA-1, thereby exerting anti-allergic effects. Vo et al. [9] studied the Fucofuroeckol-A (F-A/compound 6, see Figure 1) protective effect obtained from Ecklonia stolonifera on UVB-induced RBL-2H3 mast cell allergic reaction. They found that 50 μM F-A inhibited the fusion of granules and plasma membrane by inhibiting the increase of calcium ion concentration in mast cells, thereby inhibiting degranulation of mast cells and reducing histamine release from mast cells (release level 31%). Sugiura et al. [10] isolated and purified six compounds 2, 3, 7–10 (Figure 1) from Eisenia arborea, and studied the effect of oral administration of these compounds compared with EGCG, which is a known natural product with anti-allergic activity [11]. Their studies have shown that these compounds exhibited anti-allergic activity by inhibiting the release of chemical mediators like leukotriene B4, prostaglandin E2, and histamine, as well as inhibiting the cyclooxygenase-2 (COX-2) mRNA expression. The inhibitory activity of 3, 8, and 9 was the strongest, and the anti-allergic effect was equal to or higher than EGCG. Matsui et al. [12] isolated three compounds from Sargassum carpophyllum, and found that compounds 11, 12, and 13 (Figure 1) inhibited the release of prostaglandin D2, tumor necrosis factor-α and β-hexosaminidase in RBL-2H3 cells stimulated by DNP-HSA, with a value of 50.7, 35.9 and 43.5 μM IC50 values. β-hexosaminidase release was employed as an indicator of mast cell degranulation. At 40 μM, all three compounds significantly inhibited ROS production, and compound 13 slightly reduced the level of Ca2+.
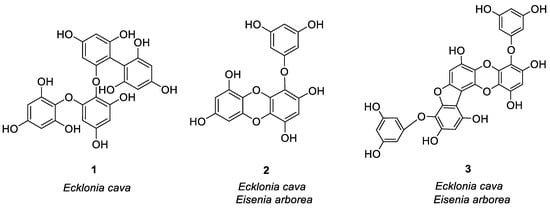
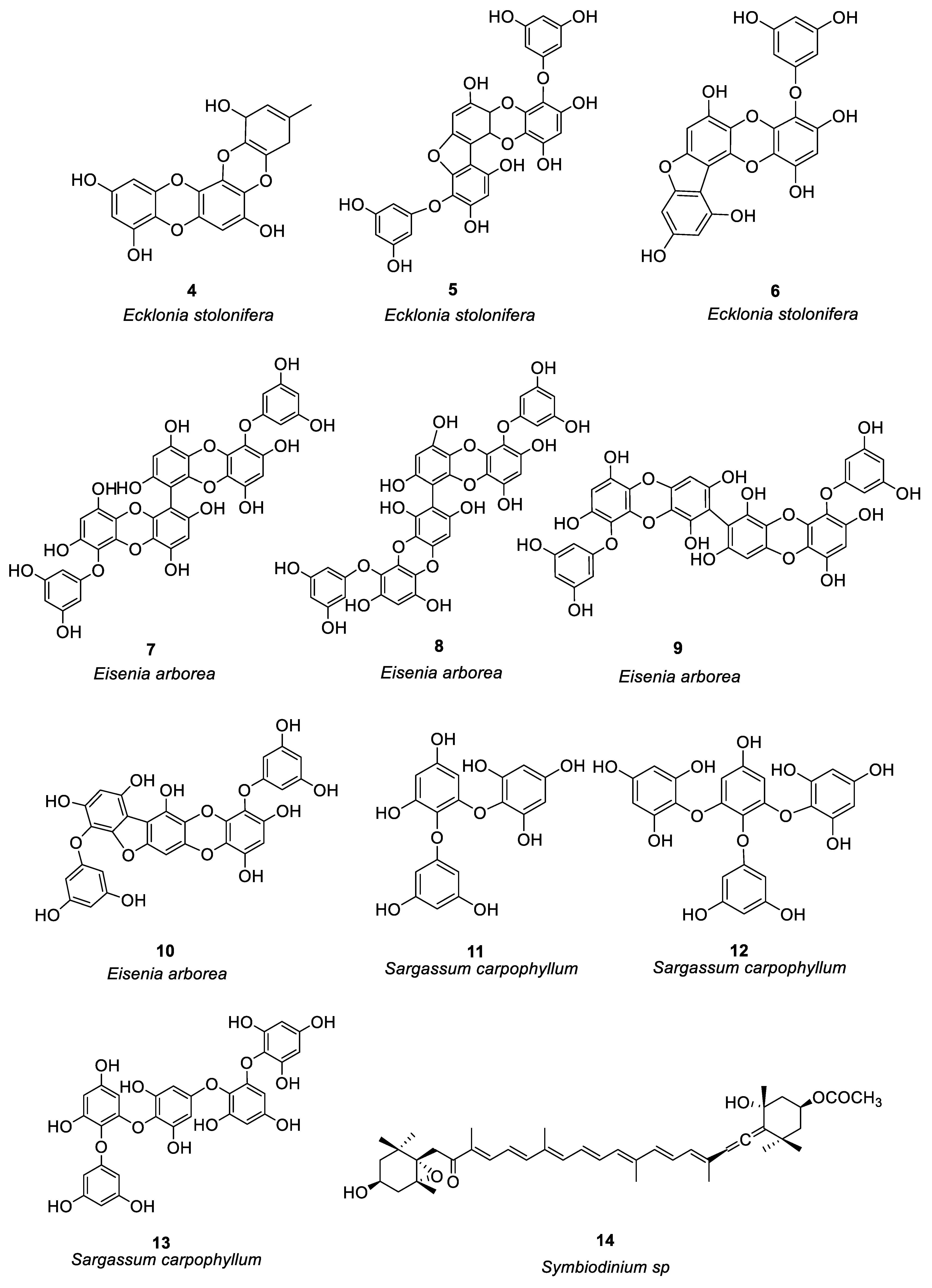
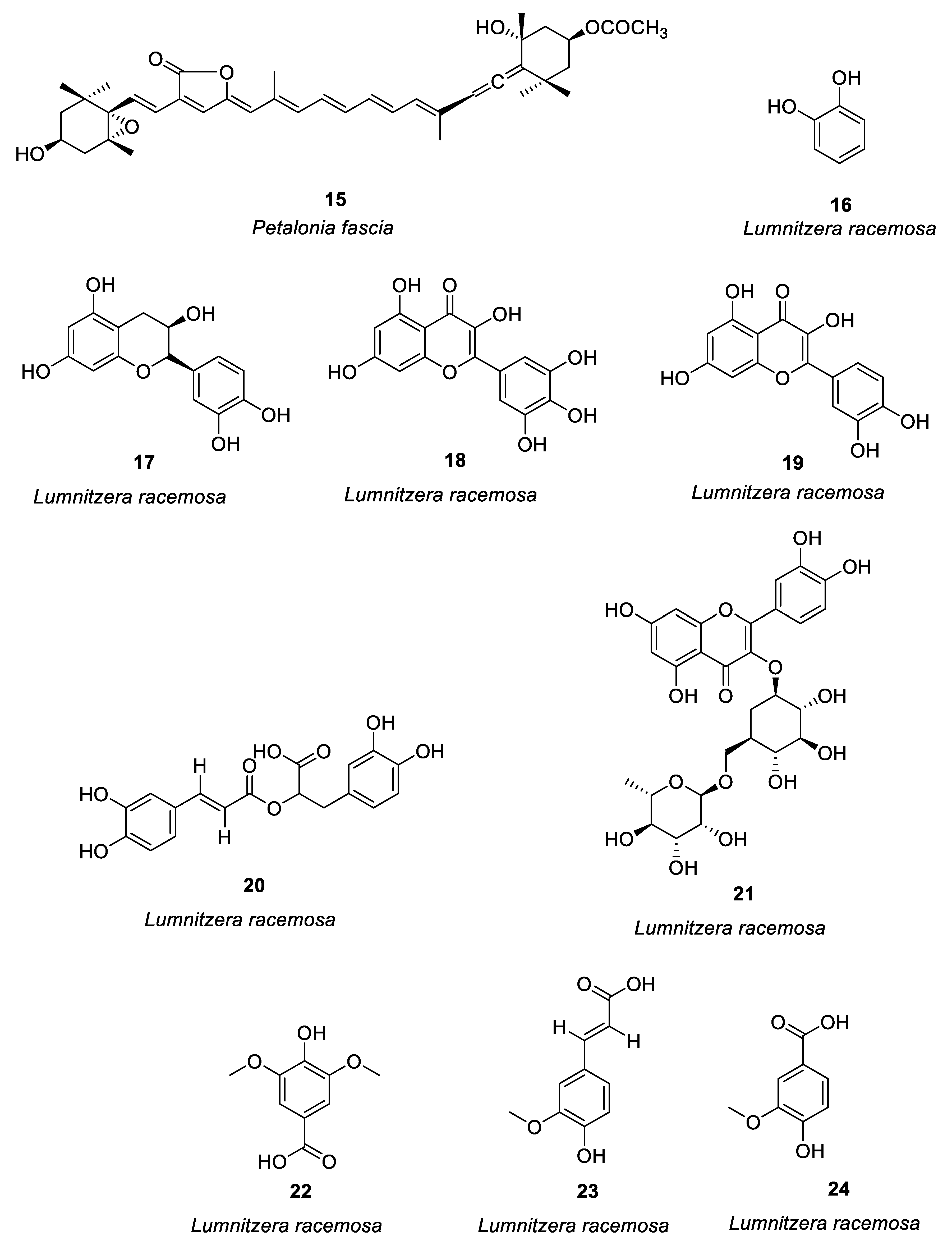
Figure 1. Structures of compounds 1–24.
Other compounds of brown algae also have good anti-allergic effects. Onodera et al. [13] compared Peridinin 14 and fucoxanthin 15 (Figure 1) isolated from Symbiodinium sp and Petalonia fascia, respectively. They found that topical application was better, and that compound 14 better inhibited delayed-type hypersensitivity compared to compound 15. Compound 14 may be a potential drug for inhibiting allergic inflammation by inhibiting the migration of ear eosinophils to eotaxin and the production of eotaxin.
1.2. Crude Extracts from Marine Plants as Potential Sources with Anti-Allergic Activity
Kim et al. [14] treated ovalbumin (OVA)-sensitized mice with Ecklonia cava (EC) extracts, and found that EC extracts significantly inhibited allergic responses before the last airway OVA challenge. IL-4, IL-5, and Th2 cytokines play an imperative role in the instigation of allergic response. Han et al. [15] studied the anti-allergic effects of ethanolic extract of copper algae on passive cutaneous anaphylaxis and IgE/BSA-mediated mouse bone marrow activation of mast cells. Studies showed that the extract of copper algae (SHE) inhibited the β-hexosaminidase and histamine release, and substantially inhibited the degranulation of bone marrow mesenchymal stem cells. In addition, flow cytometry analysis showed that SHE markedly decreased the FcεRI binding to IgE and FcεRI expression