Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manyu Chen | -- | 2033 | 2023-03-31 11:22:02 | | | |
| 2 | Dean Liu | Meta information modification | 2033 | 2023-04-03 05:06:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, M.; Hu, G.; Shen, T.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Polyacetylene Derivatives in Gas and Liquid Separation. Encyclopedia. Available online: https://encyclopedia.pub/entry/42688 (accessed on 01 March 2026).
Chen M, Hu G, Shen T, Zhang H, Sun JZ, Tang BZ. Polyacetylene Derivatives in Gas and Liquid Separation. Encyclopedia. Available at: https://encyclopedia.pub/entry/42688. Accessed March 01, 2026.
Chen, Manyu, Guangze Hu, Tanxiao Shen, Haoke Zhang, Jing Zhi Sun, Ben Zhong Tang. "Polyacetylene Derivatives in Gas and Liquid Separation" Encyclopedia, https://encyclopedia.pub/entry/42688 (accessed March 01, 2026).
Chen, M., Hu, G., Shen, T., Zhang, H., Sun, J.Z., & Tang, B.Z. (2023, March 31). Polyacetylene Derivatives in Gas and Liquid Separation. In Encyclopedia. https://encyclopedia.pub/entry/42688
Chen, Manyu, et al. "Polyacetylene Derivatives in Gas and Liquid Separation." Encyclopedia. Web. 31 March, 2023.
Copy Citation
Some substituted polyacetylenes have distorted structures and formed micropores due to the existence of rigid main chains and substituted side groups, which can be applied to the field of membrane separation.
membrane separation
substituted polyacetylenes
gas permeability
1. Introduction
With the improvement in people’s living standards and the growth of the population, people’s demand for energy is growing, which greatly promotes the rapid development of the chemical industry. However, the chemical industry inevitably releases carbon monoxide, carbon dioxide, volatile organic compound (VOC) gas and a large number of organic solvent mixture while achieving high value output, leading to global warming, water pollution and severe weather with frequent disasters. Recently, the UN Paris Agreement on Global Warming came into force, it is required that global greenhouse gas emissions should be reduced to 32 billion tons by 2030 [1]. However, the development of the chemical industry and other industries cannot be completely prohibited, which requires the development of gas separation and capture technology. To build an ecologically civilized society, it is necessary to find ways to separate mixed gases and organic liquids with low energy consumption, low cost and high efficiency.
In the field of gas separation, traditional separation methods include solvent absorption, low temperature distillation and pressure swing adsorption. Although the solvent absorption method can obtain high separation efficiency by selecting the corresponding solvent for different gases, it is inevitable that the solvent needs post-treatment and the energy consumption is large. Additionally, the separation methods of low temperature distillation and pressure swing adsorption have high energy consumption and cost. However, membrane separation technology can achieve high permeability and high separation by rationally selecting different substances for different gases, designing molecular structures and surface modifications. It has the advantages of high separation efficiency, low energy consumption, a small footprint, simple operation and no phase change [2]. In the chemical and pharmaceutical industries, organic solvents are inevitably used. Nevertheless, organic solvents cannot be directly discharged because of their toxicity and damage to the environment. Traditional separation and treatment methods such as distillation, adsorption and extraction have high energy consumption and huge costs. Therefore, membrane separation technology has become an excellent method in the field of gas separation and organic solvent separation due to its low energy consumption, low cost and simple operation.
In recent years, membrane separation technology has flourished. Researchers are moving towards the goal of high selectivity, low energy consumption and low cost. Based on the relationship between structure and performance, different types of separation membranes such as organic metal framework (MOF), covalent organic framework (COF) and polymer of intrinsic microporosity (PIM) were obtained by designing a molecular structure [3][4][5][6][7]. Since PTMSP was found to have ultra-high gas permeability, substituted polyacetylenes have become a promising candidate as permeable membranes for gas and liquid separation. Some substituted polyacetylenes show superior performance in gas permeability due to both their stiff main chain composed of alternating double bonds and the steric repulsion of spherical side groups. However, there are many restrictions on the development of gas separation membranes. Thus, significant efforts have been devoted to improve selectivity performance of substituted polyacetylenes.
