Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sohail Mumtaz | -- | 3749 | 2023-04-04 12:09:08 | | | |
| 2 | Rita Xu | -3 word(s) | 3746 | 2023-04-04 12:21:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mumtaz, S.; Khan, R.; Rana, J.N.; Javed, R.; Iqbal, M.; Choi, E.H.; Han, I. Biomedical and Environmental Applications of Nonthermal Plasma. Encyclopedia. Available online: https://encyclopedia.pub/entry/42777 (accessed on 07 March 2026).
Mumtaz S, Khan R, Rana JN, Javed R, Iqbal M, Choi EH, et al. Biomedical and Environmental Applications of Nonthermal Plasma. Encyclopedia. Available at: https://encyclopedia.pub/entry/42777. Accessed March 07, 2026.
Mumtaz, Sohail, Rizwan Khan, Juie Nahushkumar Rana, Rida Javed, Madeeha Iqbal, Eun Ha Choi, Ihn Han. "Biomedical and Environmental Applications of Nonthermal Plasma" Encyclopedia, https://encyclopedia.pub/entry/42777 (accessed March 07, 2026).
Mumtaz, S., Khan, R., Rana, J.N., Javed, R., Iqbal, M., Choi, E.H., & Han, I. (2023, April 04). Biomedical and Environmental Applications of Nonthermal Plasma. In Encyclopedia. https://encyclopedia.pub/entry/42777
Mumtaz, Sohail, et al. "Biomedical and Environmental Applications of Nonthermal Plasma." Encyclopedia. Web. 04 April, 2023.
Copy Citation
Atmospheric plasmas have led to the formation of nonthermal plasma (NTP). A number of novel plasma diagnostic approaches have been implemented and reported in order to better understand the physics of NTP. The use of NTP is a novel approach to producing reactive oxygen and nitrogen species. Plasma technology has many applications, including electrical device microfabrication, biomedicine, dentistry, agriculture, ozone generation, chemical synthesis, surface treatment, coating, and disease therapy.
cold plasma
plasma agriculture
Plasma water treatment
Plasma catalysts
Plasma antibacterial properties
Nonthermal plasma and waster water treatment
low pressure plasma and cancers
Nonthermal plasma and VOC removal
DBD plasma and dye removal
Nonthermal pla
1. Introduction
The American physicist Irving Langmuir identified plasma as a fourth state of matter in 1922 [1]. Plasmas are gases that have been totally or partially ionized. In order to remove electrons from atoms, enough energy is given to the gas, resulting in a combination of free electrons, free radicals, neutrals, and positively charged species [2][3][4]. Nonthermal plasma (NTP) or cold atmospheric plasma differs from thermal plasma because of the properties of electrons, ions, and neutrality. The charge species, reactive oxygen species (ROS), reactive nitrogen species (RNS), electromagnetic field, electric field, ability to influence pH, visible light, charged particles, neutral species, ozone, and ultraviolet (UV) radiations were all found in NTP as shown in Figure 1, making it a feasible tool for a variety of tasks [5][6][7][8][9]. The temperature of plasma electrons might reach tens of thousands of kelvin, significantly exceeding the neutral gas’ temperature of roughly room temperature. In bulk, most plasmas are electrically neutral, which adds to plasma’s unique properties and categorization as the fourth state of matter. Plasma parameters can vary dramatically depending on the energy source and amount delivered. Furthermore, NTP devices such as jet plasma, dielectric barrier discharge (DBD) plasma, and spark plasma depicted in Figure 2a often operate in a room environment, making them ideal for life science research [9][10][11][12] and a wide range of biomedical applications [13][14][15][16][17][18][19][20][21][22]. Sterilization, skin disinfection, oral/dental disease treatment, blood coagulation, wound healing, cancer therapy, and immunotherapy have all seen new possibilities in medicine by using NTP [23]. Gliding arc discharge (Figure 2d) and corona discharge (Figure 2e) devices are commonly used for environmental applications such as the removal of gaseous pollutants.
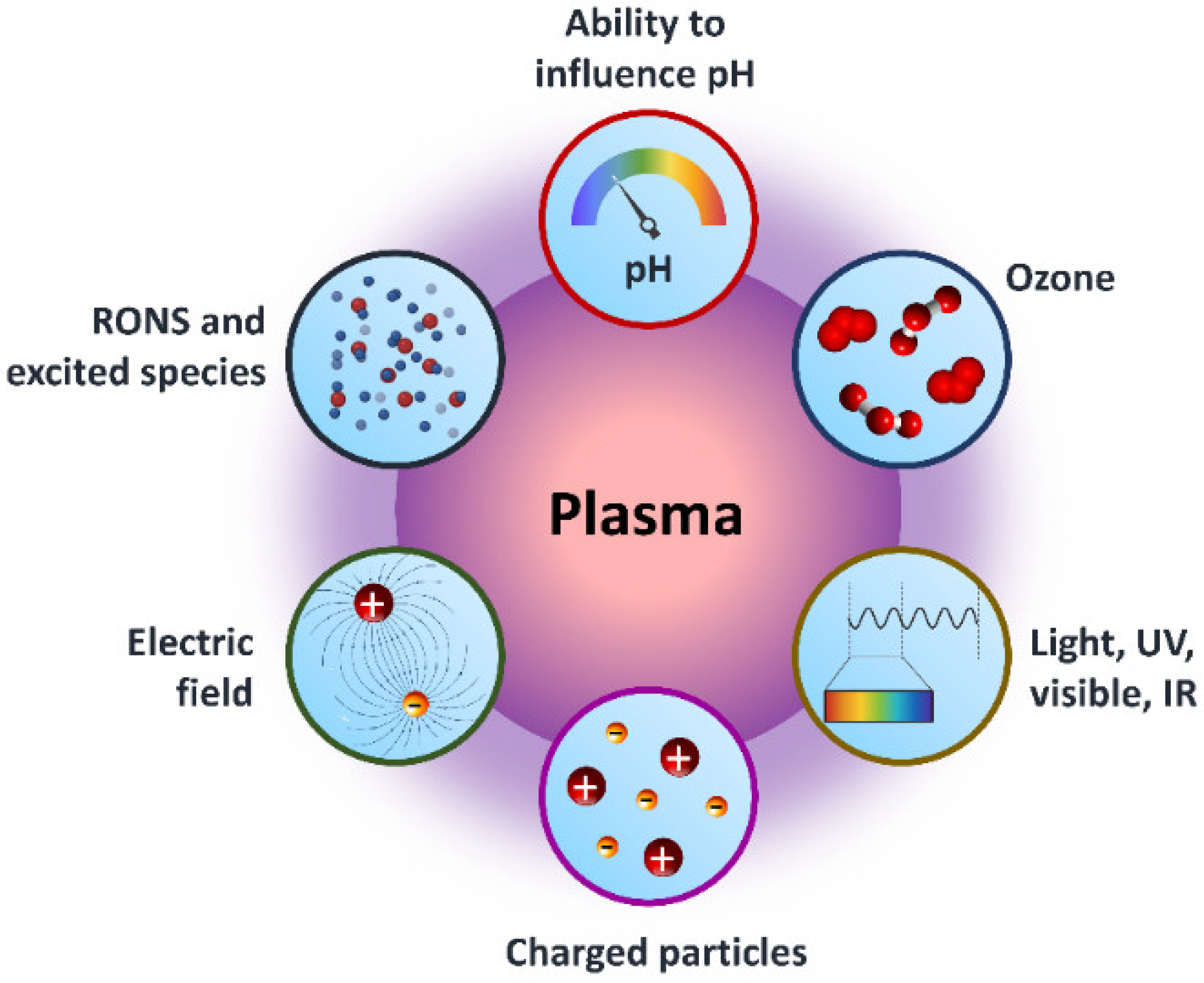
Figure 1. Graphical representation of core components of NTP for dealing with the surroundings in a variety of applications.
