
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chaiyavat Chaiyasut | -- | 2833 | 2023-03-31 09:54:52 | | | |
| 2 | Jessie Wu | Meta information modification | 2833 | 2023-03-31 10:06:11 | | |
Video Upload Options
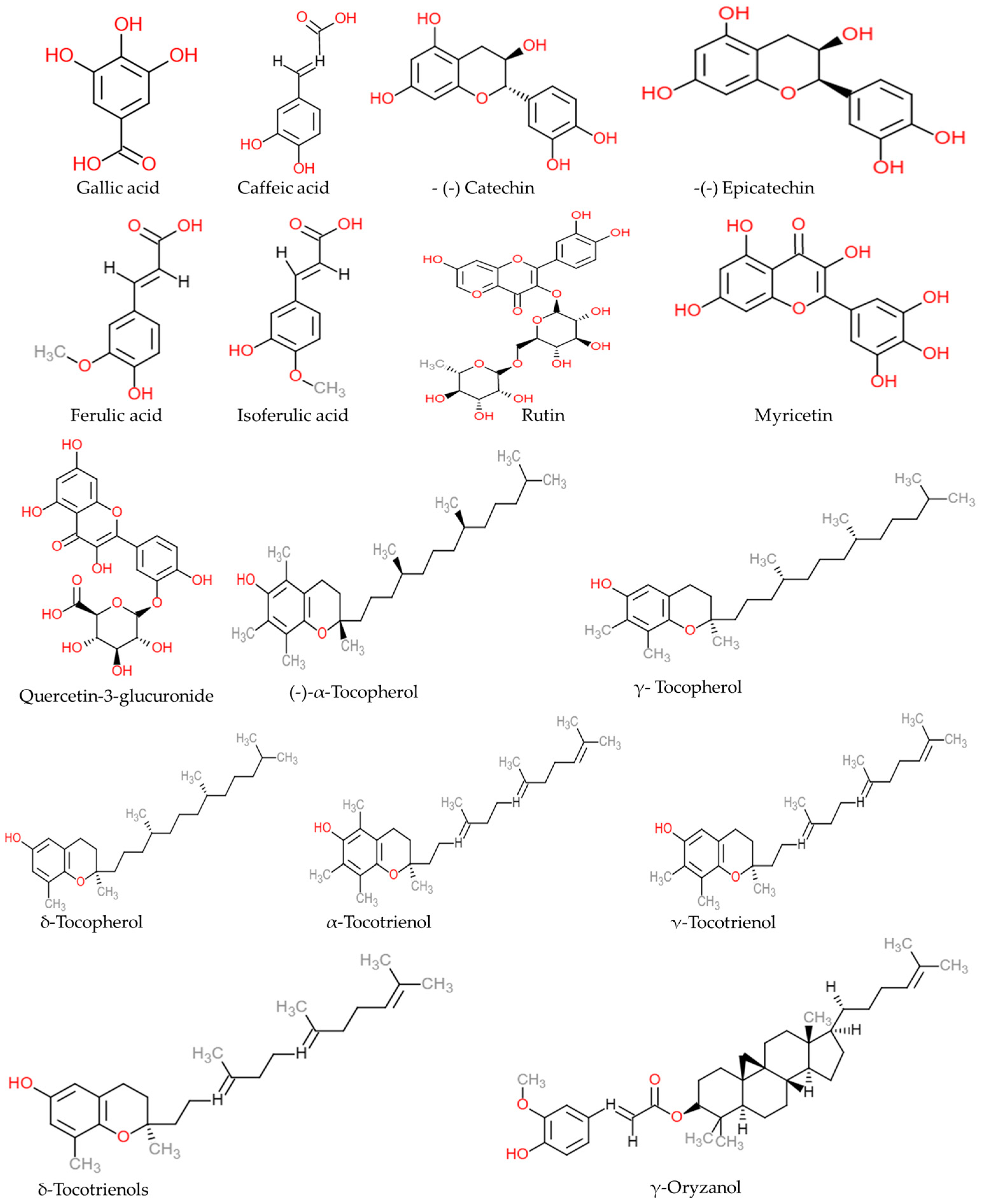
Rice is a major cereal crop and a staple food for nearly 50% of people worldwide. Rice bran (RB) is a nutrient-rich by-product of rice processing. RB is rich in carbohydrates, fibers, proteins, lipids, minerals, and several trace elements (phosphorus, calcium, magnesium, potassium, and manganese). The extraction process and storage have influenced RB extracts and RB oil’s quality. The RB composition has also varied on the rice cultivars. The color of RB indicates the richness of the bioactive compounds, especially anthocyanins. γ-oryzanol, tocopherols, tocotrienols, and unsaturated fatty acids are major components of RB oil. It has been established that RB supplementation could improve the host’s health status. Several preclinical and clinical studies have reported that RB has antioxidant, anticancer, anti-inflammatory, anticolitis, and antidiabetic properties. The beneficial biological properties of RB are partially attributed to its ability to alter the host microbiome and help to maintain and restore eubiosis. Non-communicable diseases (NCDs), including heart disease, diabetes, cancer, and lung disease, account for 74% of deaths worldwide. Obesity is a global health problem and is a major reason for the development of NCDs. The medical procedures for managing obesity are expensive and long-term health supplements are required to maintain a healthy weight. Thus, cost-effective natural adjuvant therapeutic strategy is crucial to treat and manage obesity.
1. Introduction
2. Phytochemical Composition of Rice Bran
3. Mechanisms Associated with the Anti-Obesity Property of Rice Bran

References
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096.