2. Application of Substituted Polyacetylenes in Gas Separation
Polymers are currently the most studied and widely used membrane materials. From the earliest cellulose acetate, polysulfone, polydimethylsiloxane, polyimide, polyester, polyacetylene and other gas separation membrane materials have been developed. These polymers can be divided into rubbery and glassy polymers. Polydimethylsiloxane (PDMS) and polyethylene glycol (PEG) are typical rubbery molecules with extremely high solubility coefficient of carbon dioxide. PDMS is the polymer with the best permeability for a long time. Until the emergence of poly (1-trimethylsilyl-1-propyne) (PTMSP), the oxygen permeability coefficient of PTMSP is as high as 6000 barrer, which is ten times that of PDMS. Unlike traditional glassy polymers, PTMSP has higher permeability to organic solvents and gases, which may be due to the rigid main chain and large substituents leading to the formation of micropores in PTMSP with a high free volume [8]. This type of polymer of intrinsic microporosity (PIM) has a distorted stereo conformation, and the micropores smaller than 2 nm mean that the polymer has a larger free volume, thus improving the permeability to gas. By designing the molecular structure to adjust the free volume, the introduction of different groups can change the adsorption of different molecules, which is expected to obtain high permeability and high selectivity of the separation membrane. Next, researchers will describe the development of substituted polyacetylenes in the application of separation membrane, and put forward a prospect.
Polyacetylene, as the prototype of conductive polymers, has caused a great sensation, which can be synthesized through the following route (see Figure 1), but it is difficult to put into use due to its insolubility, infusibility and instability. Subsequently, researchers have introduced different substituents to improve its performance, and in the process obtained properties such as liquid crystal, magnetic, photoluminescence, etc. In 1983, Masuda et al. found the high permeability of PTMSP, and the oxygen permeability, was much higher than that of the most permeable polymer (polydimethylsiloxane) at that time, which expanded the application of substituted polyacetylenes in gas separation [9][10][11][12][13].
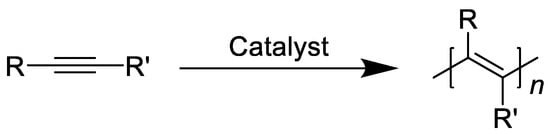
Figure 1. General synthetic route to substituted polyacetylenes.
Following this pioneering discovery, researchers have studied the permeability mechanism of PTMSP. After experimental verification, the curve of gas concentration and osmotic pressure in PTMSP presents a concave shape, that is, the adsorption isotherm of small molecule gas in glassy polymer, which can be explained by double adsorption model [14][15]: (1) The dissolution mechanism of gas in the more relaxed region is similar to that in rubbery polymer, which conforms to Henry’s law. (2) In the non-relaxation glassy region of micropores, the adsorption model following the Langmuir isotherm plays a major role. This also provides ideas for the development of more permeable substituted acetylene. Ichiraku et al. [16] studied and analyzed the reasons for the high permeability of PTMSP. Through experiments, compared with PDMS, PTMSP has a very high solubility for oxygen, carbon dioxide, nitrogen and other gases, which may be attributed to the very high free volume of the polymer. The chemical structure determines that the polymer has a high free volume. The alternating carbon–carbon double bonds make the PTMSP have a relatively high, rigid main chain. The large spherical trimethyl silane substituent limits the movement of the segments, resulting in a high free volume in the non-relaxation region.
Molecular dynamics’ simulation can help researchers to predict and analyze the experimental results to a certain extent. For example, Catlow et al. [17] obtained potential parameters by using bond energy, vibration data and structural data to study the original form of trans-polyacetylene and its mobility under different doping. Molecular dynamics simulation can also be used to analyze the gas separation performance of substituted acetylene. Clough et al. [18] calculated the single-bond rotational potential barrier of several substituted acetylenes by molecular dynamics’ simulation and calculated that the chain conformation became non-planar with the increase in the volume and number of side groups. According to their results, the rotation potential barrier of TMSP around the single bond of PTMSP main chain is about 40 kcal/mol. Thus, the torsional chain conformation will be maintained at room temperature. The dimension of the chain will change significantly only through the torsional wave in the narrow energy well. In a word, molecular dynamics’ simulation is an effective means to design a separation-capable substitute for polyacetylenes.