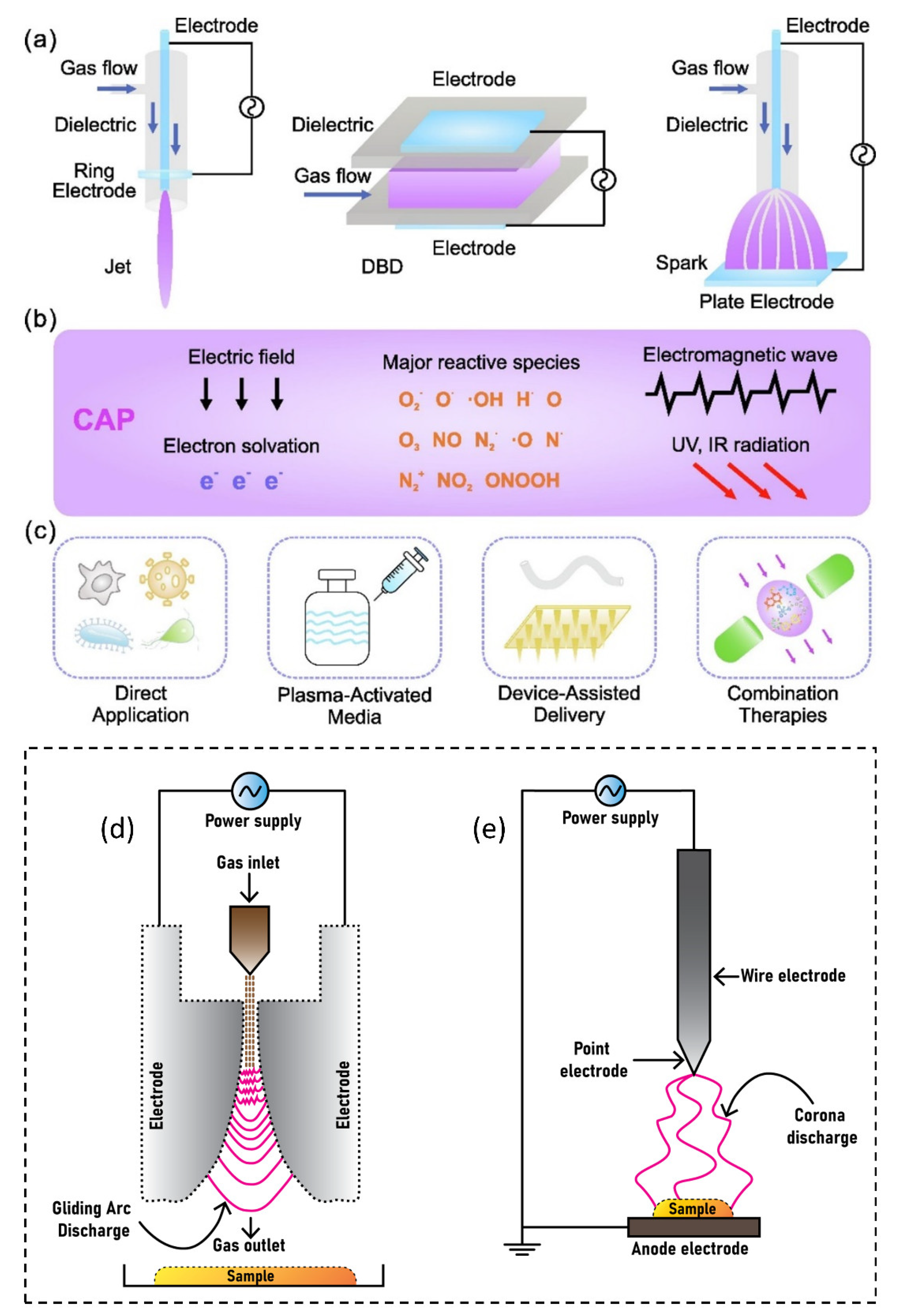
Figure 2. NTP delivery schematic for biomedical applications: (a) typical NTP devices, including plasma jet, DBD, and spark discharge; (b) plasma environment containing reactive species, electrons, other ions, emissions, waves, and physical forces; and (c) plasma delivery strategies, including direct application, plasma-activated media, device-assisted delivery, and therapeutic approaches. The most common devices used for environmental applications are (d) gliding arc discharge plasma and (e) corona discharge plasma.
1.1. Plasma
As plasma makes up 99% of the universe, it is the most prevalent kind of matter. The Sun and other stars, galaxies, solar winds, lightning, and the aurora borealis are all examples of the plasma state. Plasma televisions, neon and fluorescent lights, and plasma displays are well-known applications for man-made plasma. From Figure 1 and Figure 2c, it can be seen that plasma is made up of neutral, ionized, and/or excited charged particles, ions, and molecules, and ozone with the ability to influence the pH of solutions, as well as the existence of different ROS and RNS. A significant source of UV and vacuum UV radiation is plasma made up of several gases [24]. Plasma is a unique material treatment technology since it can employ a single component or a mix of components.
1.2. Thermal and Nonthermal Plasmas
All of the particles in a thermal plasma are at about the same temperature and completely ionized. Hot, completely ionized, and equilibrium plasma are other names for thermal plasma. Depending on the demands, equilibrium plasma is used in a variety of applications [25]. Plasma is a (partially) ionized gas made up of neutral species (molecules, radicals, excited species), ions, photons, and electrons. As the electron temperature is much higher than the temperature of heavy species (ions and neutrals) in nonthermal plasma (NTP) or non-equilibrium plasma, radicals and excited species are formed at temperatures that are closer to room temperature. This nonthermal energy distribution provides a potential route to getting around both the kinetic and thermodynamic constraints on the chemical conversion of reactants into desired products. Cold, partially ionized, atmospheric pressure, low temperature, and non-equilibrium plasma are other names for NTP. The NTP technology is suitable for treating a range of biological materials, including solids, liquids, and aerosols because it is at a low temperature when applied. There are several uses for two different types of NTP, low pressure and atmospheric pressure [26][27][28][29].
2. Generation of Reactive Species in NTP Discharge
The NTP is well recognized for generating exceptionally high concentrations of reactive species. NTP produces a variety of reactive oxygen species (ROS), including OH radicals, O2, H2O2, O, O3, and 1O2, as well as reactive nitrogen species (RNS), such as NO, NO2, N2O, N2O5, and atomic N [5]. Some of them, such as OH radicals, O, NO, N, and 1O2, have a short lifespan [19]. Helium plasma discharge was also applied by a number of studies [30][31][32][33]. The mechanisms by which ROS are produced in helium plasma jets have been well reported [34]. A number of species, including N2+, atomic (He, O) radicals, and molecular OH, were discovered in helium discharge [31][33]. The RONS, also referred to as primary reactive species, are generated by the energy released during collisions between accelerating electrons and neutrals. In the gas phase right after the collision, electrons (e−), ionized neutrals and gas (M+), excited neutrals and gas (M*), N, O, atomic H, NO, and O2*- are produced [13]. They are categorized as primary reactive species [35], and the intensities of these species are very high in the plasma region. The lifetimes of primary reactive species are relatively short; for example, the lifespan of OH radicals, NO, and O2*−, is 2.7 μs, 1.2 μs, 1.4 μs, and 1.3 μs, respectively [36]. Some of these reactive species immediately experience radiative decay, while others combine with neutrals, water molecules, and other reactive species. The main reactive species change into secondary reactive species such as H2O2, NO2, NO3, and O3 [37] in an ambient environment, as shown in Figure 3. The liquid phase (or another target) is where the RONS produced in the gas phase dissolve and form tertiary reactive species. The tertiary reactive species are formed when the RONS produced in the gas phase dissolve in the liquid phase [35]. Long-living reactive species include O3, H2O4, NO3, and NO2 because of their lifespans of a few milliseconds over several days [38]. Water can dissolve H2O2, NO2, and NO3; NO2 and NO3 are immediately converted into NO2− and NO3−, respectively [13][39]. Depending on the plasma source, working gas, power source, treatment time, and sample volume, these reactive species can reduce the pH of the target liquid by up to 2 [39]. However, whether a target is dry or aqueous affects the chemistry of ROS and RNS formation in a target [39]. The target, gas region, plasma/target interface, and discharge region of RONS formation are schematically depicted in Figure 3.
