Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maryam Ghasemi-kasman | -- | 2887 | 2023-04-01 11:53:57 | | | |
| 2 | Jessie Wu | + 101 word(s) | 2988 | 2023-04-03 05:34:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Askari, H.; Rabiei, F.; Lohrasbi, F.; Ghadir, S.; Ghasemi-Kasman, M. Molecular Mechanisms of COVID-19 on Non-Lung Organs. Encyclopedia. Available online: https://encyclopedia.pub/entry/42711 (accessed on 08 February 2026).
Askari H, Rabiei F, Lohrasbi F, Ghadir S, Ghasemi-Kasman M. Molecular Mechanisms of COVID-19 on Non-Lung Organs. Encyclopedia. Available at: https://encyclopedia.pub/entry/42711. Accessed February 08, 2026.
Askari, Hamid, Fatemeh Rabiei, Fatemeh Lohrasbi, Sara Ghadir, Maryam Ghasemi-Kasman. "Molecular Mechanisms of COVID-19 on Non-Lung Organs" Encyclopedia, https://encyclopedia.pub/entry/42711 (accessed February 08, 2026).
Askari, H., Rabiei, F., Lohrasbi, F., Ghadir, S., & Ghasemi-Kasman, M. (2023, April 01). Molecular Mechanisms of COVID-19 on Non-Lung Organs. In Encyclopedia. https://encyclopedia.pub/entry/42711
Askari, Hamid, et al. "Molecular Mechanisms of COVID-19 on Non-Lung Organs." Encyclopedia. Web. 01 April, 2023.
Copy Citation
The SARS-CoV-2 is a single-stranded RNA virus that is encapsulated. It belongs to the genus Betacoronavirus, the family Coronaviridae, the subfamily Coronavirinae, and the order Nidovirales. The genome size of the virus, which is 29.99 kB, is significant. The mechanisms of this virus on various organs such as brain, eye, and olfactory nerve and different systems such as the endocrine and gastrointestinal systems are discussed.
SARS-CoV-2
COVID-19
organ
1. Introduction
The first case of SARS-CoV-2 was officially reported in Wuhan, China, in 2019 [1]. SARS-CoV-2 is a member of the family Coronaviridae, and its RNA is encapsulated and enveloped [2]. Fusion and attachment of the virus to the cell surface are facilitated by the spike (S) glycoprotein [3]. The receptors for the virus to invade are Angiotensin-converting enzyme 2 (ACE-2) receptors. Furthermore, the host cell’s transmembrane serine protease 2 (TMPRSS2) is essential for priming the viral S protein [4].
Besides lung infection, most of the vital organs can be infected by SARS-CoV-2, for instance, the brain, olfactory nerve, eye, and endocrine and gastrointestinal systems [5]. In addition to respiratory distress, the most prominent symptom of COVID-19 that patients often experience are nausea, vertigo, and headache, which implies a possible involvement of the neurological system [6]. Also, post-COVID-19 symptoms include fatigue and cognitive impairment [7][8]. Evidence from the acute infection and the long-term effects of COVID-19 show a role for the senses of smell and taste. Mainly, they include a complete or partial lack of smell (anosmia/hyposmia) and taste (ageusia/hypogeusia) or a distorted perception of smell/taste (parosmia/parageusia) or the sense of an odor or a taste without any concomitant stimulus (phantosmia, also known as olfactory hallucination, and phantogeusia, also known as a gustatory hallucination) [8][9]. Furthermore, patients experience digestive symptoms such as vomiting, diarrhea, nausea, and abdominal discomfort [10]. In addition, recent developments suggest that COVID-19 individuals’ endocrine systems may be affected [11].
2. Ophthalmic Manifestation in COVID-19
The SARS-CoV-2 has multiple non-specific signs and symptoms. One of its non-specific symptoms is ocular involvement. Many studies have been conducted to investigate whether SARS-CoV-2 has ocular involvement or not. ACE-2 and its aid for entry, TMPRSS2, are the main entry factors for this virus that are expressed in many human organs [12][13]. However, whether cells on the ocular surface express these crucial components for cellular vulnerability to viral infection is still unclear. Western blot analysis has recently revealed that the corneal epithelium expresses TMPRSS2 and ACE-2 during photorefractive keratectomy (PRK) [14]. They also indicated the expression of ACE-2 localization on the corneal epithelium in the most superficial layer, endothelium, and corneal limbus through immunohistochemical staining [15].