- Sharma, H.R.; Chauhan, G.S.; Agrawal, K. Physico-Chemical Characteristics of Rice Bran Processed by Dry Heating and Extrusion Cooking. Int. J. Food Prop. 2004, 7, 603–614.
- Spaggiari, M.; Dall’Asta, C.; Galaverna, G.; del Castillo Bilbao, M.D. Rice Bran By-Product: From Valorization Strategies to Nutritional Perspectives. Foods 2021, 10, 85.
- Oliveira, M.S.; Feddern, V.; Kupsk, L.; Cipolatti, E.P.; Furlong, E.B.; Soares, L.A.S. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338.
- Gul, K.; Yousuf, B.; Singh, A.; Singh, P.; Wani, A.A. Rice bran: Nutritional values and its emerging potential for development of functional food-A review. Bioact. Carbohydr. Diet Fibre 2015, 6, 24–30.
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. A comprehensive review on anti-diabetic property of rice bran. Asian Pac. J. Trop. Biomed. 2018, 8, 79–84.
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Chalermpong Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501.
- Reis, N.; Castanho, A.; Lageiro, M.; Pereira, C.; Brites, C.M.; Vaz-Velho, M. Rice bran stabilisation and oil extraction using the microwave-assisted method and its effects on GABA and gamma-oryzanol compounds. Foods 2022, 11, 912.
- Bumrungpert, A.; Chongsuwat, R.; Phosat, C.; Butacnum, A. Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: A randomised double-blind controlled trial. J. Altern. Complement. Med. 2019, 25, 353–358.
- Kozuka, C.; Sunagawa, S.; Ueda, R.; Higa, M.; Tanaka, H.; Shimizu-Okabe, C.; Ishiuchi, S.; Takayama, C.; Matsushita, M.; Tsutsui, M.; et al. γ-Oryzanol protects pancreatic β-cells against endoplasmic reticulum stress in male mice. Endocrinology 2015, 156, 1242–1250.
- Francisqueti, F.V.; Minatel, I.O.; Ferron, A.J.T.; Bazan, S.G.Z.; Silva, V.D.; Garcia, J.L.; de Campos, D.H.S.; Ferreira, A.L.; Moreto, F.; Cicogna, A.C.; et al. Effect of gamma-oryzanol as therapeutic agent to prevent cardiorenal metabolic syndrome in animals submitted to high sugar-fat diet. Nutrients 2017, 9, 1299.