on the surface of BMCMCs, and regulated the expression levels of mRNA of cytokines and chemokines in IgE/BSA-stimulated BMCMCs, thereby improving activation of mast cells stimulated by immunoglobulin E/bovine serum albumin. Herath et al. [16] studied whether Sargassum horneri ethanol extract (SHE) attenuated the effects of atmospheric particulate matter (PM) exposure on asthma. By lowering mRNA levels of the transcription factors STAT5 and GATA3, they discovered that copper algae blocked Th2 polarization and decreased the expression of IL-4, IL-5, IL-13, and Th2 cytokines in lung tissue homogenates of mice with asthma caused by PM. Additionally, oral administration of SHE dramatically decreased mast cell activation, serum IgE levels, and PM-aggravated Th2 and Th17 responses in asthmatic mice. Compounds with potential anti-allergic activity are present in red algae in addition to the structure and chemical characteristics of the anti-allergic natural products in brown algae, which have also been thoroughly investigated. Jung et al. [17] used a 95% ethanol extraction method to extract Laurencia undulata (LU) and showed that it contains an enormous quantity of polyphenols, and observed its anti-asthmatic effect on ovalbumin (OVA)-induced allergic respiratory reactions in mice. The results showed that LU administered prior to the last airway OVA challenge significantly inhibited allergic reactions. Shi et al. [18] investigated the anti-allergic effects of sulfated polysaccharide from Porphyra haitanensis (PHPS) administered orally on mice that were allergic to tropomyosin (TM). The findings of that experiment revealed that PHPS can stimulate the Treg/Th1 cytokines production like IL-10 and interferon-γ in the presence or absence of allergens. Han et al. [19] applied RASP (red algae sulfated polysaccharide) to effervescent tablets for anti-allergy research, and it was acquired by extraction of Gracilaria lemaneiformis and Porphyra haitanensis. As a result of RASP treatment, serum IgE levels, mast cell protease-1, and histamine were reduced. RASP treatment can reduce IL-4, significantly increases IFN-γ, and IFN-γ as Th1 cytokines, and promotes Th1 cell differentiation, thereby regulating allergic reactions caused by Th1/Th2 immune response imbalance. The natural products found in microalgae also have anti-allergic properties. Additionally, anti-allergic compounds have also been found in green algae. Raman et al. [20] observed that the crude extract of Enteromorpha compressa reduced the level of IgE induced by food allergens such as ovalbumin and that it enhanced immune function by decreasing plasma cell generation of IgE antibodies against food allergens. Cryptomonas, another algal species, has also been shown to have anti-allergic properties. The effect of Polyopes affinis ethanol extract on Th2-mediated allergen-induced airway inflammation in an asthmatic mouse model was evaluated by Lee et al. [21]. Researchers found that continual intraperitoneal injection of P. affinis ethanol extract before the last respiratory OVA challenge significantly inhibited the response and reduced ovalbumin-specific IgE by 72%.