In contrast, the ACE-2 staining was negative on the corneal stroma. In some cases, the expression of ACE-2 was much higher in the limbus than in the superficial layer of the cornea. They also showed ACE-2 expression on the conjunctival epithelium, but the staining was negative for goblet cells in the epithelium and vascular endothelium [16]. This research proposed that the ocular surface is vulnerable to the SARS-CoV-2; however, conjunctivitis is rare in COVID-19 patients [17]. Lange and Collegues demonstrated that TMPRSS2, ACE-2, and other mediators were not considerably expressed in either healthy or diseased human conjunctival samples, making it unlikely that the SARS-CoV-2 could infect the conjunctiva through these routes [18]. Wu and Collegues conducted a study of 11 patients with ocular manifestations and positive nasopharyngeal RT-PCR and concluded that only 2 patients had positive PCR in the conjunctival swabs. This research proposed that even in patients with ocular manifestations, the positivity of conjunctival swabs is low [19]. Some hypotheses have been proposed as to how the SARS-CoV-2 gets into the ocular secretions; these include direct inoculation of the ocular surface from viral particles, emigration of the virus through the nasolacrimal duct from the pharynx, and hematogenous spread via the lacrimal gland, among others [20]. To clarify this issue, some studies have been conducted. For example, Azzolini and Collegues found SARS-CoV-2 RNA on the ocular surface even when the nasopharyngeal swab was negative [21]. Wang and Collegues demonstrated in a separate investigation that CD147 is a transmembrane glycoprotein that promotes the binding of SARS-CoV-2 to ACE-2. It facilitates the ability for the SARS-CoV-2 to enter the eye and conjunctiva [22]. It has been proposed that the retina has ACE-2 receptors. Twelve patients with positive nasopharyngeal RT-PCR for SARS-CoV-2 underwent optical coherence tomography to detect retinal alterations, and the researchers found hyper-reflective lesions at the level of the ganglion cells and the inner plexiform layer, but no vision loss. Researchers found evidence that SARS-CoV-2 might infect tissues deep inside the eye as well as those on its surface [23]. In the end, it is essential for healthcare workers to put on eye protection during contact with COVID-19 patients, as existing studies are controversial.
3. Taste Dysfunction in COVID-19
Taste dysfunction is another example of a non-specific symptom. First, the oral cavity is a crucial entry site for SARS-CoV-2 because multiple entry factors, including ACE-2, TMPRSS2 and furin, are expressed by the oral epithelial cells, taste buds, and salivary glands [24][25]. ACE-2 receptors are expressed all over the oral cavity [12]. Secondly, it has been revealed that the COVID-19 virus may attach to sialic acid receptors, an important component of the saliva, and protect glycoproteins that transport gustatory chemicals in the taste pores from early enzymatic breakdown [26]. Reduced sialylation may affect mucin function by increasing the gustatory threshold and viruses can occupy sialic acid’s binding sites, hence, accelerating the degradation of gustatory particles. Due to the strong association between smell and taste, it is also possible to have problems with both senses simultaneously [27]. However, it was reported that the frequencies of taste dysfunction are higher than olfactory dysfunction [28]. Taste bud cells are columnar sensory cells found in the tongue’s stratified epithelium and have an average life of ten days. They revive continuously from stem cells in the oral cavity [29]. Taste bud cells have receptors that can convert chemical energy into electrical energy [30]. Cranial nerves, such as the facial, glossopharyngeal, and vagus, transfer taste signals from the oral cavity to the brain stem [31]. Damage to any of these cranial nerves can cause gustatory dysfunction. The possible nerve whose damage causes dysgeusia is the chorda tympani [32]. In addition to the mechanisms discussed above, proinflammatory cytokines such as IFN- γ, and IL-6 can prevent stem cell proliferation and shorten the lifetime of taste bud cells (Figure 1) [33]. Another possibility is that the taste bud cells express ACE-2, and this leads to direct infection and cell death [34]. This results from the interaction between the virus and TLR, which induces the apoptotic process in taste bud cells [35]. Yet another possible mechanism is zinc homeostasis impairment of gustatory cells following SARS-CoV-2 infection, which can cause dysgeusia [36]. With all these COVID-19-induced taste dysfunction mechanisms described above, the exact pathophysiology of this disorder remains unknown.