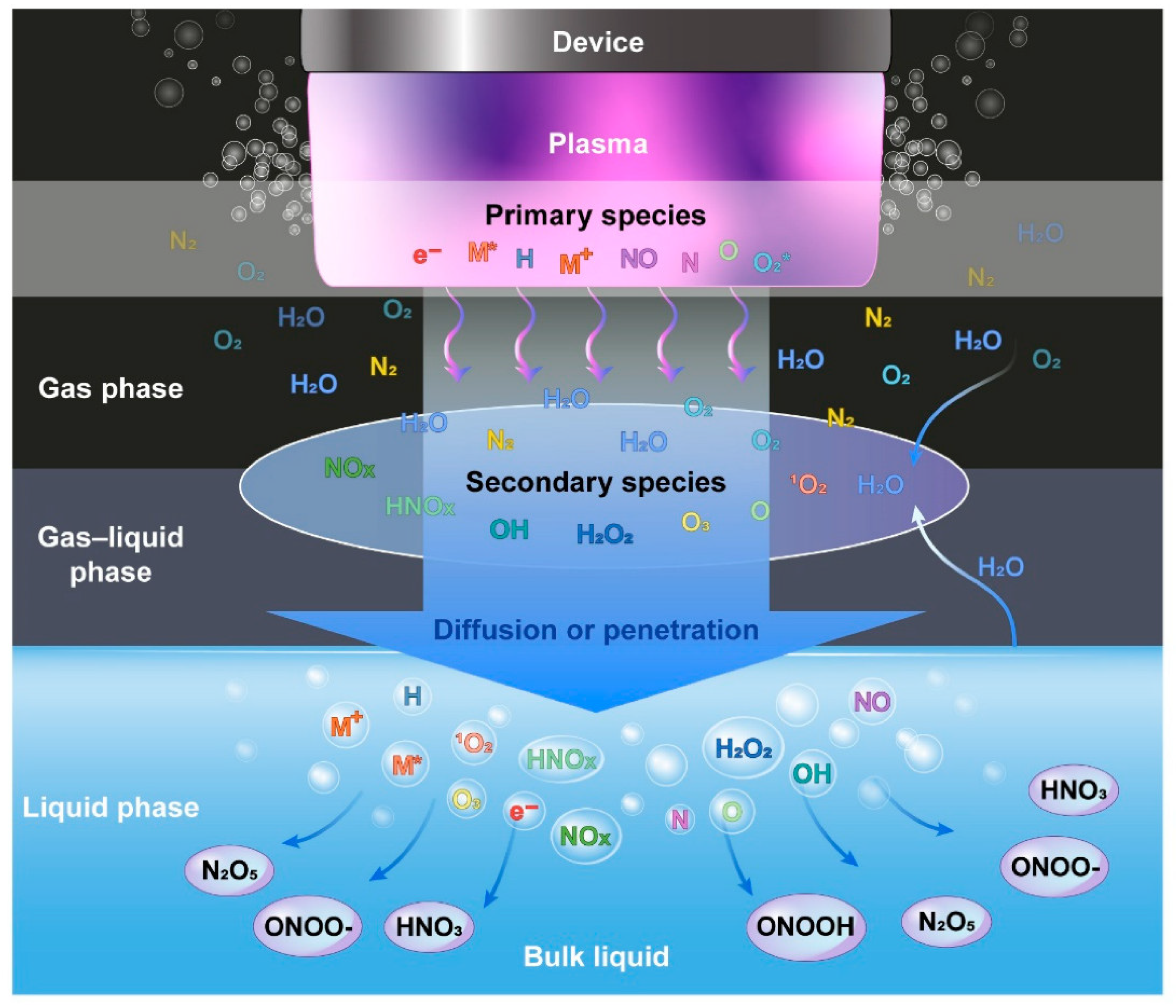
Figure 3. Graphical representation of formation of reactive species in plasma discharge, gas phase, gas–liquid phase, and liquid phase.
DBD plasma and jet plasma have been shown to produce high levels of ROS [26][40][41]. Indirect plasmas are produced between two electrodes of certain devices and transferred to the application region through a gas flow. ROS are often produced at the border of jets with the surrounding air by a variety of causes. Several authors claim that ROS generated by plasma can induce morphological alterations, membrane depolarization, lipid peroxidation, and DNA damage in cells [42][43][44][45]. The transport of reactive oxygen/nitrogen species (RONS) is the major mechanism of NTP anti-neoplastic action [46]. The quantity of reactive species created in plasma-treated liquids is extremely important in plasma therapy. Several lines of study now focus on utilizing plasma to treat cancer using the ROS generation feature [47][48][49]. In human cells, plasma treatment induces the mitochondrial membrane potential to depolarize, resulting in the generation of ROS [50]. The therapeutic effects of air plasma have been linked to the generation of RONS such as H2O2, Ox, OH, •O2, and NOx as a result of mitochondrial membrane potential depolarization and ROS accumulation [48]. Previous reports provide a more thorough explanation of plasma–liquid interactions and the plasma roadmap [23][51][52].
3. NTP Application for Cancer Treatment
The NTP technology has advanced dramatically in the previous two decades, from theoretical and experimental study to real-world implementations. The generation of plasma at atmospheric pressure and room temperatures to deal with biological systems has given rise to a new multidisciplinary field known as “plasma medicine” [2]. NTP has a variety of potentials in biomedical engineering nowadays [53]. Figure 4 depicts the interaction of NTP with the biological system, indicating the main molecular mechanisms involved in the use of LTP in cancer treatment. NTP technology has the potential to provide a less intrusive surgical procedure for removing particular cells without causing injury to the surrounding tissue. Traditional laser surgery relies on heat contact, which can result in unintentional cell death (necrosis) and lasting tissue damage. NTP contact with tissue, on the other hand, may allow selective cell elimination without necrosis [54]. Cell detachment without influencing cell viability, regulated cell death, and other interactions are examples of these interactions. It can also be employed for cosmetic approaches to dermis reticular architecture regeneration.
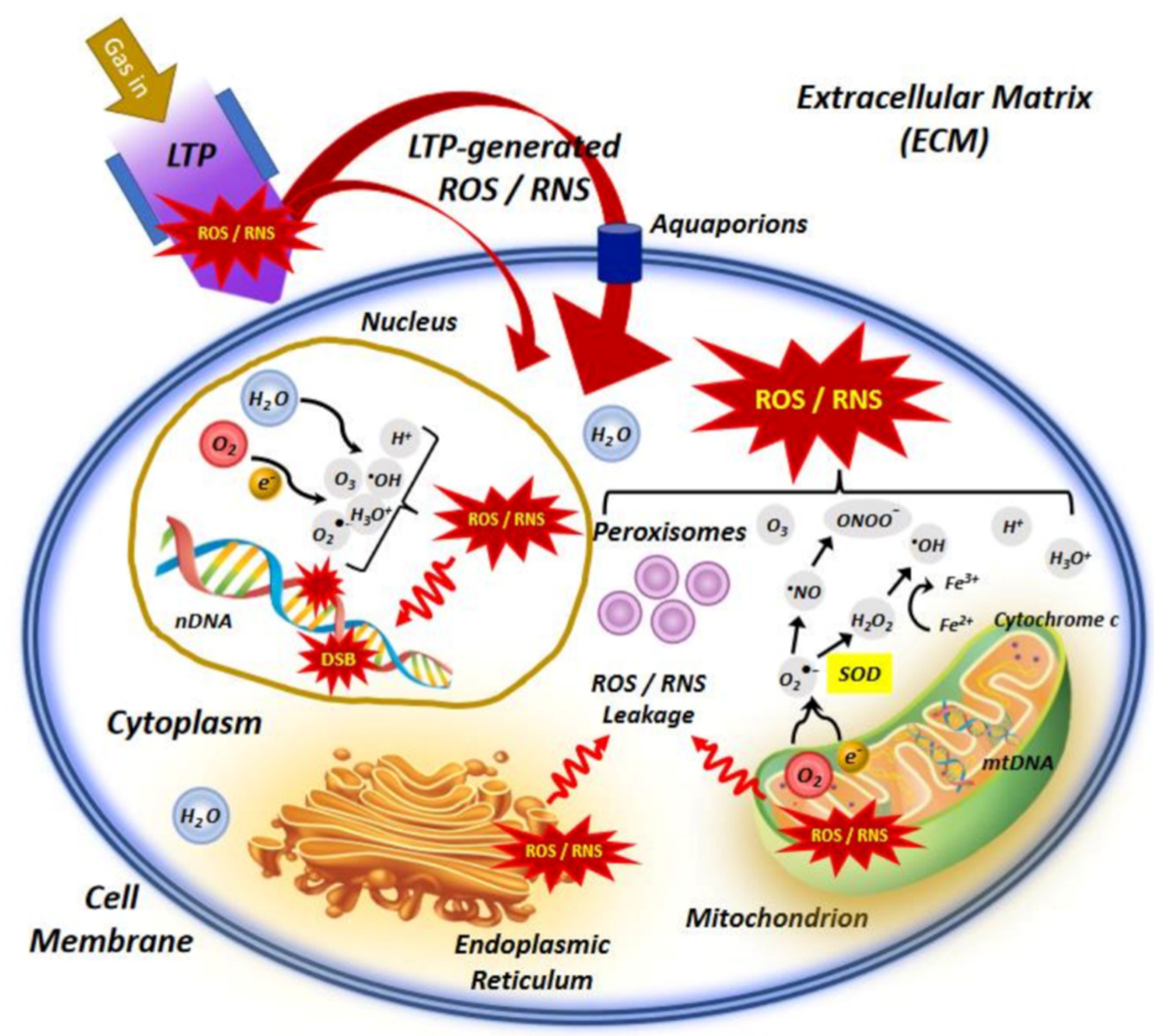
Figure 4. An illustration of the interaction of NTP or low−temperature plasma (LTP) with the cell, indicating the main molecular mechanisms involved in the use of LTP in cancer treatment.