Mangrove is a wetland woody plant community composed of evergreen trees or shrubs mainly composed of mangrove plants growing in the intertidal zone of tropical and subtropical coasts. Acharyya et al. [22] studied the anti-allergic activity of the Ethanol extract of Lumnitzera racemosa and the polyphenols related to this activity (16–24, see Figure 1). Oral administration of the ethanol extract of L. racemosa significantly reduced the number of sneezes, scratches, and nasal pain, as well as the number of lymphocytes, neutrophils, and eosinophils, and significantly inhibited TDI-induced allergic symptoms. (See Table 1 for details on compounds).
2. Marine Animals
2.1. Natural Products Derived from Marine Animals with Anti-Allergic Activity
In the study of anti-allergy, marine animals mainly include sponges, mollusks, sea cucumbers, corals, etc. A variety of marine animals, including sponges, mollusks, and fish also have anti-allergic properties. The sponge was the first multicellular animal, living in the ocean 600 million years ago, with a high capacity for filtration [23]. In mollusks, sea cucumbers and abalone are the main sources of anti-allergy compounds. Ko et al. [24] investigated the passive cutaneous anaphylaxis of gastrointestinal digestive components of the intestinal digestive digest of abalone Haliotis discus hannai and a bioactive peptide (compound 25, see Figure 2) was isolated from the gastrointestinal digestion. Histamine release could be reduced by 300μg/mL of compound 25. Mice treated with compound 25 showed significant inhibition of the immunoglobulin E-mediated PCA response. By regulating PMA + A23187, compound 25 stimulates HMC-1 cells to produce tumor necrosis factor-α, IL-1, and IL-6 reduces the release of histamine and has anti-allergic activity.
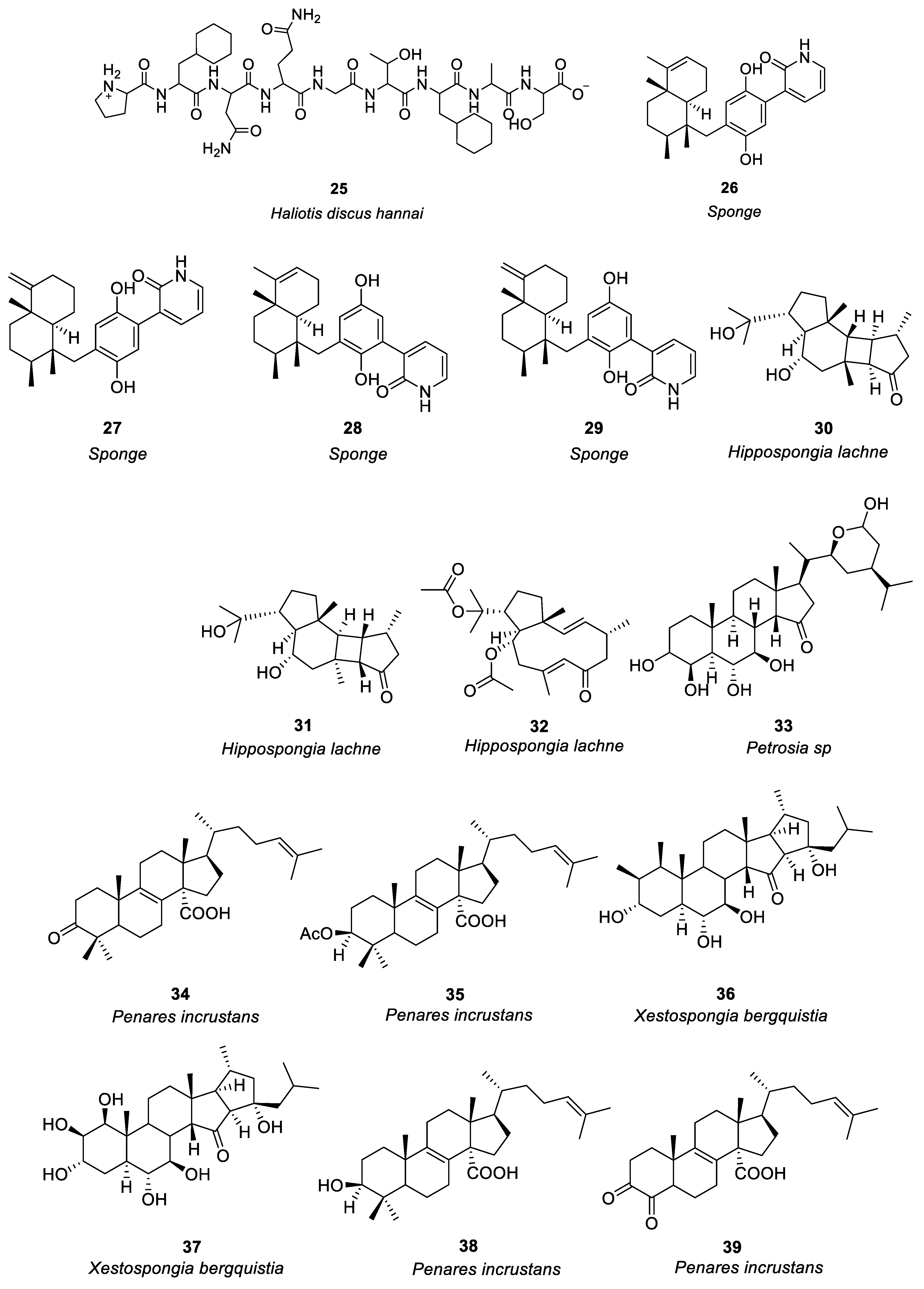
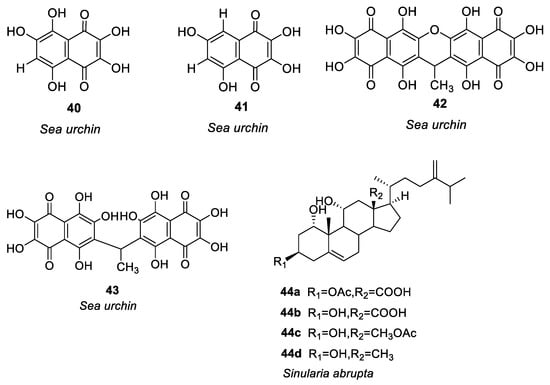
Figure 2. Structures of compounds 25–44.