- Yang, M.; Liu, S.; Zhang, C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare 2022, 10, 1616.
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231.
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252.
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862.
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365.
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946.
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398.
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905.
- Shibayama, J.; Kuda, T.; Shikano, A.; Fukunaga, M.; Takahashi, H.; Kimura, B.; Ishizaki, S. Effects of rice bran and fermented rice bran suspensions on caecal microbiota in dextran sodium sulphate-induced inflammatory bowel disease model mice. Food Biosci. 2018, 25, 8–14.
- Ito, Y.; Nakashima, Y.; Matsuoka, S. Rice bran extract containing acylated steryl glucoside fraction decreases elevated blood LDL cholesterol level in obese Japanese men. J. Med. Investig. 2015, 62, 80–84.
- Haldar, S.; Wong, L.H.; Tay, S.L.; Jacoby, J.J.; He, P.; Osman, F.; Ponnalagu, S.; Jiang, Y.R.; Lian, H.P.R.; Henry, C.J. Two blends of refined rice bran, flaxseed, and sesame seed oils affect the blood lipid profile of Chinese adults with borderline hypercholesterolemia to a similar extent as refined olive oil. J. Nutr. 2020, 150, 3141–3151.
- Hongu, N.; Kitts, D.D.; Zawistowski, J.; Dossett, C.M.; Kopeć, A.; Pope, B.T.; Buchowski, M.S. Pigmented rice bran and plant sterol combination reduces serum lipids in overweight and obese adults. J. Am. Coll. Nutr. 2014, 33, 231–238.
- Edrisi, F.; Salehi, M.; Ahmadi, A.; Fararoei, M.; Rusta, F.; Mahmoodianfard, S. Effects of supplementation with rice husk powder and rice bran on inflammatory factors in overweight and obese adults following an energy-restricted diet: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 833–843.
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813.
- Vichapong, J.; Sookserm, M.; Srijesdaruk, V.; Swatsitang, P.; Srijaranai, S. High performance liquid chromatographic analysis of phenolic compounds and their antioxidant activities in rice varieties. LWT-Food Sci. Technol. 2010, 43, 1325–1330.
- Ghasemzadeh, A.; Jaafar, H.Z.; Juraimi, A.S.; Tayebi-Meigooni, A. Comparative Evaluation of Different Extraction Techniques and Solvents for the Assay of Phytochemicals and Antioxidant Activity of Hashemi Rice Bran. Molecules 2015, 20, 10822–10838.
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 35–338.
- Zarei, I.; Brown, D.G.; Nealon, N.J.; Ryan, E.P. Rice Bran Metabolome Contains Amino Acids, Vitamins & Cofactors, and Phytochemicals with Medicinal and Nutritional Properties. Rice 2017, 10, 24.
- Saunders, R.M. The properties of rice bran as a food stuff. Cereal Foods World 1990, 35, 632–639.
- Sharif, M.K.; Butt, M.S.; Anjum, F.M.; Khan, S.H. Rice bran: A novel functional ingredient. Crit. Rev. Food Sci. Nutr. 2014, 54, 807–816.
- Zullaikah, S.; Lai, C.C.; Vali, S.R.; Ju, Y.H. A two-step acid-catalyzed process for the production of biodiesel from rice bran oil. Bioresour. Technol. 2005, 96, 1889–1896.
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653.
- Qureshi, A.A.; Salser, W.A.; Parmar, R.; Emeson, E.E. Novel tocotrienols of rice bran inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J. Nutr. 2001, 131, 2606–2618.
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104.
- Yamuangmorn, S.; Prom-u-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833.
- Charoonratana, T.; Songsak, T.; Sakunpak, A.; Pathompak, P.; Charoenchai, L. Using liquid chromatography-mass spectrometry-based metabolomics to discriminate between cold pressed rice bran oils produced from two different cultivars of Oryza sativa L. ssp. indica in Thailand. Chin. J. Chromatogr. 2015, 33, 966–973.
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Schuch, W.; Sheehy, P.J.A.; Wagner, K.H. Vitamin E-A critical review. J. Sci. Food Agric. 2000, 80, 913–938.