The goal of plasma contact with tissue is to operate below the temperature damage threshold and cause chemically specific reactions or alterations, rather than to denature the tissue. The presence of plasma, in particular, can enhance chemical processes that have the desired effect. Pressure, gas composition, and power may all be adjusted to enhance chemical reactions. As a result, finding plasma conditions that generate a positive influence on tissue without having heat treatment is crucial. NTP produces a variety of reactive species that can be employed to increase cancer cells’ oxidative stress and ultimately kill them [40][55][56]. Different ROS can be produced in plasma, and some of them can cause oxidative stress in cells [46]. As a result, it can alter any pathway that is regulated by or connected to ROS, either directly or indirectly [40][57]. Recent research has found that cancer cells generate more ROS [58] and are thus prone to oxidative stress higher than normal cells, making them more appropriate for targeting by ROS in combination with plasma technology [59][60]. Because NTP-killing activity is greater in cancer cells than in normal cells, the outcome of an NTP cancer treatment is more optimistic [60]. NTP medicinal applications have achieved this incredible breakthrough from initial discovery through fundamental scientific research to clinical applications [61].
Previous studies dealt with the optimal duration of treatment to maximize plasma’s anticancer effects [32][62][63][64]. The improvement of plasma on-time during treatment is a critical issue that needs to be addressed. The biomedical effects are directly impacted by RONS levels, which are directly impacted by plasma on-time. Recently, research on the effects of plasma on-times was conducted while keeping the duty ratio and treatment time fixed (Figure 5) [65]. The selected plasma on-times are 25, 50, 75, and 100 ms for 10% and 36% duty ratios, respectively. Figure 5a–c depict, respectively, the experimental setups, a photo taken during the preparation of PTM using a soft plasma jet, and the application of PTM to cells. Figure 5d,e show that the NOx concentration in PTM corresponds to the plasma on-time, in 10% and 36% duty ratios. Similarly, Figure 5f,g show the concentrations of H2O2. Figure 5h,i indicate the viability of the U87-MG cell line when PTM was applied. It is interesting to note that by increasing the plasma on-time, the levels of ROS/RNS dramatically increased in PTM and significantly impacted the viability and ATP levels of the U87-MG cell line. The findings of this study offer a significant indication of progress by introducing the optimization of plasma on-time to enhance the effectiveness of the soft plasma jet for biomedical applications [65]. It is interesting to observe from this research that the ROS/RNS levels can be altered to suit needs by adjusting the plasma on-time within the fixed duty ratio and treatment time.
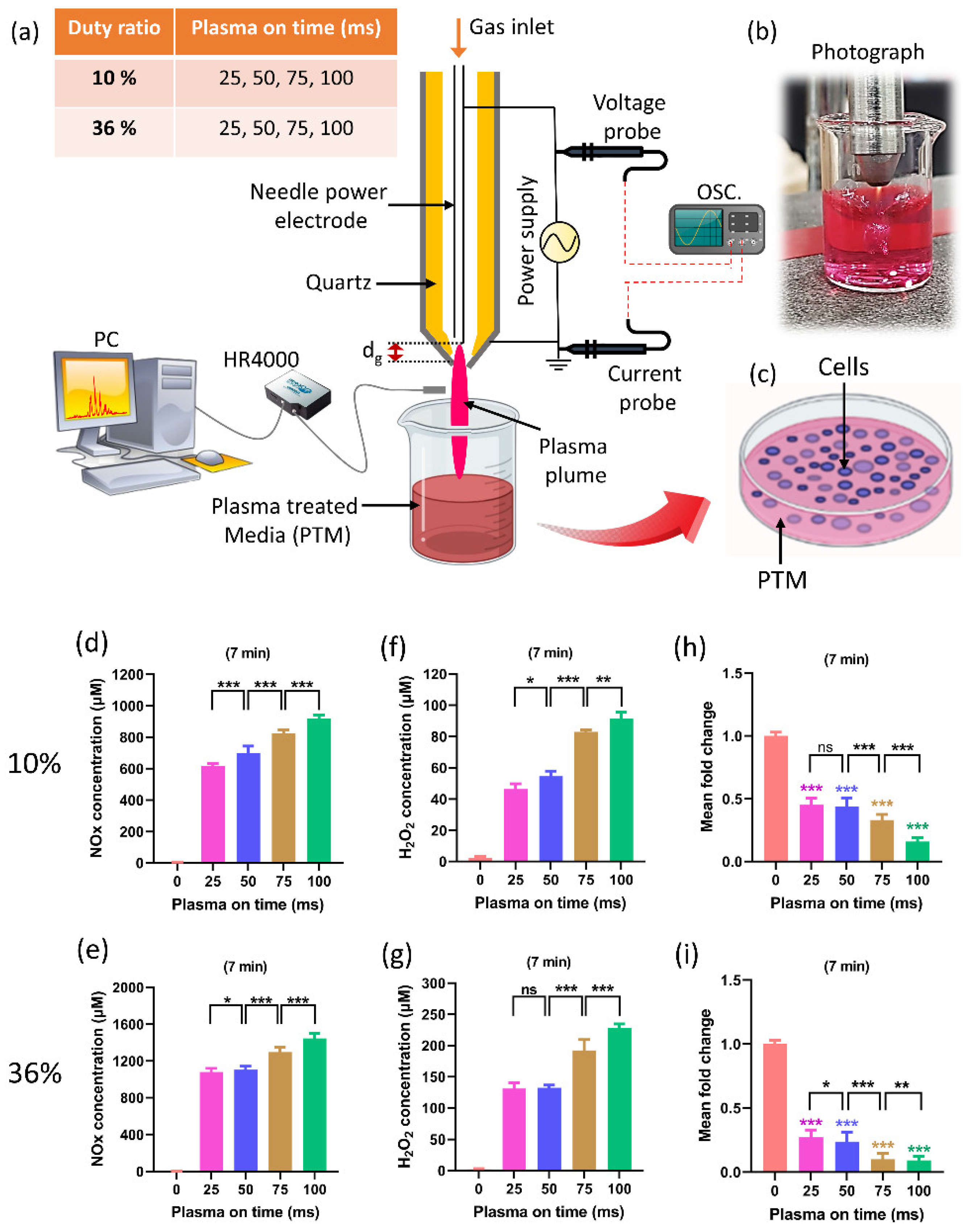
Figure 5. (a) The schematic of soft plasma jet and experimental setup. When preparing PTM, two fixed duty ratios of 10% and 36% were kept, as well as a fixed treatment time of 7 min; only the plasma on-time was changed (25, 50, 75, and 100 ms). (b) Photograph of soft jet during treatment, and (c) the application of PTM to the U87-MG cell line. (d,e) The NOx content in PTM by changing the plasma on-time when duty ratio (10% and 36%) and treatment time are fixed. (f,g) H2O2 content corresponding to plasma on-time in 10% and 36% duty ratio. (h,i) The cell viability by changing the plasma on-time. The NOx and H2O2 content significantly increased when only plasma on-time increased to 25, 50, 75, and 100 ms which shows further decline in the viability of brain cancer cells. The significance of treatment groups indicated by * p < 0.05, ** p < 0.01, *** p < 0.001, ns—not significant.