Jiao et al. [25] identified anti-allergic terpenoids isolated from the marine sponge Dysidea villosa and they found that four compounds, 26–29 (Figure 2), suppressed the release of degranulation marker β-hexosaminidase with IC50 values of 8.2, 10.2, 19.9 and 16.2 μM, respectively, in a dose-dependent manner. As a result of antigen stimulation, the production of LTB 4 and IL-4 in RBL-2H3 mast cells was dose-dependently inhibited. Compound 26 demonstrated the greatest anti-allergic activity out of the four compounds. In some studies it has been shown that mast cell activation is inhibited by compound 26 by inhibiting the signaling pathway of Syk/PLCγ-1, thereby inhibiting mast cell degranulation and down-regulating LTB 4 and IL-4 production. Hong et al. [26] isolated three compounds (including 30–32, see Figure 2) from the South China Sea sponge Hippospongia lachne to find that they inhibited IgE-stimulated RBL-2H3 cells from releasing β-hexosaminidase. It was found that compounds 30 and 31 had higher β-aminocaproic glycosidase inhibitory activity. LTB4 production by activated RBL-2H3 cells was significantly inhibited by compounds 30 and 31 with IC50 values of 49.37 and 23.91 μM, respectively. Andrew et al. [27] found that the marine sponge Petrosia sp. contained a sterol-like compound called IZP-94005 (Compound 33 as shown in Figure 2). Both in vivo and in vitro allergic reactions were studied using ovalbumin-induced bronchoconstriction and smooth muscle contractions. Based on a concentration-dependent inhibition of OVA-stimulated sensitized tracheal ring response, IZP-94005 had an IC50 of 10 μM. A substantial lowering in histamine release was observed after the application of IZP-94005. Shoji et al. [28] isolated two new triterpenoids with 14 carboxyl groups from the Okinawan marine sponge Penares incrustans. Anti-IgE-induced histamine release from rat peritoneal mast cells was inhibited by compounds 34 and 35 (Figure 2) with IC50 values of 1.5 μM and 10 μM, respectively. It was found that compound 34 was 17 times more potent in nature than disodium cromoglycerate (DSCG). Takei et al. [29] characterized the Okinawan marine sponge Xestospongia bergquistia and isolated different terpenoids from it. Dose-dependent inhibition of the release of histamine from mast cells in male Wistar rats was observed with compounds 36 and 37 (Figure 2). Release of histamine from IgE-activated mast cells was blocked by compounds 36 and 37 at 100 μM each. PI-PLC activity and inhibition of IP3 production were initiated by compound 36 in a dose-dependent manner. Aside from inhibiting calcium mobilization in intracellular calcium stores, compound 36 also inhibited calcium influx. Isolation of two terpenoids from the Okinawan marine sponge Penares incrustans was also performed by Takei et al. [30]. It was shown that compounds 38 and 39 (Figure 2) inhibited anti-IgE-induced histamine release in Wistar rats. At 100 μM, the anti-IgE-induced histamine release was inhibited at 90.7 ± 2.3%, 0.5 and 1.5 μM IC50, respectively. There was a dose-dependent inhibition of PLA2 (phospholipase A2) activity with both compounds. This system was able to measure the IC50 values for PLA2 activity at 2 and 0.1 μM, respectively. (See Table 1 for details on compounds).
Pozharitskaya et al. [31] isolated and studied the anti-allergic effect of compounds (40–43, see Figure 2) of green sea urchin shell pigment. Green sea urchin shell pigment compounds had a dose-dependent inhibitory effect on histamine-induced ileum contraction in guinea pigs, ID50 = 1.2μg/mL. The inhibitory effect on the ocular allergic inflammation model was better than that of the reference drug olopatadine.
Most of the compounds isolated from soft corals belong to terpenoids, which mainly have cytotoxicity and anti-tumor activity, especially lactone diterpenoids, while compounds with anti-allergic activity account for a minority [32]. Shoji et al. [33] isolated four compounds (44a–44d, see Figure 2) from the soft coral Sinularia abrupta. Compounds 44a–44d inhibited anti-IgE-induced histamine release from rat peritoneal mast cells in a dose-dependent manner. The IC50 values of 44a–44d were 0.04, 0.6, 1.5, and 0.2 μM, respectively. It is 6500 times more potent than the well-known anti-allergic drug sodium cromoglycate (IC50 = 262 μM).
2.2. Crude Extracts from Marine Animals as Potential Sources with Anti-Allergic Activity
A research study by Kim et al. [34] examined the ability of oral administration of LMW-AV (low molecular weight peptides) acquired from gastrointestinal digestion of Abalone viscera (AV) to treat (AD) atopic dermatitis in a dermatitis-induced model stimulated with Dermatophagoides farinae. In AD-like lesions, LMW-AV inhibited the expression of chemokines and cytokines related to Th2, and it inhibited serum IgE levels. Eosinophils were decreased as a result of oral LMW-AV treatment, skin thickness was reduced, mast cell infiltration into the epidermis was inhibited, and skin edema was reduced.
Lee et al. [35] investigated the anti-allergic activity of sea cucumber and demonstrated that the liquid salting-out extract of sea cucumber activated and recruited regulatory T and Treg cells that improved allergic airway inflammation. Moreover, sea cucumber extract rich in palmitoleic acid inhibited IgE better than extracts poor in palmitoleic acid, whereas palmitoleic acid lowers serum total immunoglobulin E (IgE) concentrations.