In addition to the extended research on PTMSP, researchers have also explored other disubstituted polyacetylenes with permeability based on the strategy of large substituents and rigid main chains. By introducing spherical substituents, such as trimethylsilyl, trimethyl germanyl and tert-butyl, similar structures and distorted conformations can be obtained. Large benzene ring substituents can hinder chain stacking and make the resulting polymers have a high free volume, such as poly[1-(trimethyl germanyl)-1-propyne] (PTMGP), poly(4-methyl-2-pentyne) (PMP), poly[1-phenyl-2-(p-trimethylsilyl) ethynylene] (PTMSDPA)(see Figure 2) and other permeable disubstituted acetylenes which were summarized by Masuda et al. [8][19][20][21]. In summary, the main goal of improving the gas separation performance of polymers is to increase selectivity based on high gas permeability [22] and to solve the problem of the aging of glassy polymers with high free volume (e.g., PTMSP [23]).
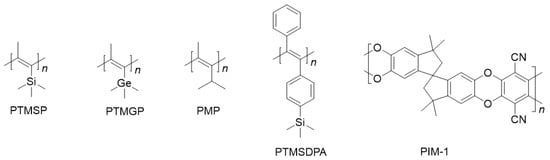
Figure 2. Representative disubstituted polyacetylenes and PIM-1 with good gas permeability.
There are two main methods to improve selectivity. One is to obtain a mixed membrane by mixing multiple components, and the other is to obtain a self-supporting membrane by chemical modification, which includes halogenation, surface cross-linking, copolymerization and other methods. Taking PTMSP as an example, the composite membrane obtained by coating a highly selective polybenzodioxane (PIM-1) on a highly permeable cross-linked PTMSP by Ilya et al. [24] has high CO2/N2 selectivity. The separation factor α = 35.8–55.7, which is higher than PIM-1 (α = 18.5) and cross-linked PTMSP (α = 3.7). Among them, Viktoriya et al. [25] modified PTMSP by introducing ionic-liquid butyl imidazolium bromide with high CO2 solubility as a sub-substituent. The selectivity was doubled and the permeability was not reduced much, which was closer to the upper limit curve in the Robeson diagram than the original PTMSP. On the basis of the previous introduction of bromine and fluorine atoms, Kossov et al. [26] successfully improved the selectivity to CO2 by introducing chlorine atoms on the side chains of PTMSP and PMP, but the permeability was reduced compared with the initial polymer. This is consistent with Robeson’s law, that is, there is a “trade-off” relationship between permeability and selectivity.
To tackle the aging problem of glassy/microporous polymers, one first needs to understand the aging mechanism. Taking porous polymer PTMSP as an example [27], its aging mechanism can be divided into three parts: (1) Physical aging, which means with the passage of time, the polymer chains relaxes, free volume decreases and bulk density becomes larger, resulting in a significant reduction in permeability; (2) Absorption aging. The polymer membrane absorbs nonvolatile impurities resulting in a decrease in free volume. (3) Chemical aging. This means that the polymer absorbs impurities in the environment to form oxygen-containing groups. At high temperatures, the main chain even breaks to form a low-molecular-weight oxygen-containing polymer. This polar oxygen-containing group leads to an increase in bulk density and poor polymer permeability. Moreover, it is found that the thinner the film, the faster the aging rate, because more free volume is exposed to the surface, which will relax faster. Physical aging is very common in porous polymers with high free volume. Although physical aging can be eliminated by dissolving and re-forming the membrane, absorption aging can also be eliminated by dissolving, reprecipitating and then dissolving the reprecipitate and re-forming the membrane. However, these processes will undoubtedly increase energy consumption and cost, so aging still provides a major resistance to commercial membranes.