- Abidi, S.L. Tocol-derived minor constituents in selected plant seed oils. J. Am. Oil Chem. Soc. 2003, 80, 327–333.
- Wisetkomolmat, J.; Arjin, C.; Satsook, A.; Seel-Audom, M.; Ruksiriwanich, W.; Prom-U-Thai, C.; Sringarm, K. Comparative Analysis of Nutritional Components and Phytochemical Attributes of Selected Thai Rice Bran. Front. Nutr. 2022, 9, 833730.
- Wang, M.; Hettiarachchy, N.S.; Qi, M.; Burks, W.; Siebenmorgen, T. Preparation and functional properties of rice bran protein isolate. J. Agric. Food Chem. 1999, 47, 411–416.
- Fabian, C.; Ju, Y.H. A review on rice bran protein: Its properties and extraction methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 816–827.
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769.
- Lee, S.K.; Jang, I.S.; Kim, M.K.; Park, S.K.; Lee, W.Y.; Youn, K.S.; Bae, D.H. Changes in functional properties of rice bran and sesame meal proteins through chemical modifications. Food Sci. Biotechnol. 2004, 13, 555–560.
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J. Food Sci. Technol. 2014, 51, 3878–3885.
- Rao, R.S.P.; Muralikrishna, G. Non-starch polysaccharide–phenolic acid complexes from native and germinated cereals and millet. Food Chem. 2004, 84, 527–531.
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Identification and Quantification of Phenolic Acids and Anthocyanins as Antioxidants in Bran, Embryo and Endosperm of White, Red and Black Rice Kernels (Oryza sativa L.). J. Cereal Sci. 2014, 59, 211–218.
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Effect of Extraction Conditions on Phenolic Content, Anthocyanin Content and Antioxidant Activity of Bran Extracts from Thai Rice Cultivars. J. Cereal Sci. 2019, 86, 86–91.
- Aziz, S.; Elfahmi, Y.; Soemardji, A.A.; Sukrasno, S. Anti-Hypercholesterolemic Agent from Indonesian Rice Bran. Int. J. Res. Pharm. Sci. 2019, 10, 2733–2738.
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous Determination of Tocols, γ-Oryzanols, Phytosterols, Squalene, Cholecalciferol and Phylloquinone in Rice Bran and Vegetable Oil Samples. Food Chem. 2019, 271, 630–638.
- Sapwarobol, S.; Saphyakhajorn, W.; Astina, J. Biological Functions and Activities of Rice Bran as a Functional Ingredient: A Review. Nutr. Metab. Insights 2021, 14, 1–11.
- Ruksiriwanich, W.; Manosroi, J.; Abe, M.; Manosroi, W.; Manosroi, A. 5αReductase type 1 inhibition of Oryza sativa bran extract prepared by supercritical carbon dioxide fluid. J. Supercrit. Fluids 2011, 59, 61–71.
- Wang, W.; Guo, J.; Zhang, J.; Peng, J.; Liu, T.; Xin, Z. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49.
- Wu, F.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463.
- Ahmadifard, N.; Murueta, J.H.; Abedian-Kenari, A.; Motamedzadegan, A.; Jamali, H. Comparison the effect of three commercial enzymes for enzymatic hydrolysis of two substrates (rice bran protein concentrate and soy-been protein) with SDS PAGE. J. Food Sci. Technol. 2016, 53, 1279–1284.
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2013, 152, 46–55.
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306.
- Ding, C.; Liu, Q.; Li, P.; Pei, Y.; Tao, T.; Wang, Y.; Yan, W.; Yang, G.; Shao, X. Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice. Food Chem. 2019, 274, 384–391.
- Zhao, G.; Zhang, R.; Dong, L.; Huang, F.; Liu, L.; Deng, Y.; Ma, Y.; Zhang, Y.; Wei, Z.; Xiao, J.; et al. A Comparison of the Chemical Composition, In Vitro Bioaccessibility and Antioxidant Activity of Phenolic Compounds from Rice Bran and Its Dietary Fibres. Molecules 2018, 23, 202.