Fish have a rich diversity due to their complex living environment. There is a variety of biological activities associated with different parts of fish. Willemsen [36] found that fish oil has an effect on decreasing allergic symptoms when high n-3LCPUFA intake is coupled with low n-6PUFA intake, whereas TH2 and TH1 reactions are reduced by N-3LCPUFA (fish oil), Treg frequency increases, and IgE level is reduced, which indicates that this oil has the potential for anti-allergic activity. Aryani et al. [37] examined the anti-allergic properties of charcoal from the inedible part of Channa pleurophthalmus Blkr, (Kerandang fish), and anti-hyaluronidase activity was determined by anti-hyaluronidase test. Based on the results, caudal fin charcoal extract exhibited the highest inhibitory effect and pectoral fin charcoal extract exhibited the lowest inhibitory effect. With four mg/mL, ethyl acetate extract concentration of caudal fin charcoal showed the greatest inhibitory effect on hyaluronidase. A potential anti-allergic drug can be developed from its non-edible parts.
3. Marine Microorganisms
Natural Products Derived from Marine Microorganisms with Anti-Allergic Activity
Harunari et al. [38] studied the activity of Hyaluromycin 45 (Figure 3), a new member of the rubromycin family isolated from marine-derived Streptomyces sp., which is composed of γ-rubromycin core structure with 2-amino-3-hydroxycyclopent-2-enone (C5N) unit as amide substituent of the carboxyl group. The enzyme hyaluromycin imparts a major role in allergic responses and in mast cell degranulation. Researchers found that hyaluromycin had a 25-fold higher inhibitory effect against hyaluronidase than the plant terpenoid glycyrrhizic acid with 14 μM IC50 value, therefore providing new insights in the development of anti-allergic drugs. Niu et al. [39] isolated a polyketone compound 46 (Figure 3) from a deep-sea-derived fungus Graphostroma sp. and tested its biological activity in IgE-mediated rat basophilic leukemia-2H3 cells. Compound 46 can also be isolated from the fermentation broth of Streptomyces sp. The findings showed that compound 46 significantly inhibited histamine release and degranulation in RBL-2H3 cells, with a 13.7 μM IC50 value. It was found that the methyl group present at C-3, the C-6 hydroxyl group, and the methoxy group at C-7 were essential for anti-food allergy activity. They also isolated eight tetracyclic diterpenoids from the deep-sea fungus Botryotinia fuckeliana in the western Pacific [40]. Compound 47 (Figure 3) was found with a novel 6/6/5/5 tetracyclic carbon skeleton. Compared with loratadine (positive control and IC50 = 0.1 mM), compound 47 showed anti-allergic effects in RBL-2H3 cells (IC50 = 0.2 mM).
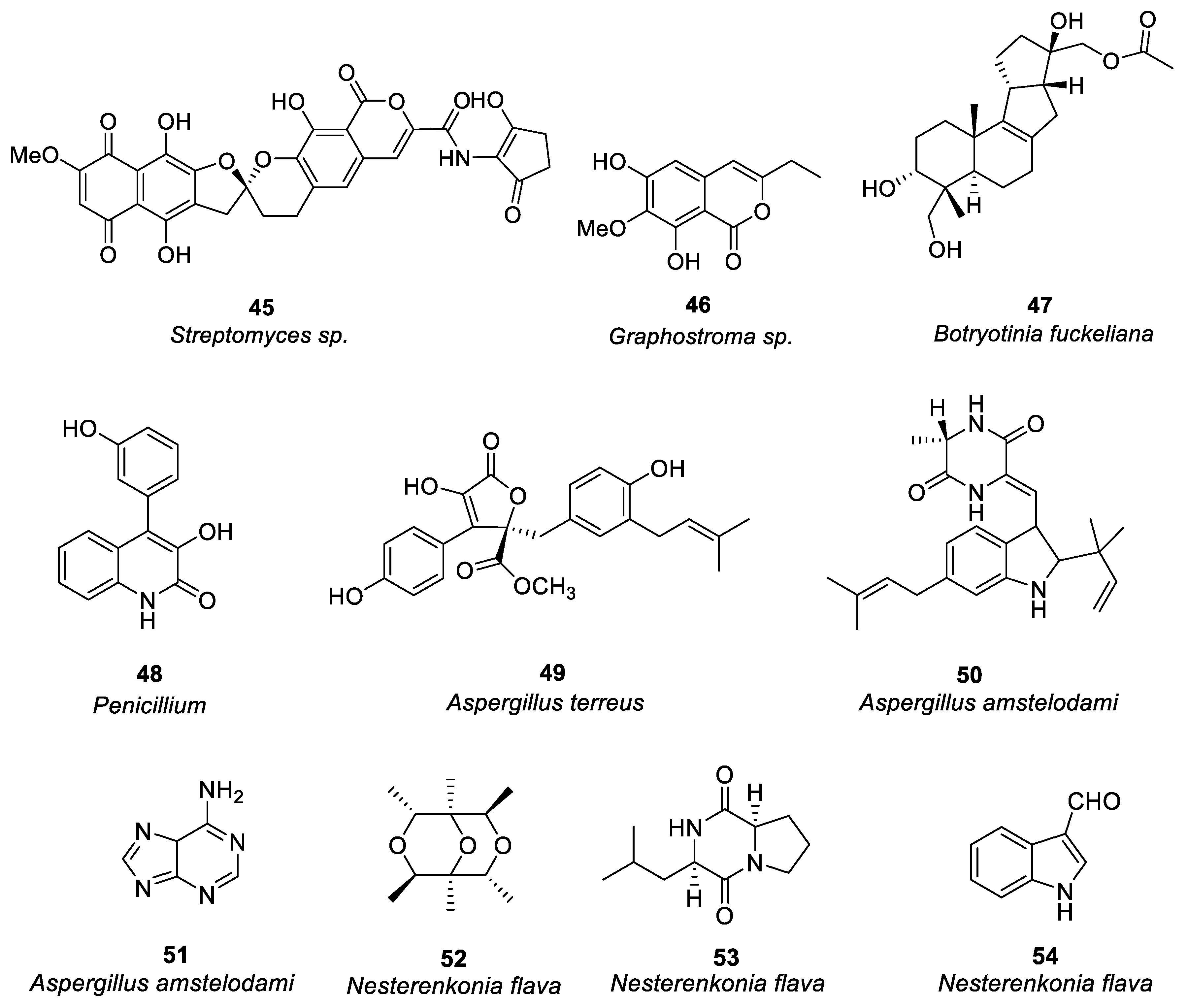
Figure 3. Structures of compounds 45–54.