A new cancer treatment modality is being developed by the multidisciplinary field of plasma medicine, which combines plasma physics, chemistry, biology, and clinical medicine. In order to specifically target malignant cells for the prevention of cell proliferation and tumor progression, it primarily relies on the use of low-temperature plasmas in atmospheric pressure. A comprehensive discussion is given of intracellular mechanisms of action, important pathways, and the selectivity of NTP against cancer cells as shown in Figure 4. The in vivo, in vitro, and in ovo experiments are all studied using the NTP technology. Figure 6 depicts two recent in vivo and in ovo experiments using NTP for anticancer purposes. According to reports and widespread knowledge, the NTP raises the rate of apoptosis in cancer cells when compared to the control group (Figure 6a) [66].
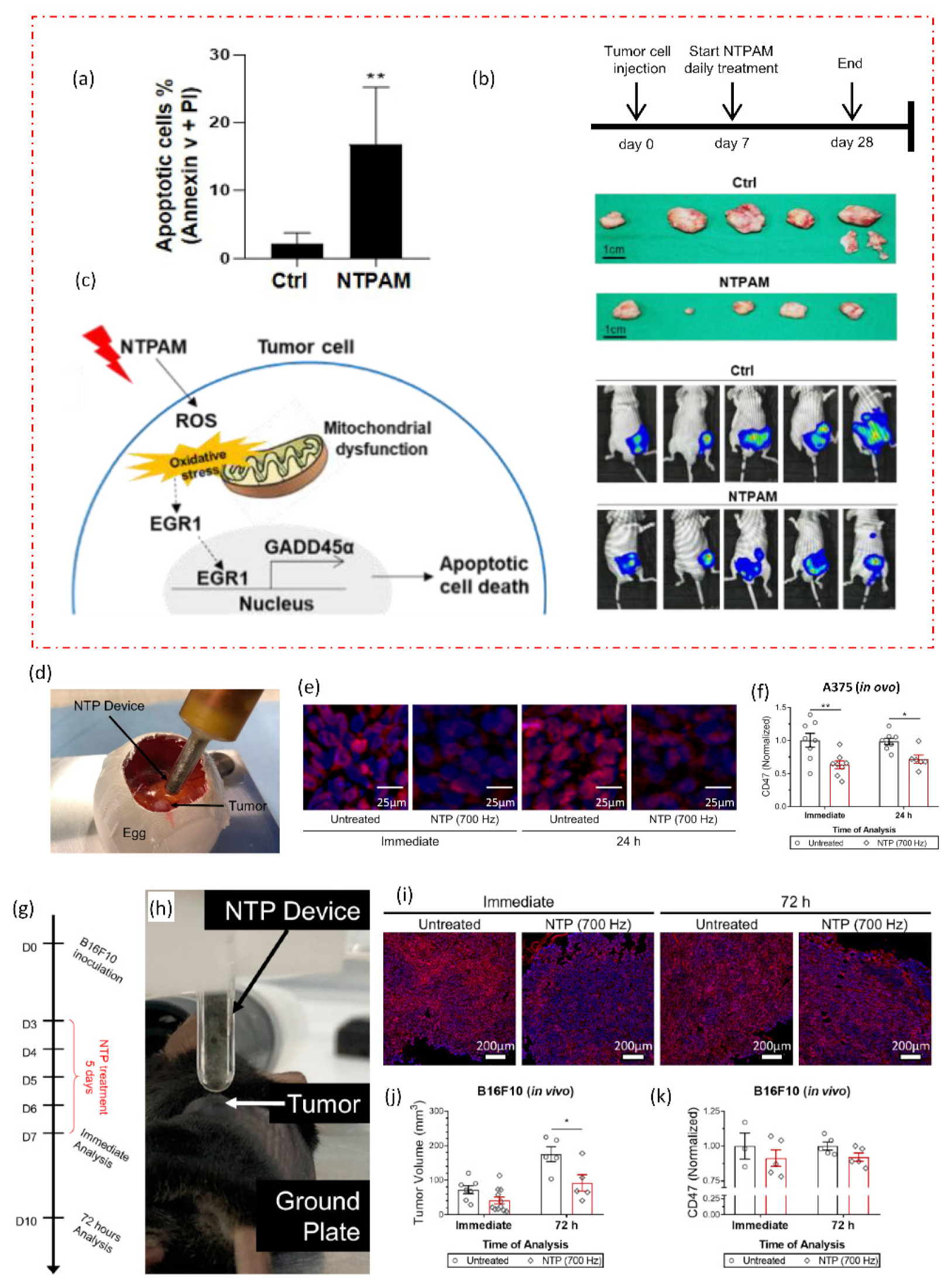
Figure 6. NTP applications in in vivo and in ovo studies. (a) The effects of nonthermal plasma-activated medium (NTPAM) on apoptosis in thyroid cancer (THCA) cells, which exhibit an increased percentage of apoptosis following NTPAM treatment. NTPAM has anticancer activity in an in vivo xenograft model. (b) Diagrammatic representation of the in vivo experimental plan. Following the injection of mice with FRO-Luc cells, final tumor images of cancer cells tracked with the IVIS imaging system, and last tumor images. (c) potential mechanism according to study, the THCA ROS/EGR1/GADD45α axis is induced by NTPAM. In an in ovo model, NTP treatment reduced CD47 expression in the A375 melanoma tumors. (d) Illustration of fertilized eggs that have been treated directly with NTP. Tumors were removed immediately after treatment or 24 h later, sectioned, and (e) stained with CD47 (red) and counterstained with DAPI (blue). (f) CD47 quantification was compared to untreated. In vivo, NTP treatment decreased tumor volume and marginally decreased CD47 expression. (g,h) Experimental design and NTP treatment directly for 5 days after developing B16F10 melanoma tumors in mice. (i) Tumors were removed either immediately after treatment or 72 h after treatment. (j) Following treatment, tumor volumes were also decreased. (k) CD47 quantification. The significance of treatment groups indicated by * p < 0.05, ** p < 0.01, ns—not significant.
There is still a lack of understanding of the molecular mechanisms underlying NTP’s therapeutic effect on thyroid cancer. Understanding the anticancer effects of NTP-activated medium (NTPAM) on thyroid cancer cells and clarifying the signaling mechanisms causing NTPAM-induced thyroid cancer cell death was explored in a recent study [66]. The in vivo analysis demonstrates that, when compared to control groups, NTPAM-treated groups had significantly lower tumor weights (Figure 6b). The findings of the reported study showed that NTPAM inhibited THCA cell growth more potently than the control did and that ROS controlled EGR1/GADD45α to mediate NTPAM-induced apoptotic cell death (Figure 6c). Recent research offers a fresh understanding of the basic chemical mechanisms underlying NTP–cancer cell interactions as well as a previously unrecognized benefit of current NTP cancer therapy: lowering immunosuppressive signals on the surface of cancer cells (Figure 6g–k) [67]. Researchers can better explain many disagreements by having a thorough understanding of the underlying mechanisms. This includes choosing the best plasma parameters to regulate the combination and concentration of reactive species, delivering plasma to deep-lying tumors, and figuring out the best plasma dose to achieve particular clinical translational outcomes. Designing low-temperature plasma sources that satisfy medical device technical standards is a unique approach for cancer therapy in clinical trials, but it still has to be safer and more effective.
4. Role of Plasma Technology in Food Decontamination and Storage
4.1. Microbial Inactivation
Plasma technology’s source of ROS and RNS is mainly concerned with increasing the yields of cultivars by stimulating seedling growth and inactivating microorganisms to increase the shelf life of food storage and fulfill the scarcity of food from all over the world [68][69]. Several treatment methods increased the concentration of intrabacterial reactive species such as H2O2, NO3, NO2, and O3. These reactive species are caused by oxidative stress, which prevented the ability of biofilms to regenerate [70]. The inactivation of E. coli bacteria was reported due to the UV radiation produced during the PAW [71]. The PAW has bactericidal properties due to the RONS. Conidium is an asexually generated fungal spore, a notorious plant disease inhibited by the reactive species in the PAW [72]. Most crops are destroyed by serious diseases by wall-less bacteria called phytoplasmas. Yellow grapevines can easily become infected with phytoplasma [73]. Phytoplasma caused disease in yellow grapevines controlled by the plasma-treated liquids which stimulate the defensive enzymes stilbene synthase and phenylalanine ammonia. The presence of RONS in PAW solution results in oxidative stress, which activates the stilbene metabolic pathway and increases the antioxidant properties. Resultantly, the research shows that PAW treatment can be used to enhance plants’ resistance to diseases [74].