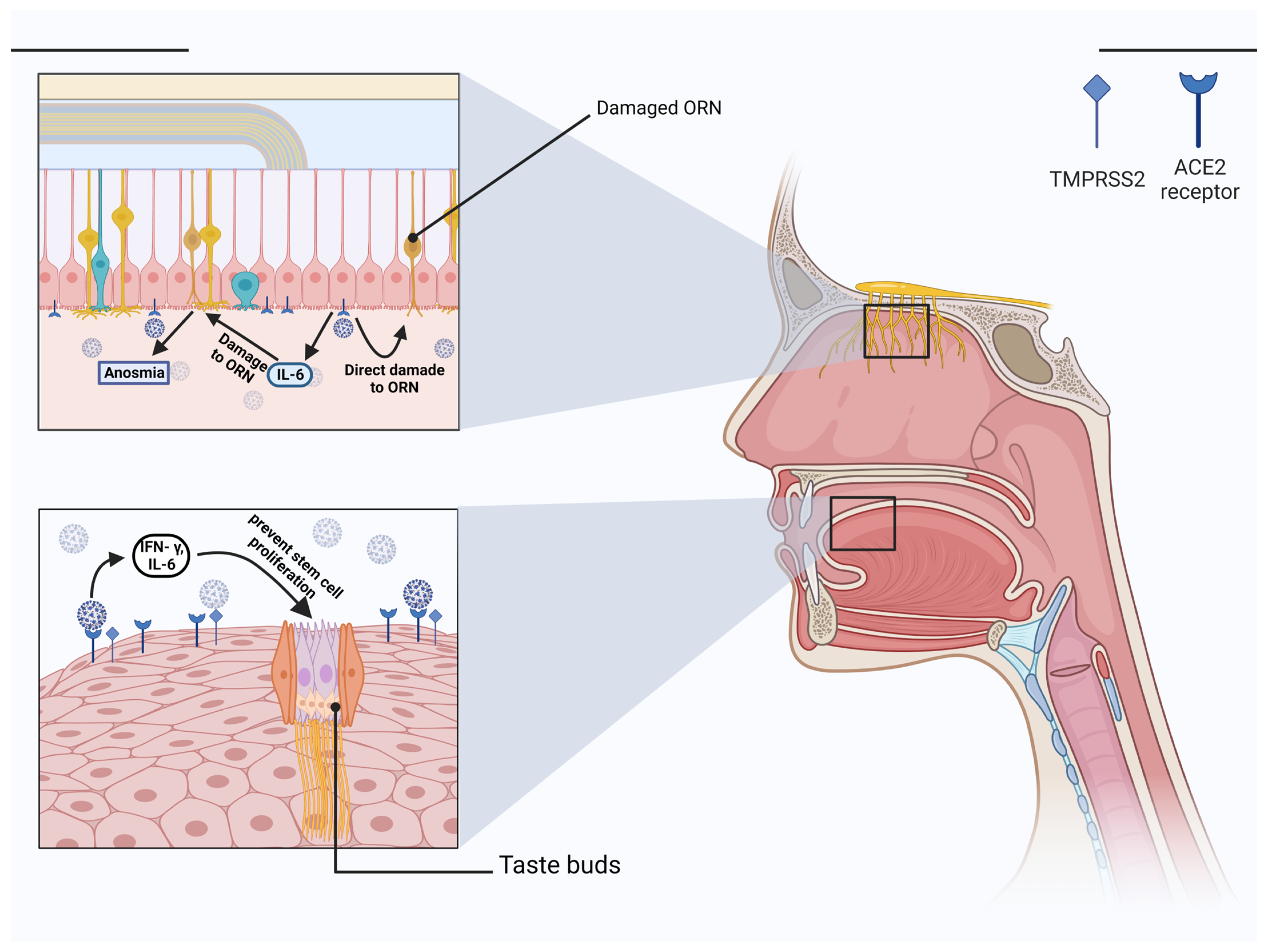
Figure 1. Taste and olfactory dysfunction in COVID-19. IFN- γ and IL-6 are two examples of proinflammatory cytokines that can inhibit stem cells growth and reduce the lifespan of taste bud cells. Another sense that plays a role besides taste is smell. In contrast to ORN and olfactory bulb cells, sustentacle cells express high levels of ACE-2 and TMPRSS2. Loss of cilia on ORN due to infection of the sustentacular cells can interfere with the transmission of the odorous stimulus. Anosmia caused by COVID-19 was also linked to IL-6, proving the importance of this cytokine in the disease’s etiology. (Created with BioRender.com).