Shu et al. [41] isolated an alkaloid 48 (Figure 3) from Penicillium, i.e., deep-sea fungus. Their study revealed that compound 48 significantly reduced β-hexose release and histamine in RBL-2H3 cells induced by ovalbumin (OVA) in a dose-dependent manner (IC50 = 6.67 μg/ml), and it had no cytotoxic effect on RBL-2H3. There was a dose-dependent decrease in mast cell protease-1, histamine, immunoglobulin E, and tumor necrosis factor-α levels, and an increase in IL-10 production. The increase of calcium ions is the key process of MC secretory granule translocation. Compound 48 significantly inhibited the accumulation of calcium ions in RBL-2H3 cells in a dose-dependent manner, thereby blocking the activation of macrophages and inhibiting mast cell degranulation.
Uras et al. [42] purified butyrolactone I (compound 49, see Figure 3) from Aspergillus terreus. Inhibition of calcium ion carrier A23187 and antigen-induced degranulation is manifested by its significant anti-allergic activity, with 39.7 and 41.6 μM, IC50 values. Elsbaey et al. [43] isolated two compounds (50, 51, see Figure 3) from the white bean culture of the endophytic fungus Aspergillus amstelodami. Anti-allergic activity of quercetin was determined in 100 μM RBL-2H3. Both compounds significantly reduced the release of β-hexosaminidase and had no significant cytotoxicity to cells. These compounds may have some anti-allergic effects, although they have a lower efficacy than quercetin. Xie et al. [44] isolated a new cyclic ether compound nesterenkoniane (52) and 12 known compounds from Nesterenkonia flava, an actinomycete originating from the deep sea (see Figure 3). By employing IgE-mediated rat mast cell RBL-2H3 as a model, cyclo-(D)-proline-(D)-leucine (compound 53, see Figure 3) and indole-3-carbaldehyde (compound 54, see Figure 3) showed significant anti-allergic activity with 69.95 and 57.12 μg/mL IC50 values, respectively. (See Table 1 for details on compounds).
Table 1. Research Overview of Marine Natural Products with Anti-allergy Activities.
| Source of Compounds | The Sources of Isolation | Number of Compounds | Range of Dosage | Structure Type | Test System | Targets/Pathway/Process Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Marine Plants | Ecklonia cava | Compound 1–3 | 100 μM | Polyphenol | Human basophilic KU812F cells and RBL-2H3 cells | FcεRI and IgE binding activity, histamine release, degranulation of cell |
[6] |
| Ecklonia stolonifera | Compound 4–5 | 50 μM | Polyphenol | Human basophilic KU812F cells | The expression of FcεRI, intracellular Ca2+ |
[8] | |
| Ecklonia stolonifera Okamura | Compound 6 | 50 μM | Polyphenol | RBL-2H3 mast cell | Ca2+ concentration, mast cell degranulation, histamine release |
[9] | |
| Eisenia arborea | Compound 2,3,7–10 | 10–200 µM | Polyphenol | DNP-BSA-induced RBL-2H3 mast cell |
Release of histamine, leukotriene B4 and prostaglandin E2, H1 receptor |
[10] | |
| Sargassum carpophyllum | Compound 11–13 | 40 μM | Polyphenol | DNP-HSA-induced RBL-2H3 cells | Release of β-hexosaminidase, mast cell degranulation |
[12] | |
| Symbiodinium sp., Petalonia fascia | Compound 14–15 | 50 μg | Carotenoid | BALB/cAJc1 mice | Migration of eosinophils | [13] | |
| Lumnitzera racemosa | Compound 16–24 | / | (Ethanol extract) | Toluene 2,4-diisocyanate (TDI)-induced allergic model mice |
IgE | [22] | |
| Marine Animals | Haliotis discus hannai | Compound 25 | 50 mg/kg | Polypeptide | Passive cutaneous anaphylaxis in mice | Histamine release, FcεRI and IgE binding activity | [24] |
| Sponge | Compound 26–29 | 250 μg/mL | Terpenoids | RBL-2H3 mast cells | β-hexosaminidase, Syk/PLCγ-1, mast cell degranulation | [25] | |
| Hippospongia lachne | Compound 30–32 | 200 μg/mL | (Ethanol extract) | IgE-stimulated RBL-2H3 cells | β-hexosaminidase | [26] | |
| Petrosia sp. | Compound 33 | 3–30 μM | Sterol | OVA-induced mice | Histamine release levels | [27] | |
| Penares incrustans | Compound 34–35 | 0–10 μM | Triterpenoids | Anti-IgE-induced mast cells | Histamine release | [28] | |
| Xestospongia bergquistia, Penares incrustans |
Compound 36–39 | 100 μM | Terpenoids | Anti-IgE-induced male Wistar rats’ mast cells | IP3 production, Histamine release, intracellular Ca2+, PLA2 | [29][30] | |
| Green sea urchin | Compound 40–43 | 1.2 μg/mL | Polyhydroxy-1,4-naphthoquinone | Histamine-induced guinea pigs | β-hexosaminidase | [31] | |
| Sinularia abrupta | Compound 44 | 0.04–1.5 μM | Polyhydroxysteroid | Anti-IgE-induced mice | Mast cell, histamine release | [33] | |
| Marine Microorganisms | Streptomyces sp. | Compound 45 | / | Macrolide | / | Mast cell degranulation, hyaluronidase | [38] |
| Graphostroma sp. Botryotinia fuckeliana |
Compound 46–47 | 0–200 μM | Tetracyclic diterpenoids | RBL-2H3 cells | Histamine release, mast cell degranulation | [39][40] | |
| Penicillium | Compound 48 | 20 mg/kg | Quinoline alkaloid | OVA-induced RBL-2H3 cells | β-hexose and histamine, mast cell degranulation, IgE | [41] | |
| Aspergillus terreus | Compound 49 | 100 μM | Hemiterpenes | RBL-2H3 cells | β-hexosaminidase, IgE | [42] | |
| Aspergillus amstelodami | Compound 50–51 | 100 μM | β-lactams, adenine | RBL-2H3 cells | β-hexosaminidase | [43] | |
| Nesterenkonia flava | Compound 52–54 | 1.0–80.0 µg/mL | Cycloethers, diketopiperazine, alkaloid | RBL-2H3 cells | IgE, β-hexosaminidase | [44] |
References
- Carpenter, L.J.; Malin, G.; Liss, P.S.; Küpper, F.C. Novel biogenic iodine-containing trihalomethanes and other short-lived halocarbons in the coastal East Atlantic. Glob. Biogeochem. Cycles 2000, 14, 1191–1204.
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25.
- Anis, M.; Ahmed, S.; Hasan, M.M. Algae as nutrition, medicine and cosmetic: The forgotten history, present status and future trends. World J. Pharm. Pharm. Sci. 2017, 6, 1934–1959.