- Sompong, R.; Siebenhandl-Ehn, S.; Linsberger-Martin, G.; Berghofer, E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011, 124, 132–140.
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Anthocyanins in Thai rice varieties: Distribution and pharmacological significance. Int. Food Res. J. 2018, 25, 2024–2032.
- Maisuthisakul, P.; Changchub, L. Effect of Extraction on Phenolic Antioxidant of Different Thai Rice (Oryza sativa L.) Genotypes. Int. J. Food Prop. 2014, 17, 855–865.
- Huang, Y.P.; Lai, H.M. Bioactive Compounds and Antioxidative Activity of Colored Rice Bran. J. Food Drug Anal. 2016, 24, 564–574.
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice Secondary Metabolites: Structures, Roles, Biosynthesis, and Metabolic Regulation. Molecules 2018, 23, 3098.
- Mohanlal, S.; Maney, S.K.; Santhoshkumar, T.R.; Jayalekshmy, A. Tricin 40 -O-(erythro-β-guaiacylglyceryl) ether and tricin 40 -O-(threo-β-guaiacylglyceryl) ether isolated from Njavara (Oryza sativa L. var. Njavara), induce apoptosis in multiple tumor cells by mitochondrial pathway. J. Nat. Med. 2013, 67, 528–533.
- Saito, T.; Abe, D.; Sekiya, K. Sakuranetin induces adipopenesis of 3T3-L1 cells through enhanced expression of PPARγ2. Biochem. Biophys. Res. Commun. 2008, 372, 835–839.
- Zhang, X.F.; Hung, T.M.; Phuong, P.T.; Ngoc, T.M.; Min, B.-S.; Song, K.-S.; Seong, Y.H.; Bai, K.H. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch. Pharm. Res. 2006, 29, 1102–1108.
- Miyazawa, M.; Kinoshita, H.; Okuno, Y. Antimutagenic activity of sakuranetin from Prunus jamasakura. J. Food Sci. 2003, 68, 52–56.
- Grecco, S.S.; Reimao, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.; Romoff, P.; Ferreira, M.J.P.; Favero, O.A.; Lago, J.H.G. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC (Asteraceae). Exp. Parasitol. 2012, 130, 141–145.
- Chen, X.; Yang, Y.; Yang, X.; Zhu, G.; Lu, X.; Jia, F.; Diao, B.; Yu, S.; Ali, A.; Zhang, H.; et al. Investigation of flavonoid components and their associated antioxidant capacity in different pigmented rice varieties. Food Res. Int. 2022, 161, 111726.
- Hao, J.; Zhu, H.; Zhang, Z.; Yang, S.; Li, H. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99.
- Eder, R. Pigments. In Food Analysis by HPLC, 2nd ed.; Nollet, L.M.L., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2000; pp. 825–880.
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933.
- Chumpolsri, W.; Wijit, N.; Boontakham, P.; Nimmanpipug, P.; Sookwong, P.; Luangkamin, S.; Wongpornchai, S. Variation of Terpenoid Flavor Odorants in Bran of Some Black and White Rice Varieties Analyzed by GC×GC-MS. J. Food Nutr. Res. 2015, 3, 3–120.
- Tochitani, S.; Maehara, Y.; Kawase, T.; Tsukahara, T.; Shimizu, R.; Watanabe, T.; Maehara, K.; Asaoka, K.; Matsuzaki, H. Fermented rice bran supplementation ameliorates obesity via gut microbiota and metabolism modification in female mice. J. Clin. Biochem. Nutr. 2022, 70, 160–174.
- Anikisetty, M.; Gopala Krishna, A.G.; Panneerselvam, V.; Kamatham, A.N. Diacylglycerol (DAG) rich rice bran and sunflower oils modulate lipid profile and cardiovascular risk factors in Wistar rats. J. Funct. Foods 2018, 40, 117–127.
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472.