4. COVID-19 and the Endocrine System
COVID-19 could exert adverse impacts on different organs, including endocrine dysfunction. The expression of the ACE-2 receptor is responsible for the cellular entrance of COVID-19 via interaction with the S protein. The TMPRSS 2 facilitates the binding of S protein and ACE-2 receptor. ACE-2 receptor expresses widely in the endocrine glands such as the thyroid, pancreas, adrenal glands, pituitary, and testis. Different mechanisms contribute to endocrine damage caused by COVID-19, which include direct viral injury, immune-mediated damage due to cytokine storm, endothelial injury, and the renin–angiotensin–aldosterone system (RAAS) dysfunction. ACE-2 negatively affect RAAS regulation by inactivation of angiotensin (Ang) 1 and 2. The efficacy of ACE-2 reduces during COVID-19 which leads to an elevated level of Ang2 and aldosterone. This RAAS over-activation induces insulin resistance and vascular damage [36][37].
In this step, the pathophysiological mechanisms of COVID-19 on the endocrine system are reviewed:
4.1. Hypothalamic-Pituitary Axis (HPA)
COVID-19 could damage HPA via direct or indirect injury. The expression of the ACE-2 receptor results in COVID-19 invasion into neurons and glial cells. A virus genome was detected in the autopsy samples which showed edema and neuronal degeneration of the hypothalamus [37][38]. Cytokine storms, including IL-11, IL-6, and TNF-α, cause indirect injury to the HPA. These cytokines increase the secretion of corticotroph-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH) from the hypothalamus and pituitary, respectively, and affect the adrenal to release more cortisol. This neuroinflammation induces hemorrhage, necrosis, and edema in the hypothalamus and pituitary [39][40].
Moreover, it has been shown that the spike protein of COVID-19 binds to gonadotropin-releasing hormone receptor (GnRHR) and G-protein-coupled-receptor (GPCR) in the brain and blocks them. GPCRs and GnRHRs act by binding to G proteins. Activation of GPCRs and GNRHRs are responsible for cortisol levels and the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), respectively. Inhibition of these receptors disrupts the neuroendocrine system and its effects on peripheral endocrine glands [41]. Furthermore, virus infiltration and cytokine effects impair the hormonal axis mentioned below.
4.2. Thyroid
The thyroid gland regulates metabolism by secreting triiodothyronine (T3) and thyroxine (T4). Studies showed that COVID-19 infects the thyroid and presents in different clinical manifestations such as sick euthyroid syndrome, Graves’ disease, Hashimoto’s syndrome, subacute thyroiditis, and silent thyroiditis. In addition to lymphocyte infiltration in thyroid tissue, ACE-2 receptor β expression provides a target for COVID-19 invasion [36]. Different mechanisms are suggested for thyroid changes in COVID-19 infection. Direct destruction of thyroid follicular and parafollicular cells could induce primary hypothyroidism with a high level of thyroid-stimulating hormone (TSH) and decrease free T4. However, there is no evidence of morphological changes in follicular cells.
On the other hand, the effects of COVID-19 on HPA imply a reduction in TSH level, which leads to a low level of total 3,5,3′-triiodothyronine (TT3) and low free T4. The severity of the infection correlates with the magnitude of the decline. Also, non-thyroidal illness syndrome (NTIS) in critical patients caused low free T3 with normal or low TSH. It is related to deiodinase activity changes due to systemic inflammation [42][43].
4.3. Pancreas
ACE-2 and TMPRSS2 are expressed in the pancreas, and its endocrine section can be a target for COVID-19. Studies demonstrated that injury of islet cells leads to diabetes and hyperglycemia. Several mechanisms have been proposed for blood glucose imbalance, including β cells cytotoxicity by COVID-19, systemic inflammation, and glucocorticoid drugs [36][37]. Production of inflammatory factors involving MCP-1, inducible protein-10, and interleukin-1β destruct β cells during COVID-19 infection [37][39].