- Laura, B. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 98, 317–333.
- Khuanjing, T.; Ongnok, B.; Maneechote, C.; Siri-Angkul, N.; Prathumsap, N.; Arinno, A.; Chunchai, T.; Arunsak, B.; Chat-tipakorn, S.C.; Chattipakorn, N. Acetylcholinesterase inhibitor ameliorates doxorubicin-induced cardiotoxicity through reducing RIP1-mediated necroptosis. Pharmacol. Res. 2021, 173, 105882.
- Li, Y.; Lee, S.-H.; Le, Q.-T.; Kim, M.-M.; Kim, S.-K. Anti-allergic Effects of Phlorotannins on Histamine Release via Binding Inhibition between IgE and FcεRI. J. Agric. Food Chem. 2008, 56, 12073–12080.
- Han, E.J.; Kim, H.S.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Jeon, Y.J.; Jee, Y.; Lee, J.; Kim, T.; Shim, S.Y.; Ahn, G. Eckol from Ecklonia cava Suppresses Immunoglobulin E-mediated Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Nutrients 2020, 12, 1361.
- Shim, S.-Y.; Choi, J.-S.; Byun, D.-S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorganic Med. Chem. 2009, 17, 4734–4739.
- Vo, T.S.; Kim, S.-K.; Ryu, B.; Ngo, D.; Yoon, N.-Y.; Bach, L.G.; Hang, N.T.N. The Suppressive Activity of Fucofuroeckol-A Derived from Brown Algal Ecklonia stolonifera Okamura on UVB-Induced Mast Cell Degranulation. Mar. Drugs 2018, 16, 1.
- Sugiura, Y.; Usui, M.; Katsuzaki, H.; Imai, K.; Kakinuma, M.; Amano, H.; Miyata, M. Orally Administered Phlorotannins from Eisenia arborea Suppress Chemical Mediator Release and Cyclooxygenase-2 Signaling to Alleviate Mouse Ear Swelling. Mar. Drugs 2018, 16, 267.
- Singha, B.; Shankarb, S.; Srivastavaa, R. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical Biochemical applications. Biochem. Pharmacol. 2011, 82, 1807–1821.
- Matsui, T.; Ito, C.; Itoigawa, M.; Shibata, T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022, 377, 131992.
- Onodera, K.-I.; Konishi, Y.; Taguchi, T.; Kiyoto, S.; Tominaga, A. Peridinin from the Marine Symbiotic Dinoflagellate, Symbiodinium sp., Regulates Eosinophilia in Mice. Mar. Drugs 2014, 12, 1773–1787.
- Kim, S.-K.; Lee, D.-Y.; Jung, W.-K.; Kim, J.-H.; Choi, I.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; Lee, C.M.; Yea, S.S.; et al. Effects of Ecklonia cava ethanolic extracts on airway hyperresponsiveness and inflammation in a murine asthma model: Role of suppressor of cytokine signaling. Biomed. Pharmacother. 2008, 62, 289–296.
- Han, E.J.; Kim, H.-S.; Sanjeewa, K.K.A.; Jung, K.; Jee, Y.; Jeon, Y.-J.; Fernando, I.P.S.; Ahn, G. Sargassum horneri as a Functional Food Ameliorated IgE/BSA-Induced Mast Cell Activation and Passive Cutaneous Anaphylaxis in Mice. Mar. Drugs 2020, 18, 594.
- Herath, K.H.I.N.M.; Kim, H.J.; Mihindukulasooriya, S.P.; Kim, A.; Kim, H.J.; Jeon, Y.-J.; Jee, Y. Sargassum horneri extract containing mojabanchromanol attenuates the particulate matter exacerbated allergic asthma through reduction of Th2 and Th17 response in mice. Environ. Pollut. 2020, 265 Pt B, 114094.
- Jung, W.-K.; Choi, I.; Oh, S.; Park, S.-G.; Seo, S.-K.; Lee, S.-W.; Lee, D.-S.; Heo, S.-J.; Jeon, Y.-J.; Je, J.-Y.; et al. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 293–297.
- Shi, C.; Pan, T.; Cao, M.; Liu, Q.; Zhang, L.; Liu, G. Suppression of Th2 immune responses by the sulfated polysaccharide from Porphyra haitanensis in tropomyosin-sensitized mice. Int. Immunopharmacol. 2015, 24, 211–218.
- Han, J.; Liu, B.; Liu, Q.-M.; Zhang, Y.-F.; Liu, Y.-X.; Liu, H.; Cao, M.-J.; Liu, G.-M. Red Algae Sulfated Polysaccharides Effervescent Tablets Attenuated Ovalbumin-Induced Anaphylaxis by Upregulating Regulatory T cells in Mouse Models. J. Agric. Food Chem. 2019, 67, 11911–11921.
- Raman, B.V.; Rao, D.N.; Radhakrishnan, T.M. Enteromorpha compressa (L.) Grevillean edible green alga as a source of antiallergic principle (S). J. Clin. Biochem. 2004, 19, 105–109.
- Lee, D.S.; Park, W.S.; Heo, S.J.; Cha, S.H.; Kim, D.; Jeon, Y.J.; Park, S.G.; Seo, S.K.; Choi, J.S.; Park, S.J.; et al. Polyopes affinis alleviates airway inflammation in a murine model of allergic asthma. J. Biosci. 2011, 36, 869–877.
- Acharyya, R.N.; Mithila, S.; Rani, S.; Islam, A.; Golder, M.; Ahmed, K.S.; Hossain, H.; Dev, S.; Das, A.K. Anti-allergic and Anti-hyperglycemic Potentials of Lumnitzera racemose Leaves: In vivo and In silico Studies. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2022, 1–12.
- Pita, L.; Rix, L.; Slaby, B.M.; Franke, A.; Hentschel, U. The sponge holobiont in a changing ocean: From microbes to ecosystems. Microbiome 2018, 6, 46.
- Ko, S.-C.; Lee, D.-S.; Park, W.S.; Yoo, J.S.; Yim, M.-J.; Qian, Z.-J.; Lee, C.-M.; Oh, J.; Jung, W.-K.; Choi, I.-W. Anti-allergic effects of a nonameric peptide isolated from the intestine gastrointestinal digests of abalone (Haliotis discus hannai) in activated HMC-1 human mast cells. Int. J. Mol. Med. 2015, 37, 243–250.