4.2. Effect of NTP on Biofilms
Biofilms are multicellular communities of microbial cells, connected to a surface indefinitely and enveloped by a matrix predominantly consisting of polysaccharides, extracellular nucleic acid, and protein. In nature, more than 95% of the bacteria exist as a biofilm which favors their growth on solid surfaces [75]. Bacterial biofilm formation involves many steps. Quorum sensing is a special type of signaling required which occurs between microbial cells, and the transcription of a different set of genes is also a basic requirement for biofilm formation. Bacterial inactivation or bio-decontamination, as well as the sterilization of surfaces by NTP, have gained a lot of interest in recent years [76]. It is well-known that NTP has antibacterial effectivity and can inactivate planktonic bacteria, yeast, and spores. The effects of plasma application on biofilms seem to be a promising new direction in biofilm removal technology [77]. Since NTP, particularly atmospheric-pressure plasma jets (APPJs), are frequently operated at temperatures close to room temperature, they can be used directly on heat-sensitive materials such as human tissues. Due to the APPJs’ transitory character and accompanying thermal non-equilibrium, the plasma chemistry is improved, and charged species are transported to the targets quickly and efficiently [78]. The reactive species formed by plasmas are expected to comprise a combination of charged particles and chemically active species producing ultraviolet (UV) light (e.g., O3, O2, NO, H2O2, and OH) which contribute to the anti-microbial effects by inflicting damage on DNA, lipids, proteins, etc., leading to cellular structure degradation [79]. Bacterial biofilms consist of DNA, proteins, and polysaccharides which are affected or damaged by plasma-generated reactive species which are made up of charged particles and chemically active species and the generation of UV radiation.
4.3. Sustaining Food Freshness and Storage
NTP technology has been suggested as an advanced method to increase food freshness and food storage for longer use. The freshness of freshly cut lettuce was reported to be maintained for a long time after washing with PAW. The lettuce tissue organelles were sustained as fresh properties such as color, texture, and taste after washing with PAW treatment [80][81]. PAW treatment has the ability to maintain the freshness of fresh-cut kiwifruit. Kiwifruits were sprayed with PAW solution or deionized water and submerged in S. aureus suspension. The reactive species in PAW generated oxidative stress, which in turn damaged the membrane of bacterial cells and ultimately lead to cell death. Furthermore, PAW treatment had no detrimental effects on the kiwifruits’ quality. They were less affected by reactive species oxidative species damage due to the antioxidant enzymes in PAW [82]. Plasma therapy effectively suppressed B. cinerea spore germination and mycelial development in vitro, as well as gray mold decomposition in blueberries infected with B. cinerea during postharvest storage [83]. Figure 7 shows the action of NTP on bacterial cell structures, resulting in functional loss and sterilization which helps in food preservation.
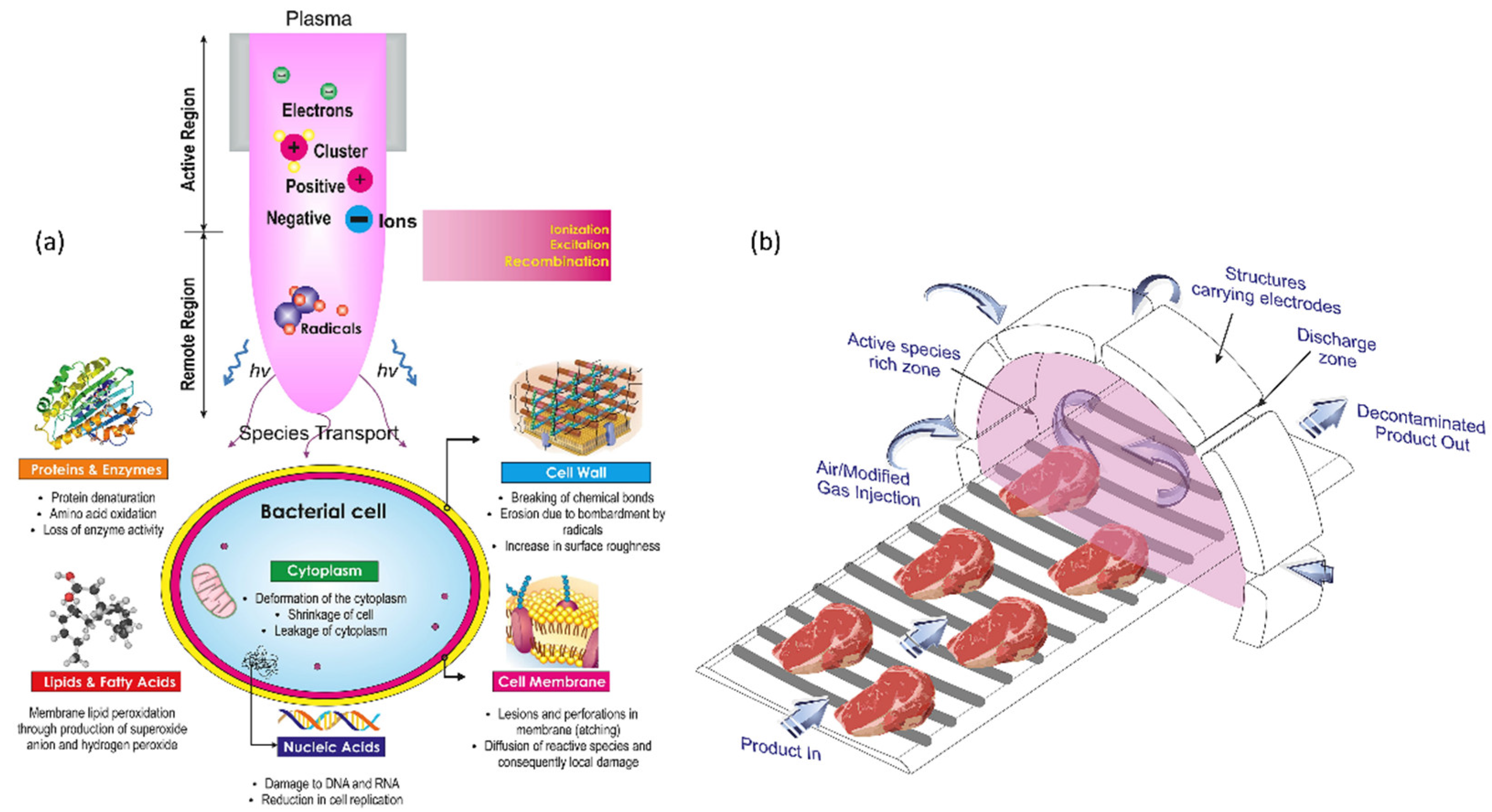
Figure 7. (a) A diagram depicting the action of NTP on bacterial cell structures, resulting in functional loss and sterilization. (b) Conceptual model for an industrial-level continuous NTP disinfecting unit.
The microbial inactivation in a liquid substrate is mostly caused by oxidative stressors brought on by the reactive species, and NTP is regarded as an improved oxidation approach. Typically, seed treatment is conducted to guard against infections that could harm seeds during the early stages of seedling development. If this phase is skipped, pathogens, insects, and plant diseases will only attack, disrupting the germination process and plant growth. However, some seed treatments that use chemicals can be extremely detrimental since they expose people to chemicals when treating large amounts of seeds, which can lead to inadvertent poisoning, damage to the seeds, and risk for farmers or workers. The presence of microorganisms (bacteria and fungus) that will impact crops is the industry’s top concern right now. Recently, the agriculture sector has implemented a number of methods to limit the growth of bacteria and fungi on crops. The creation of bacterially resistant strains, the use of chemical fumigants, the drainage of the soil, and particular plowing techniques are a few of the methods used to manage bacteria in the agriculture sector. The denaturation of proteins, lipids, and other micronutrients during some of these reactions, however, could result in unfavorable alterations to the nutritional quality of food. Furthermore, when applying this technology for food applications, it is important to thoroughly research the genotoxicity of reactive species. The application of cold plasma for microbial eradication can be used across a variety of food substrates, including cheese, fruits, and meat products. In addition, it is utilized to modify the rate at which seeds germinate. It is an environmentally friendly method that is utilized as an alternative to conventional methods for food preservation and other possible uses.
References
- Chen, Z.; Chen, G.; Obenchain, R.; Zhang, R.; Bai, F.; Fang, T.; Wang, H.; Lu, Y.; Wirz, R.E.; Gu, Z. Cold atmospheric plasma delivery for biomedical applications. Mater. Today 2022, 54, 153–188.
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60.
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 33001.
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. Technol. 2000, 9, 441–454.