More factors in β cells facilitate COVID-19 entry. NRP1 and high mobility group box 1 (HMGB1) help bind ACE-2 and COVID-19. HMGB1 is a chromatin regulator that controls ACE-2 expression. Moreover, other molecules similar to SMARCA4, a non-fermenting chromatin-remodeling complex, and RIPK3 as a necroptosis kinase interfere in COVID-19 entry via ACE-2 expression. It has been shown DPP-4, an amino peptide in glucose metabolism, is an alternative receptor for COVID-19 in the pancreas. Due to viral injury, hypoinsulinemia and hyperglycemia can be observed in COVID-19 patients [43]. Shaharuddin and Collegues investigated the expression of inflammatory genes in induced pluripotent stem cells (iPSC)-derived pancreatic cultures infected by COVID-19. Data showed inflammatory genes such as nuclear factor kappa B (NFKB)-1, C-X-C motif chemokine Ligand (CXCL)-12, and signal transducer and activator of transcription (STAT)-3 were upregulated in the infected cells. Additionally, infected endocrine cells of the pancreas showed dysfunction in insulin secretion [44].
4.4. Adrenal Gland
The expression of ACE-2 receptor and TMPRSS2 zona fasciculate and zona reticularis provides a target for COVID-19 invasion. Besides direct injury of the virus, CD3+ and CD8+ lymphocyte infiltration, acute fibrinoid necrosis in arterioles, and adrenal vein thrombosis damage the adrenal gland. SARS-COV produces molecules similar to ACTH, resulting in presenting auto-antibodies against host ACTH. These antibodies could destroy ACTH and cause adrenal insufficiency (AI). Also, different studies reported clinical manifestations of AI in COVID-19 patients [36][42].
5. Gastrointestinal (GI) Disorders of COVID19
One of the extrapulmonary manifestations of COVID-19 is GI symptoms. GI symptoms can be presented during different phases of the disease. Most clinical presentations of the disease are diarrhea, loss of appetite, nausea, vomiting, and abdominal pain. Stool examination of patients with GI symptoms demonstrated viral RNA. An autopsy from a patient with COVID-19 has shown necrosis and shedding of GI epithelium from the esophagus to the intestine. Moreover, stenosis of the small intestine has been reported [45]. In addition to GI organs, COVID-19 could lead to liver injury that presents by dysregulation of liver function tests, including aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), and elevation of bilirubin [46][47]. Different mechanisms are proposed for GI injury:
5.1. Direct Injury
ACE-2 and TMPRSS2 are presented on epithelial cells especially on type 2 epithelial cells in the duodenum, jejunum, ileum, cecum, and colon. ACE-2 on enterocytes preserves amino acid homeostasis and antimicrobial molecules. ACE-2 maintains the B0AT1, an amino acid transporter, and controls the uptake of tryptophan in the gut which interferes with immunity, including suppressing inflammatory cytokines, keeping the intestinal junction, and preventing dysbiosis. COVID-19 invasion of enterocytes leads to ACE-2 downregulation which causes RAAS inhibition, a decrease of the anti-inflammatory role of the intestinal mucosa, cellular metabolic condition, and cellular viability. The damage of enterocytes expressing ACE-2 and malabsorption may explain diarrhea in patients with COVID-19 [48]. Yamada and colleagues designed a model of iPSC-derived small intestinal epithelial cells (iPSC-SIECs) infected by COVID-19 and investigated the expression of pro-inflammatory genes electrical resistance (TEER). They demonstrated COVID-19 infection triggers the expression of various inflammatory genes, including CC motif chemokine ligand 2 (CCL2), CCL3, CCL5, CXCL10, IL-6, and IL-1β. Also, it has been reported that TEER was reduced, indicating disruption of tight junction between cells via downregulation of the expression of CLDN1 and ZO-3 genes. The impairment of the intestinal barrier leads to GI symptoms [49].
5.2. Enteric Nervous System (ENS) Dysfunction
Studies suggested the involvement of ENS in COVID-19 infection. This virus can enter the CNS via ENS with access to splanchnic and vagal nerves. ACE-2 and TMPRSS2 are expressed on the ENS in submucosal and myenteric plexus that can function as a target for COVID-19 invasion and GI damage. Moreover, the inflammation affects ENS, which induces GI symptoms such as nausea and vomiting [50].