- Jiao, W.-H.; Cheng, B.-H.; Shi, G.-H.; Chen, G.-D.; Gu, B.-B.; Zhou, Y.-J.; Hong, L.-L.; Yang, F.; Liu, Z.-Q.; Qiu, S.-Q.; et al. Dysivillosins A–D, Unusual Anti-allergic Meroterpenoids from the Marine Sponge Dysidea villosa. Sci. Rep. 2017, 7, 8947.
- Hong, L.-L.; Yu, H.-B.; Wang, J.; Jiao, W.-H.; Cheng, B.-H.; Yang, F.; Zhou, Y.-J.; Gu, B.-B.; Song, S.-J.; Lin, H.-W. Unusual Anti-allergic Diterpenoids from the Marine Sponge Hippospongia lachne. Sci. Rep. 2017, 7, 43138.
- Bramley, A.M.; Langlands, J.M.; Jones, A.K.; Burgoyne, D.L.; Li, Y.; Andersen, R.J.; Salari, H. Effects of IZP-94005 (contignasterol) on antigen-induced bronchial responsiveness in ovalbumin-sensitized guinea-pigs. Br. J. Pharmacol. 1995, 115, 1433.
- Shoji, N.; Umeyama, A.; Motoki, S.; Arihara, S.; Ishida, T.; Nomoto, K.; Kobayashi, J.; Takei, M. Potent Inhibitors of Histamine Release, Two Novel Triterpenoids from the Okinawan Marine Sponge Penares incrustans. J. Nat. Prod. 1992, 55, 1682–1685.
- Takei, M.; Umeyama, A.; Shoji, N.; Arihara, S.; Endo, K. Mechanism of inhibition of IgE-dependent histamine release from rat mast cells by xestobergsterol A from the Okinawan marine spongeXestospongia bergquistia. Cell Mol. Life Sci. 1993, 49, 145–149.
- Takei, M.; Umeyama, A.; Shoji, N.; Arihara, S.; Endo, K. Mechanism of Inhibition of IgE-Dependent Histamine Release from Rat Mast Cells by Penasterol and Penasterone. J. Pharm. Sci. 1995, 84, 228–230.
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Ivanova, S.A.; Kosman, V.M.; Makarov, V.G.; Bazgier, V.; Berka, K.; Otyepka, M.; Ulrichová, J. Antiallergic Effects of Pigments Isolated from Green Sea Urchin (Strongylocentrotus droebachiensis) Shells. Planta Medica 2013, 79, 1698–1704.
- Chen, W.-T.; Li, Y.; Guo, Y.-W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237.
- Shoji, N.; Umeyama, A.; Takei, M.; Arihara, S. Potent inhibitors of histamine release: Polyhydroxylated sterols from the okinawan soft coral Sinularia abrupta. J. Pharm. Sci. 1994, 83, 761–762.
- Kim, T.-H.; Heo, S.-Y.; Oh, G.-W.; Park, W.S.; Choi, I.-W.; Qian, Z.-J.; Jung, W.-K. Anti-Allergic Effect of Low Molecular Weight Digest from Abalone Viscera on Atopic Dermatitis-Induced NC/Nga. Mar. Drugs 2021, 19, 634.
- Lee, D.-I.; Kang, S.A.; Anisuzzaman; Jeong, U.-C.; Jin, F.; Kang, S.-J.; Lee, J.-Y.; Yu, H.S. Sea Cucumber Lipid-Soluble Extra Fraction Prevents Ovalbumin-Induced Allergic Airway Inflammation. J. Med. Food 2018, 21, 21–29.
- Willemsen, L.E. Dietary n-3 long chain polyunsaturated fatty acids in allergy prevention and asthma treatment. Eur. J. Pharmacol. 2016, 785, 174–186.
- Aryani, A.; Suprayitno, E.; Sasmito, B.B.; Hardoko, H. Characterization and identification of charcoal of inedible Kerandang fish (Channa pleurophthalmus Blkr) body parts and potential antiallergenic properties. Veter World 2020, 13, 1480–1486.
- Harunari, E.; Imada, C.; Igarashi, Y.; Fukuda, T.; Terahara, T.; Kobayashi, T. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Mar. Drugs 2014, 12, 491–507.
- Niu, S.; Liu, Q.; Xia, J.-M.; Xie, C.-L.; Luo, Z.-H.; Shao, Z.; Liu, G.-M.; Yang, X.-W. Polyketides from the Deep-Sea-Derived Fungus Graphostroma sp. MCCC 3A00421 Showed Potent Antifood Allergic Activities. J. Agric. Food Chem. 2018, 66, 1369–1376.
- Niu, S.; Xie, C.L.; Xia, J.M.; Liu, Q.M.; Peng, G.; Liu, G.M.; Yang, X.W.; Botryotins, A.-H. Tetracyclic Diterpenoids Representing Three Carbon Skeletons from a Deep-Sea-Derived Botryotinia fuckeliana. Org. Lett. 2020, 22, 580–583.
- Shu, Z.; Liu, Q.; Xing, C.; Zhang, Y.; Zhou, Y.; Zhang, J.; Liu, H.; Cao, M.; Yang, X.; Liu, G. Viridicatol Isolated from Deep-Sea Penicillium Griseofulvum Alleviates Anaphylaxis and Repairs the Intestinal Barrier in Mice by Suppressing Mast Cell Activation. Mar. Drugs 2020, 18, 517.
- Uras, I.S.; Ebada, S.S.; Korinek, M.; Albohy, A.; Abdulrazik, B.S.; Wang, Y.H.; Chen, B.H.; Horng, J.T.; Lin, W.; Hwang, T.L.; et al. Anti-Inflammatory, Antiallergic, and COVID-19 Main Protease (M(pro)) Inhibitory Activities of Butenolides from a Marine-Derived Fungus Aspergillus terreus. Molecules 2021, 26, 3354.
- Elsbaey, M.; Sallam, A.; El-Metwally, M.; Nagata, M.; Tanaka, C.; Shimizu, K.; Miyamoto, T. Melanogenesis Inhibitors from the Endophytic Fungus Aspergillus amstelodami. Chem. Biodivers 2019, 16, e1900237.
- Xie, C.-L.; Liu, Q.; Xia, J.-M.; Gao, Y.; Yang, Q.; Shao, Z.-Z.; Liu, G.; Yang, X.-W. Anti-Allergic Compounds from the Deep-Sea-Derived Actinomycete Nesterenkonia flava MCCC 1K00610. Mar. Drugs 2017, 15, 71.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
770
Revisions:
2 times
(View History)
Update Date:
06 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