- Ghimire, B.; Lee, G.J.; Mumtaz, S.; Choi, E.H. Scavenging effects of ascorbic acid and mannitol on hydroxyl radicals generated inside water by an atmospheric pressure plasma jet. AIP Adv. 2018, 8, 75021.
- Lamichhane, P.; Veerana, M.; Lim, J.S.; Mumtaz, S.; Shrestha, B.; Kaushik, N.K.; Park, G.; Choi, E.H. Low-Temperature Plasma-Assisted Nitrogen Fixation for Corn Plant Growth and Development. Int. J. Mol. Sci. 2021, 22, 5360.
- Lamichhane, P.; Adhikari, B.C.; Nguyen, L.N.; Paneru, R.; Ghimire, B.; Mumtaz, S.; Lim, J.S.; Hong, Y.J.; Choi, E.H. Sustainable nitrogen fixation from synergistic effect of photo-electrochemical water splitting and atmospheric pressure N2 plasma. Plasma Sources Sci. Technol. 2020, 29, 45026.
- Han, I.; Rana, J.N.; Kim, J.-H.; Choi, E.H.; Kim, Y. A Non-thermal Biocompatible Plasma-Modified Chitosan Scaffold Enhances Osteogenic Differentiation in Bone Marrow Stem Cells. Pharmaceutics 2022, 14, 465.
- Lim, J.S.; Hong, Y.J.; Ghimire, B.; Choi, J.; Mumtaz, S.; Choi, E.H. Measurement of electron density in transient spark discharge by simple interferometry. Results Phys. 2021, 20, 103693.
- Lu, X.; Laroussi, M.; Puech, V. On atmospheric-pressure non-equilibrium plasma jets and plasma bullets. Plasma Sources Sci. Technol. 2012, 21, 34005.
- Yoon, S.; Jeong, K.; Mumtaz, S.; Choi, E.H. Electromagnetic pulse shielding effectiveness of circular multi-waveguides for fluids. Results Phys. 2020, 16, 102946.
- Kuchenbecker, M.; Bibinov, N.; Kaemlimg, A.; Wandke, D.; Awakowicz, P.; Viöl, W. Characterization of DBD plasma source for biomedical applications. J. Phys. D Appl. Phys. 2009, 42, 45212.
- Lamichhane, P.; Paneru, R.; Nguyen, L.N.; Lim, J.S.; Bhartiya, P.; Adhikari, B.C.; Mumtaz, S.; Choi, E.H. Plasma-assisted nitrogen fixation in water with various metals. React. Chem. Eng. 2020, 5, 2053–2057.
- Mumtaz, S.; Uhm, H.; Lim, J.S.; Choi, E.H. Output-Power Enhancement of Vircator Based on Second Virtual Cathode Formed by Wall Charge on a Dielectric Reflector. IEEE Trans. Electron Devices 2022, 69, 2043–2050.
- Shaw, P.; Kumar, N.; Mumtaz, S.; Lim, J.S.; Jang, J.H.; Kim, D.; Sahu, B.D.; Bogaerts, A.; Choi, E.H. Evaluation of non-thermal effect of microwave radiation and its mode of action in bacterial cell inactivation. Sci. Rep. 2021, 11, 14003.
- Tornin, J.; Labay, C.; Tampieri, F.; Ginebra, M.-P.; Canal, C. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures. Nat. Protoc. 2021, 16, 2826–2850.
- Mumtaz, S.; Bhartiya, P.; Kaushik, N.; Adhikari, M.; Lamichhane, P.; Lee, S.-J.; Kaushik, N.K.; Choi, E.H. Pulsed high-power microwaves do not impair the functions of skin normal and cancer cells in vitro: A short-term biological evaluation. J. Adv. Res. 2020, 22, 47–55.
- Zhang, H.; Xu, S.; Zhang, J.; Wang, Z.; Liu, D.; Guo, L.; Cheng, C.; Cheng, Y.; Xu, D.; Kong, M.G.; et al. Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer. Biomaterials 2021, 276, 121057.
- Lamichhane, P.; Ghimire, B.; Mumtaz, S.; Paneru, R.; Ki, S.H.; Choi, E.H. Control of hydrogen peroxide production in plasma activated water by utilizing nitrification. J. Phys. D Appl. Phys. 2019, 52, 265206.
- Nguyen, L.N.; Kaushik, N.; Lamichhane, P.; Mumtaz, S.; Paneru, R.; Bhartiya, P.; Kwon, J.S.; Mishra, Y.K.; Nguyen, L.Q.; Kaushik, N.K.; et al. In situ plasma-assisted synthesis of polydopamine-functionalized gold nanoparticles for biomedical applications. Green Chem. 2020, 22, 6588–6599.
- Lim, J.S.; Kim, D.; Ki, S.; Mumtaz, S.; Shaik, A.M.; Han, I.; Hong, Y.J.; Park, G.; Choi, E.H. Characteristics of a Rollable Dielectric Barrier Discharge Plasma and Its Effects on Spinach-Seed Germination. Int. J. Mol. Sci. 2023, 24, 4638.
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288.
- Adamovich, I.; Agarwal, S.; Ahedo, E.; Alves, L.L.; Baalrud, S.; Babaeva, N.; Bogaerts, A.; Bourdon, A.; Bruggeman, P.J.; Canal, C.; et al. The 2022 Plasma Roadmap: Low temperature plasma science and technology. J. Phys. D Appl. Phys. 2022, 55, 373001.
- Machala, Z.; Pavlovich, M.J. A New Phase in Applied Biology. Trends Biotechnol. 2018, 36, 577–578.
- von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.-D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320.
- Li, Y.; Ho Kang, M.; Sup Uhm, H.; Joon Lee, G.; Ha Choi, E.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781.
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31.
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291.
- Jenns, K.; Sassi, H.P.; Zhou, R.; Cullen, P.J.; Carter, D.; Mai-Prochnow, A. Inactivation of foodborne viruses: Opportunities for cold atmospheric plasma. Trends Food Sci. Technol. 2022, 124, 323–333.
- Yan, W.; Xia, Y.; Bi, Z.; Song, Y.; Wang, D.; Liu, D. Numerical investigation of underwater discharge generated in a single helium bubble at atmospheric pressure. Phys. Plasmas 2019, 26, 23504.
- Lunov, O.; Zablotskii, V.; Churpita, O.; Chánová, E.; Syková, E.; Dejneka, A.; Kubinová, Š. Cell death induced by ozone and various non-thermal plasmas: Therapeutic perspectives and limitations. Sci. Rep. 2014, 4, 7129.
- Cheng, X.; Sherman, J.; Murphy, W.; Ratovitski, E.; Canady, J.; Keidar, M. The Effect of Tuning Cold Plasma Composition on Glioblastoma Cell Viability. PLoS ONE 2014, 9, e98652.
- Zhang, H.; Xu, Z.; Shen, J.; Li, X.; Ding, L.; Ma, J.; Lan, Y.; Xia, W.; Cheng, C.; Sun, Q.; et al. Effects and Mechanism of Atmospheric-Pressure Dielectric Barrier Discharge Cold Plasmaon Lactate Dehydrogenase (LDH) Enzyme. Sci. Rep. 2015, 5, 10031.
- Yonemori, S.; Nakagawa, Y.; Ono, R.; Oda, T. Measurement of OH density and air–helium mixture ratio in an atmospheric-pressure helium plasma jet. J. Phys. D Appl. Phys. 2012, 45, 225202.
- Lu, X.; Keidar, M.; Laroussi, M.; Choi, E.; Szili, E.J.; Ostrikov, K. Transcutaneous plasma stress: From soft-matter models to living tissues. Mater. Sci. Eng. R Rep. 2019, 138, 36–59.
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332.
- Kim, S.; Kim, C.-H. Applications of Plasma-Activated Liquid in the Medical Field. Biomedicines 2021, 9, 1700.
- Kondeti, V.S.S.K.; Phan, C.Q.; Wende, K.; Jablonowski, H.; Gangal, U.; Granick, J.L.; Hunter, R.C.; Bruggeman, P.J. Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic. Biol. Med. 2018, 124, 275–287.
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59.
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194.
- Ja Kim, S.; Min Joh, H.; Chung, T.H. Production of intracellular reactive oxygen species and change of cell viability induced by atmospheric pressure plasma in normal and cancer cells. Appl. Phys. Lett. 2013, 103, 153705.