So, how to avoid aging? According to the aging mechanism, it can be found that inhibiting chain stacking or “freezing the polymer high free volume” is theoretically an effective way to attenuate aging. At present, there are two main ways, one is to improve the rigidity of the polymer structure through the design of the structure or cross-linking, copolymerization and other modification methods; the other is to support microporosity by adding additives [28]. However, rigid chemical structures and cross-linking methods that increase the rigidity of the polymer do not necessarily improve aging, because rigid polymer chains are not equivalent to restricted chain motion [29][30]. Therefore, additives are usually added to prevent pore collapse and maintain high free volume. In 2014, Lau et al. [31] demonstrated that tetrakis(4-bromophenyl) methane self-condensed to form carbon-based microporous arrays (PAF-1) through the Yamamoto coupling reaction. PAF-1 is a porous aromatic skeleton particle with a pore diameter of about 1.2 nm, which is attractive for polyacetylene side chains or large-volume chemical structures. On the other hand, the similar attraction of chemical groups makes the aromatic hydrogen energy of PAF-1 and the methyl group of PTMSP attract each other. With the increase in time, PAF-1 can prevent the collapse of the pores, but the large pore size becomes smaller, so that large gas molecules cannot pass through, small molecule gas is not affected, which is conducive to H2/N2 separation [32]. However, the difficulty of synthesizing PAF-1 makes the membrane costly and difficult to apply. In order to reduce costs, Lau et al. [33] developed a hyper-crosslinking additive (p-DCX) similar to the structure of PAF-1 in 2016, reducing costs on the basis of improving aging
References
- Du, J.; Duan, L. The united nations environment programme issued the 2018 emission gap report. World Agric. 2019, 98.
- Fei, W.; Ai, N.; Chen, J. Capture and separation of greenhouse gases Co2—The challenge and opportunity for separation technology. Chem. Ind. Eng. Prog. 2005, 24, 1–4.
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504.
- Ding, S.Y.; Wang, W. Covalent organic frameworks (cofs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568.
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; Mckeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269.
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663.
- Wang, H.J.; Wang, M.D.; Liang, X.; Yuan, J.Q.; Yang, H.; Wang, S.Y.; Ren, Y.X.; Wu, H.; Pan, F.S.; Jiang, Z.Y. Organic molecular sieve membranes for chemical separations. Chem. Soc. Rev. 2021, 50, 5468–5516.
- Nagai, K.; Masuda, T.; Nakagawa, T.; Freeman, B.D.; Pinnau, I. Poly and related polymers: Synthesis, properties and functions. Prog. Polym. Sci. 2001, 26, 721–798.
- Ponomarenko, M.V.; Kalinovich, N.; Serguchev, Y.A.; Bremer, M.; Roschenthaler, G.V. Synthesis of pentafluoro-lambda(6)-sulfanyl substituted acetylenes for novel liquid crystals. J. Fluor. Chem. 2012, 135, 68–74.
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene. J. Chem. Soc. Chem. Commun. 1977.
- Ko, K.C.; Cho, D.; Lee, J.Y. Systematic approach to design organic magnetic molecules: Strongly coupled diradicals with ethylene coupler. J. Phys. Chem. A 2012, 116, 6837–6844.
- Chen, J.W.; Xie, Z.L.; Lam, J.; Law, C.; Tang, B.Z. Silole-containing polyacetylenes. Synthesis, thermal stability, light emission, nanodimensional aggregation, and restricted intramolecular rotation. Macromolecules 2003, 36, 1108–1117.
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly—A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas-permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474.
- Plate, N.A.; Bokarev, A.K.; Kaliuzhnyi, N.E.; Litvinova, E.G.; Khotimskii, V.S.; Volkov, V.V.; Yampolskii, Y.P. Gas and vapor permeation and sorption in poly(trimetylsilylpropyne). J. Membr. Sci. 1991, 60, 13–24.
- Paul, D.R. Gas sorption and transport in glassy-polymers. Ber. Bunsen-Ges. Phys. Chem. Chem. Phys. 1979, 83, 294–302.
- Ichiraku, Y.; Stern, S.A.; Nakagawa, T. An investigation of the high gas-permeability of poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 1987, 34, 5–18.
- Sese, G.; Catlow, C.; Vessal, B. Molecular-dynamics simulations of polyacetylene. Mol. Simul. 1992, 9, 99–113.