5.3. Immune-Mediated Injury
In some patients with COVID-19 that presented digestive symptoms, the RNA of virus has not been detected. It may suggest the indirect injury of the GI system by COVID-19. Respiratory tract infection by COVID-19 causes intestinal injury through the gut-lung axis. CD4+ T cells derived lung entry small intestine by CC chemokine receptor 9 (CCR9+). CCL25 supports the recruitment of CD4+ T cells. Gut-lung axis impacts the intestinal immunity and disturbs the homeostasis of flora [48][51]. In addition, cytokine storms including IFN-γ, IL-2, IL-7, and ΤΝF-α, cause intestinal inflammation and diarrhea. The detection of fecal calprotectin indicates systemic inflammation in COVID-19, which involves the digestive system [50].
5.4. Liver Injury
The elevation of liver enzymes is reported in 7.6%–39% of patients with COVID-19 [52]. Histopathological study showed necrosis and degeneration of hepatic cells during COVID-19 infection [53]. The expression of ACE-2 and TMPRSS2 on hepatocytes, cholangiocytes, and sinusoidal epithelial cells provides a potential for direct injury by COVID-19. This viral invasion disturbs the transportation of bile acid and cholangiocytes barrier.
On the other hand, hypoxia due to pneumonia damages liver function. The increase of inflammatory cytokines such as ILs, IFN-γ, and ΤΝF-α involves in liver damage [46][47]. Table 1 summarizes different mechanisms involved in COVID-19 infection.
Table 1. Description of different mechanisms involved in COVID-19 infection.
| Systems (Disorders) | Factors Involved | Mechanisms | Reference |
|---|---|---|---|
| CNS (Cerebrovascular diseases) | MCP-1, chemokine, IL-6, IL-1β, TNF-α, and NETs |
|
[54][55][56] |
| Skeletal muscle and Neuromuscular Junction (Myasthenia gravis) | Proinflammatory cytokines |
|
[57] |
| Olfactory Nerve (smell dysfunction) | IL-6, IFN-γ, MCP-1, and IP-10 |
|
[44] |
| Ophthalmic manifestation | ACE-2 receptors, TMPRSS2, CD147, |
|
[58] |
| Taste manifestation | IFN- γ, and IL-6 |
|
[26][32][35] |
| Endocrine System (sick euthyroid syndrome, hypothyroidism, diabetes and hyperglycemia) | IL-1, IL-6, and TNFα, the spike protein, MCP-1, inducible protein-10, and inter-leukin-1β |
|
[36][37][39][40][41][44] |
| GI System (diarrhea, loss of appetite, nausea, vomiting, and abdominal pain) | CCL2, CCL3, CCL5, CXCL10, IL-6, and IL-1β, IFN-γ, IL-2, IL-7, and ΤΝF-α |
|
[46][47][50][59] |
References
- Ozma, M.A.; Maroufi, P.; Khodadadi, E.; Köse, Ş.; Esposito, I.; Ganbarov, K.; Dao, S.; Esposito, S.; Dal, T.; Zeinalzadeh, E. Clinical manifestation, diagnosis, prevention and control of SARS-CoV-2 (COVID-19) during the outbreak period. Infez. Med. 2020, 28, 153–165.
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154.
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278.
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiat. 2020, 7, 875–882.
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069.
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135.
- Ercoli, T.; Masala, C.; Pinna, I.; Orofino, G.; Solla, P.; Rocchi, L.; Defazio, G. Qualitative smell/taste disorders as sequelae of acute COVID-19. Neurol. Sci. 2021, 42, 4921–4926.
- Wee, L.E.; Chan, Y.F.Z.; Teo, N.W.Y.; Cherng, B.P.Z.; Thien, S.Y.; Wong, H.M.; Wijaya, L.; Toh, S.T.; Tan, T.T. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur. Arch. Otorhinolaryngol. 2020, 277, 2389–2390.
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001.
- Puig-Domingo, M.; Marazuela, M.; Giustina, A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine 2020, 68, 2–5.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Sungnak, W.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687.
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454.
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006, 210, 288–297.
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637.
- Zhou, L.; Xu, Z.; Castiglione, G.M.; Soiberman, U.S.; Eberhart, C.G.; Duh, E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020, 18, 537–544.
- Lange, C.; Wolf, J.; Auw-Haedrich, C.; Schlecht, A.; Boneva, S.; Lapp, T.; Horres, R.; Agostini, H.; Martin, G.; Reinhard, T. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J. Med. Virol. 2020, 92, 2081–2086.