- Akter, M.; Lim, J.S.; Choi, E.H.; Han, I. Non-Thermal Biocompatible Plasma Jet Induction of Apoptosis in Brain Cancer Cells. Cells 2021, 10, 236.
- Li, Y.; Liu, Y.J.; Wang, S.B.; Choi, E.H.; Han, I. Non-Thermal Bio-Compatible Plasma Induces Osteogenic Differentiation of Human Mesenchymal Stem/Stromal Cells With ROS-Induced Activation of MAPK. IEEE Access 2020, 8, 36652–36663.
- Kumar, N.; Singh, A.K. Reactive oxygen species in seminal plasma as a cause of male infertility. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 565–572.
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS production in response to high-power microwave pulses induces p53 activation and DNA damage in brain cells: Radiosensitivity and biological dosimetry evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861.
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270.
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301.
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.-S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, e86173.
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995.
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.-S. Atmospheric-Pressure Plasma Jet Induces Apoptosis Involving Mitochondria via Generation of Free Radicals. PLoS ONE 2011, 6, e28154.
- Samukawa, S.; Hori, M.; Rauf, S.; Tachibana, K.; Bruggeman, P.; Kroesen, G.; Whitehead, J.C.; Murphy, A.B.; Gutsol, A.F.; Starikovskaia, S.; et al. The 2012 Plasma Roadmap. J. Phys. D Appl. Phys. 2012, 45, 253001.
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002.
- Laroussi, M.; Kong, M.; Morfill, G.; Stolz, W. Plasma Medicine: Applications of Low-Temperature Gas Plasmas in Medicine and Biology; Cambridge University Press: Cambridge, UK, 2012.
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; van den Bedem, L.J.M.; van der Laan, E.P.; Steinbuch, M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006, 15, S169–S180.
- Ratovitski, E.A.; Cheng, X.; Yan, D.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. Anti-Cancer Therapies of 21st Century: Novel Approach to Treat Human Cancers Using Cold Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 1128–1137.
- Pai, K.; Timmons, C.; Roehm, K.D.; Ngo, A.; Narayanan, S.S.; Ramachandran, A.; Jacob, J.D.; Ma, L.M.; Madihally, S.V. Investigation of the Roles of Plasma Species Generated by Surface Dielectric Barrier Discharge. Sci. Rep. 2018, 8, 16674.
- Kim, C.-H.; Bahn, J.H.; Lee, S.-H.; Kim, G.-Y.; Jun, S.-I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538.
- Chien, P.-C.; Chen, C.-Y.; Cheng, Y.-C.; Sato, T.; Zhang, R.-Z. Selective inhibition of melanoma and basal cell carcinoma cells by short-lived species, long-lived species, and electric fields generated from cold plasma. J. Appl. Phys. 2021, 129, 163302.
- Golpour, M.; Alimohammadi, M.; Mohseni, A.; Zaboli, E.; Sohbatzadeh, F.; Bekeschus, S.; Rafiei, A. Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Appl. Sci. 2022, 12, 128.
- Choi, E.H.; Kaushik, N.K.; Hong, Y.J.; Lim, J.S.; Choi, J.S.; Han, I. Plasma bioscience for medicine, agriculture and hygiene applications. J. Korean Phys. Soc. 2022, 80, 817–851.
- Laroussi, M. Cold Plasma in Medicine and Healthcare: The New Frontier in Low Temperature Plasma Applications. Front. Phys. 2020, 8, 74.
- Choi, E.H. Cold Atmospheric Plasma Sources for Cancer Applications and Their Diagnostics. In Plasma Cancer Therapy; Keidar, M., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 53–73. ISBN 978-3-030-49966-2.
- Sato, T.; Yokoyama, M.; Johkura, K. A key inactivation factor of HeLa cell viability by a plasma flow. J. Phys. D Appl. Phys. 2011, 44, 372001.
- Bekeschus, S.; Masur, K.; Kolata, J.; Wende, K.; Schmidt, A.; Bundscherer, L.; Barton, A.; Kramer, A.; Bröker, B.; Weltmann, K.-D. Human Mononuclear Cell Survival and Proliferation is Modulated by Cold Atmospheric Plasma Jet. Plasma Process. Polym. 2013, 10, 706–713.
- Mumtaz, S.; Rana, J.N.; Lim, J.S.; Javed, R.; Choi, E.H.; Han, I. Effect of Plasma On-Time with a Fixed Duty Ratio on Reactive Species in Plasma-Treated Medium and Its Significance in Biological Applications. Int. J. Mol. Sci. 2023, 24, 5289.
- Jung, S.-N.; Oh, C.; Chang, J.W.; Liu, L.; Lim, M.A.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Won, H.-R.; Lee, S.E.; et al. EGR1/GADD45α Activation by ROS of Non-Thermal Plasma Mediates Cell Death in Thyroid Carcinoma. Cancers 2021, 13, 351.
- Lin, A.; Razzokov, J.; Verswyvel, H.; Privat-Maldonado, A.; De Backer, J.; Yusupov, M.; Cardenas De La Hoz, E.; Ponsaerts, P.; Smits, E.; Bogaerts, A. Oxidation of Innate Immune Checkpoint CD47 on Cancer Cells with Non-Thermal Plasma. Cancers 2021, 13, 579.
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210.
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50.
- Xu, Z.; Zhou, X.; Yang, W.; Zhang, Y.; Ye, Z.; Hu, S.; Ye, C.; Li, Y.; Lan, Y.; Shen, J. In vitro antimicrobial effects and mechanism of air plasma-activated water on Staphylococcus aureus biofilm. Plasma Process. Polym. 2020, 17, 1900270.
- Lukes, P.; Clupek, M.; Babicky, V.; Sunka, P. Ultraviolet radiation from the pulsed corona discharge in water. Plasma Sources Sci. Technol. 2008, 17, 24012.
- Šimečková, J.; Krčma, F.; Klofáč, D.; Dostál, L.; Kozáková, Z. Influence of Plasma-Activated Water on Physical and Physical–Chemical Soil Properties. Water 2020, 12, 2357.
- Weintraub, P.G.; Jones, P. Phytoplasmas: Genomes, Plant Hosts and Vectors; CABI: Wallingford, UK, 2009; ISBN 1845935314.
- Liu, X.; Li, Y.; Wang, S.; Huangfu, L.; Zhang, M.; Xiang, Q. Synergistic antimicrobial activity of plasma-activated water and propylparaben: Mechanism and applications for fresh produce sanitation. LWT 2021, 146, 111447.
- Chen, T.-P.; Su, T.-L.; Liang, J. Plasma-Activated Solutions for Bacteria and Biofilm Inactivation. Curr. Bioact. Compd. 2017, 13, 59–65.
- Foest, R.; Schmidt, M.; Becker, K. Microplasmas, an emerging field of low-temperature plasma science and technology. Int. J. Mass Spectrom. 2006, 248, 87–102.
- Koban, I.; Holtfreter, B.; Hübner, N.-O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965.
- Xu, Z.; Shen, J.; Zhang, Z.; Ma, J.; Ma, R.; Zhao, Y.; Sun, Q.; Qian, S.; Zhang, H.; Ding, L.; et al. Inactivation Effects of Non-Thermal Atmospheric-Pressure Helium Plasma Jet on Staphylococcus aureus Biofilms. Plasma Process. Polym. 2015, 12, 827–835.
- Ikawa, S.; Kitano, K.; Hamaguchi, S. Effects of pH on Bacterial Inactivation in Aqueous Solutions due to Low-Temperature Atmospheric Pressure Plasma Application. Plasma Process. Polym. 2010, 7, 33–42.
- Schnabel, U.; Handorf, O.; Stachowiak, J.; Boehm, D.; Weit, C.; Weihe, T.; Schäfer, J.; Below, H.; Bourke, P.; Ehlbeck, J. Plasma-functionalized water: From bench to prototype for fresh-cut lettuce. Food Eng. Rev. 2021, 13, 115–135.
- Yu, N.-N.; Ketya, W.; Choi, E.-H.; Park, G. Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+. Int. J. Mol. Sci. 2022, 23, 6668.
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of nonthermal plasma-activated water on quality and antioxidant activity of fresh-cut kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817.
- Hu, X.; Sun, H.; Yang, X.; Cui, D.; Wang, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Potential use of atmospheric cold plasma for postharvest preservation of blueberries. Postharvest Biol. Technol. 2021, 179, 111564.
More
Information
Subjects:
Food Science & Technology; Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
04 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