- Clough, S.B.; Sun, X.F.; Tripathy, S.K.; Baker, G.L. Molecular-dynamics simulation of substituted polyacetylenes. Macromolecules 1991, 24, 4264–4269.
- Morisato, A.; Pinnau, I. Synthesis and gas permeation properties of poly(4-methyl-2-pentyne). J. Membr. Sci. 1996, 121, 243–250.
- Tsuchihara, K.; Masuda, T.; Higashimura, T. Polymerization of silicon-containing diphenylacetylenes and high gas-permeability of the product polymers. Macromolecules 1992, 25, 5816–5820.
- Toy, L.G.; Nagai, K.; Freeman, B.D.; Pinnau, I.; He, Z.; Masuda, T.; Teraguchi, M.; Yampolskii, Y.P. Pure-gas and vapor permeation and sorption properties of polyacetylene] (ptmsdpa). Macromolecules 2000, 33, 2516–2524.
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185.
- Yampolskii, Y.P.; Shishatskii, S.M.; Shantorovich, V.P.; Antipov, E.M.; Kuzmin, N.N.; Rykov, S.V.; Khodjaeva, V.L.; Plate, N.A. Transport characteristics and other physicochemical properties of aged poly(1-(trimethylsilyl)-1-propyne). J. Appl. Polym. Sci. 1993, 48, 1935–1944.
- Borisov, I.; Bakhtin, D.; Luque-Alled, J.M.; Rybakova, A.; Makarova, V.; Foster, A.B.; Harrison, W.J.; Volkov, V.; Polevaya, V.; Gorgojo, P.; et al. Synergistic enhancement of gas selectivity in thin film composite membranes of pim-1. J. Mater. Chem. A 2019, 7, 6417–6430.
- Polevaya, V.; Geiger, V.; Bondarenko, G.; Shishatskiy, S.; Khotimskiy, V. Chemical modification of poly(1-trimethylsylil-1-propyne) for the creation of highly efficient Co2-selective membrane materials. Materials 2019, 12, 2763.
- Kossov, A.A.; Geiger, V.Y.; Matson, S.M.; Litvinova, E.G.; Polevaya, V.G. Synthesis and gas-transport properties of poly(1-trimethylsilyl-1-propyne)- and poly(4-methyl-2-pentyne)-based chlorinated polyacetylenes for membrane separation of carbon dioxide. Membr. Membr. Technol. 2019, 1, 212–219.
- Starannikova, L.; Khodzhaeva, V.; Yampolskii, Y. Mechanism of aging of poly and its effect on gas permeability. J. Membr. Sci. 2004, 244, 183–191.
- Bakhtin, D.S.; Kulikov, L.A.; Bondarenko, G.N.; Vasilevskii, V.P.; Maksimov, A.L.; Volkov, A.V. Stabilization of gas transport properties of composite membranes with a thin ptmsp selective layer by adding porous aromatic framework nanoparticles and simultaneous polymer crosslinking. Pet. Chem. 2018, 58, 790–796.
- Kelman, S.D.; Rowe, B.W.; Bielawski, C.W.; Pas, S.J.; Hill, A.J.; Paul, D.R.; Freeman, B.D. Crosslinking poly and its effect on physical stability. J. Membr. Sci. 2008, 320, 123–134.
- Carta, M.; Malpass-Evans, R.; Croad, M.; Rogan, Y.; Jansen, J.C.; Bernardo, P.; Bazzarelli, F.; Mckeown, N.B. An efficient polymer molecular sieve for membrane gas separations. Science 2013, 339, 303–307.
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.; Sprouster, D.J.; et al. Ending aging in super glassy polymer membranes. Angew. Chem. Int. Edit. 2014, 53, 5322–5326.
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M.R. Gas-separation membranes loaded with porous aromatic frameworks that improve with age. Angew. Chem. Int. Edit. 2015, 54, 2669–2673.
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked additives for ageless gas-separation membranes. Angew. Chem. Int. Edit. 2016, 55, 1998–2001.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
631
Revisions:
2 times
(View History)
Update Date:
03 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