- Wu, P.; Duan, F.; Luo, C.; Liu, Q.; Qu, X.; Liang, L.; Wu, K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020, 138, 575–578.
- Seah, I.; Agrawal, R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395.
- Liu, Z.; Sun, C.B. Conjunctiva is not a preferred gateway of entry for SARS-CoV-2 to infect respiratory tract. J. Med. Virol. 2020, 92, 1410–1412.
- Wang, K.; Chen, W.; Zhou, Y.-S.; Lian, J.-Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.-Y.; Geng, J.-J. CD147-spike proteinis a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 2020, 283, 1–10.
- Marinho, P.M.; Marcos, A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610.
- Usami, Y.; Hirose, K.; Okumura, M.; Toyosawa, S.; Sakai, T. Brief communication: Immunohistochemical detection of ACE2 in human salivary gland. Oral. Sci. Int. 2021, 18, 101–104.
- Lechien, J.R.; Radulesco, T.; Calvo-Henriquez, C.; Chiesa-Estomba, C.M.; Hans, S.; Barillari, M.R.; Cammaroto, G.; Descamps, G.; Hsieh, J.; Vaira, L. ACE2 & TMPRSS2 expressions in head & neck tissues: A systematic review. Head Neck Pathol. 2021, 15, 225–235.
- Milanetti, E.; Miotto, M.; Di Rienzo, L.; Nagaraj, M.; Monti, M.; Golbek, T.W.; Gosti, G.; Roeters, S.J.; Weidner, T.; Otzen, D.E. In-silico evidence for a two receptor based strategy of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 690655.
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp. Brain Res. 2005, 166, 345–357.
- Roper, S.D.; Chaudhari, N. Taste buds: Cells, signals and synapses. Nat. Rev. Neurosci. 2017, 18, 485–497.
- Roper, S.D. Taste buds as peripheral chemosensory processors. Semin Cell Dev. Biol. 2013, 24, 71–79.
- Breslin, P.A. An evolutionary perspective on food and human taste. Curr. Biol. 2013, 23, R409–R418.
- Finsterer, J.; Stollberger, C. Causes of hypogeusia/hyposmia in SARS-CoV2 infected patients. J. Med. Virol. 2020, 92, 1793.
- Cohn, Z.J.; Kim, A.; Huang, L.; Brand, J.; Wang, H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010, 11, 72.
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X. High expression of ACE2 receptor of 2019-nCoV onthe epitelial cells of oral mucosa. Int. J. Oral. Sci. 2020, 12, 8.
- Wang, H.; Zhou, M.; Brand, J.; Huang, L. Inflammation and taste disorders: Mechanisms in taste buds. Ann. N. Y Acad. Sci. 2009, 1170, 596–603.
- Clark, H.L.; Jhingran, A.; Sun, Y.; Vareechon, C.; de Jesus Carrion, S.; Skaar, E.P.; Chazin, W.J.; Calera, J.A.; Hohl, T.M.; Pearlman, E. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J. Immunol. 2016, 196, 336–344.
- Esmaeilzadeh, A.; Elahi, R.; Siahmansouri, A.; Maleki, A.J.; Moradi, A. Endocrine and metabolic complications of COVID-19: Lessons learned and future prospects. J. Mol. Endocrinol. 2022, 69, R125–R150.
- Abdel-Moneim, A.; Hosni, A. Insights into the possible impact of COVID-19 on the endocrine system. Arch. Physiol. Biochem. 2021, 1–9.
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261.
- Garg, M.K.; Gopalakrishnan, M.; Yadav, P.; Misra, S. Endocrine Involvement in COVID-19: Mechanisms, Clinical Features, and Implications for Care. Indian J. Endocrinol. Metab. 2020, 24, 381–386.
- Hornick, M.G.; Olson, M.E.; Jadhav, A.L. SARS-CoV-2 Psychiatric Sequelae: A Review of Neuroendocrine Mechanisms and Therapeutic Strategies. Int. J. Neuropsychopharmacol. 2022, 25, 1–12.
- Elkazzaz, M.; Ahmed, A.; Abo-Amer, Y.E.; Hydara, T.; Haikal, A.; Razek, D.; Eltayb, W.A.; Wang, X.; Karpiński, T.M.; Hamza, D.; et al. In Silico Discovery of GPCRs and GnRHRs as Novel Binding Receptors of SARS-CoV-2 Spike Protein Could Explain Neuroendocrine Disorders in COVID-19. Vaccines 2022, 10, 1500.