- Justo, M.L.; Claro, C.; Vila, E.; Herrera, M.D.; Rodriguez-Rodriguez, R. Microvascular disorders in obese Zucker rats are restored by a rice bran diet. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 524–531.
- Park, H.; Yu, S.; Kim, W. Rice bran oil attenuates chronic inflammation by inducing m2 macrophage switching in high-fat diet-fed obese mice. Foods 2021, 10, 359.
- Parklak, W.; Munkong, N.; Somnuk, S.; Somparn, S.; Naowaboot, J.; Yoysungnoen, B.; Lerdvuthisopon, N. Rice bran water extract attenuates pancreatic abnormalities in high-fat diet-induced obese rats. Trop. J. Pharm. Res. 2017, 16, 819–825.
- Munkong, N.; Lonan, P.; Mueangchang, W.; Yadyookai, N.; Kanjoo, V.; Yoysungnoen, B. Red rice bran extract attenuates adipogenesis and inflammation on white adipose tissues in high-fat diet-induced obese mice. Foods 2022, 11, 1865.
- Munkong, N.; Thim-Uam, A.; Pengnet, S.; Hansakul, P.; Somparn, N.; Naowaboot, J.; Tocharus, J.; Tocharus, C. Effects of red rice bran extract on high-fat diet-induced obesity and insulin resistance in mice. Prev. Nutr. Food Sci. 2022, 27, 180–187.
- Tun, S.; Spainhower, C.J.; Cottrill, C.L.; Lakhani, H.V.; Pillai, S.S.; Dilip, A.; Chaudhry, H.; Shapiro, J.I.; Sodhi, K. Therapeutic efficacy of antioxidants in ameliorating obesity phenotype and associated comorbidities. Front. Pharmacol. 2020, 11, 1234.
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694.
- Justo, M.L.; Claro, C.; Zeyda, M.; Stulnig, T.M.; Herrera, M.D.; Rodríguez-Rodríguez, R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. Eur. J. Nutr. 2016, 55, 2011–2019.
- Shibayama, J.; Goto, M.; Kuda, T.; Fukunaga, M.; Takahashi, H.; Kimura, B. Effect of rice bran fermented with Saccharomyces cerevisiae and Lactobacillus plantarum on gut microbiome of mice fed high-sucrose diet. Benef. Microbes. 2019, 10, 811–821.
- Wang, O.; Liu, J.; Cheng, Q.; Guo, X.; Wang, Y.; Zhao, L.; Zhou, F.; Ji, B. Effects of ferulic acid and γ-oryzanol on high-fat and high-fructose diet-induced metabolic syndrome in rats. PLoS ONE 2015, 10, e0118135.
- Duansak, N.; Piyabhan, P.; Srisawat, U.; Naowaboot, J.; Lerdvuthisopon, N.; Schmid-Schönbein, G. The effect of rice bran extract on arterial blood pressure, hepatic steatosis, and inflammation in mice fed with a high-fat diet. J. Nutr. Metab. 2020, 2020, 8374287.
- Duansak, N.; Schmid-Schönbein, G.W.; Srisawat, U. Anti-obesity effect of rice bran extract on high-fat diet-induced obese mice. Prev. Nutr. Food Sci. 2022, 27, 172–179.
- Tamura, M.; Hori, S.; Hoshi, C.; Nakagawa, H. Effects of rice bran oil on the intestinal microbiota and metabolism of isoflavones in adult mice. Int. J. Mol. Sci. 2012, 13, 10336–10349.
- Zhao, G.; Zhang, R.; Huang, F.; Dong, L.; Liu, L.; Jia, X.; Chi, J.; Ma, Y.; Deng, M.; Chen, Y.; et al. Hydrolyzed bound phenolics from rice bran alleviate hyperlipidemia and improve gut microbiota dysbiosis in high-fat-diet fed mice. Nutrients 2022, 14, 1277.
- Chen, T.; Shen, M.; Yu, Q.; Chen, Y.; Wen, H.; Lu, H.; Chen, S.; Xie, J. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 2022, 397, 133768.