- Piticchio, T.; Le Moli, R.; Tumino, D.; Frasca, F. Relationship between betacoronaviruses and the endocrine system: A new key to understand the COVID-19 pandemic-A comprehensive review. J. Endocrinol. Investig. 2021, 44, 1553–1570.
- Chen, W.; Tian, Y.; Li, Z.; Zhu, J.; Wei, T.; Lei, J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology 2021, 162, bqab004.
- Shaharuddin, S.H.; Wang, V.; Santos, R.S.; Gross, A.; Wang, Y.; Jawanda, H.; Zhang, Y.; Hasan, W.; Garcia, G., Jr.; Arumugaswami, V.; et al. Deleterious Effects of SARS-CoV-2 Infection on Human Pancreatic Cells. Front. Cell Infect. Microbiol. 2021, 11, 678482.
- Saeed, U.; Piracha, Z.Z.; Uppal, S.R.; Waheed, Y.; Uppal, R. SARS-CoV-2 induced hepatic injuries and liver complications. Front. Cell Infect. Microbiol. 2022, 12, 726263.
- Campbell, P.T.; Fix, O.K. Coronavirus Disease-2019 and Implications on the Liver. Clin. Liver Dis. 2023, 27, 27–45.
- Alnamshan, M.M. Potential histopathological and immunological effects of SARS-CoV-2 on the liver. Braz. J. Biol. 2022, 82, e262008.
- Li, S.; Zhou, Y.; Yan, D.; Wan, Y. An Update on the Mutual Impact between SARS-CoV-2 Infection and Gut Microbiota. Viruses 2022, 14, 1774.
- Yamada, S.; Noda, T.; Okabe, K.; Yanagida, S.; Nishida, M.; Kanda, Y. SARS-CoV-2 induces barrier damage and inflammatory responses in the human iPSC-derived intestinal epithelium. J. Pharmacol. Sci. 2022, 149, 139–146.
- Marasco, G.; Lenti, M.V.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Di Sabatino, A.; Barbara, G. Implications of SARS-CoV-2 infection for neurogastroenterology. Neurogastroenterol. Motil. 2021, 33, e14104.
- Chen, T.H.; Hsu, M.T.; Lee, M.Y.; Chou, C.K. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses 2022, 14, 1188.
- Zhong, P.; Xu, J.; Yang, D.; Shen, Y.; Wang, L.; Feng, Y.; Du, C.; Song, Y.; Wu, C.; Hu, X.; et al. COVID-19-associated gastrointestinal and liver injury: Clinical features and potential mechanisms. Signal. Transduct. Target Ther. 2020, 5, 256.
- Dawood, R.M.; Maher Salum, G.; Abd El-Meguid, M. The Impact of COVID-19 on Liver Injury. Am J Med Sci. 2022, 363, 94–103.
- Glass, W.G.; Subbarao, K.; Murphy, B.; Murphy, P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004, 173, 4030–4039.
- Robertson, J.; Beaulieu, J.M.; Doroudchi, M.M.; Durham, H.D.; Julien, J.P.; Mushynski, W.E. Apoptotic death of neurons exhibiting peripherin aggregates is mediated by the proinflammatory cytokine tumor necrosis factor-alpha. J. Cell Biol. 2001, 155, 217–226.
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179.
- Welch, C.; Greig, C.; Masud, T.; Wilson, D.; Jackson, T.A. COVID-19 and Acute Sarcopenia. Aging Dis. 2020, 11, 1345–1351.
- Willcox, M.D.; Walsh, K.; Nichols, J.J.; Morgan, P.B.; Jones, L.W. The ocular surface, coronaviruses and COVID-19. Clin. Exp. Optom. 2020, 103, 418–424.
- Lei, H.Y.; Ding, Y.H.; Nie, K.; Dong, Y.M.; Xu, J.H.; Yang, M.L.; Liu, M.Q.; Wei, L.; Nasser, M.I.; Xu, L.Y.; et al. Potential effects of SARS-CoV-2 on the gastrointestinal tract and liver. Biomed. Pharm. 2021, 133, 111064.
More
Information
Subjects:
Infectious Diseases
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
619
Revisions:
2 times
(View History)
Update Date:
03 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




