You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sagar Salave | -- | 4108 | 2023-03-30 11:34:32 | | | |
| 2 | Catherine Yang | Meta information modification | 4108 | 2023-03-31 02:56:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Karunakaran, B.; Gupta, R.; Patel, P.; Salave, S.; Sharma, A.; Desai, D.; Benival, D.; Kommineni, N. Types of Lipid-Based Nanocarriers for Vaccine Delivery. Encyclopedia. Available online: https://encyclopedia.pub/entry/42640 (accessed on 30 December 2025).
Karunakaran B, Gupta R, Patel P, Salave S, Sharma A, Desai D, et al. Types of Lipid-Based Nanocarriers for Vaccine Delivery. Encyclopedia. Available at: https://encyclopedia.pub/entry/42640. Accessed December 30, 2025.
Karunakaran, Bharathi, Raghav Gupta, Pranav Patel, Sagar Salave, Amit Sharma, Dhruv Desai, Derajram Benival, Nagavendra Kommineni. "Types of Lipid-Based Nanocarriers for Vaccine Delivery" Encyclopedia, https://encyclopedia.pub/entry/42640 (accessed December 30, 2025).
Karunakaran, B., Gupta, R., Patel, P., Salave, S., Sharma, A., Desai, D., Benival, D., & Kommineni, N. (2023, March 30). Types of Lipid-Based Nanocarriers for Vaccine Delivery. In Encyclopedia. https://encyclopedia.pub/entry/42640
Karunakaran, Bharathi, et al. "Types of Lipid-Based Nanocarriers for Vaccine Delivery." Encyclopedia. Web. 30 March, 2023.
Copy Citation
Lipid-based vaccine delivery systems such as the conventional liposomes, virosomes, bilosomes, vesosomes, pH-fusogenic liposomes, transferosomes, immuno-liposomes, ethosomes, and lipid nanoparticles have gained a remarkable interest in vaccine delivery due to their ability to render antigens in vesicular structures, that in turn prevents its enzymatic degradation in vivo. The particulate form of lipid-based nanocarriers confers immunostimulatory potential, making them ideal antigen carriers.
vaccine
liposomes
antigen
mRNA vaccine
DNA vaccine
lipid-based carriers
1. Liposomes
Conventional liposomes have exhibited excellent potential for the delivery of vaccines due to their properties such as biodegradability, biocompatibility, and the versatility to load both hydrophilic and lipophilic components [1]. The size of liposomes typically ranges from 20 nm to a few micrometers in diameter with the phospholipid bilayer being around 4 to 5 nm thick [2]. The first ever liposome-based commercial product Doxil® comprising the therapeutically active agent, doxorubicin hydrochloride, was approved for the treatment of AIDS-related Kaposi’s sarcoma by the USFDA in 1995. The use of Doxil® for around 20 years has been successful due to its superiority over conventional doxorubicin formulations in terms of its unique pharmacokinetics and bio-distribution. This can be attributed to the enhanced permeation and retention effect of liposomes. The resultant lowering of side effects, in particular, marked by a drastic decrease in doxorubicin-associated cardiotoxicity along with enhanced circulation half-life of the pegylated-liposomes has improved compliance and the quality of life of patients to a remarkable extent [3]. This eventually paved way for the approval of several liposome-based products that are currently marketed for various indications including neoplastic meningitis (Depocyt®), acute lymphoblastic leukemia (Marqibo®), and severe fungal infections (Ambisome®) [4]. In terms of vaccine delivery, liposomes can provide controlled release of the encapsulated antigens and are amenable to a wide range of chemical modifications that enable the attachment of targeting ligands to the surface. Further, the ease of modifying its physicochemical properties such as size, surface charge, and lipid composition has made liposomes a popular nanocarrier in vaccine delivery. The efficiency of antigen loading, apart from the choice of liposomal preparation methods and physicochemical properties of the bilayer, is also dependent on certain characteristics of the antigen such as the polarity and partition coefficient (Log P) [1]. In general, drugs with a negative Log P value are highly hydrophilic and are encapsulated in the aqueous compartment of the liposome, whereas drugs with a high Log P value are lipophilic and embedded in the lipidic bilayer. Hydrophilic components are generally not encapsulated with high efficiency and demonstrate leakage owing to their lesser affinity towards the lipid phase. Several modifications have been attempted to improve the affinity of hydrophilic components that includes conjugation with polymers, pairing with surfactants that carry an opposite charge, and the use of lipophilic prodrugs [5]. In the case of vaccine delivery systems, the antigens can be embedded within the lipid bilayer or encapsulated in the aqueous core, or covalently conjugated/adsorbed on the surface of liposomes. The location of antigens in the liposomes has a profound impact on the subsequent processing and presentation pathway. Surface-adsorbed antigens are recognized easily by the B-cells, while antigens that are encapsulated within the liposomes, need to be degraded before it is available for recognition by the immune system. A comparative study between encapsulated antigens and surface-adsorbed antigens demonstrated that haemagglutinin adsorbed onto the surface of the liposomes was more immunogenic than the ones encapsulated within the liposomes [1]. However, despite the numerous advantages of conventional liposomes, the lack of stability of liposomes in vivo is an issue of major concern, that results in bilayer rupture, leakage, and pre-mature release of the antigens. Further, the shelf-life of a liposomal product is dependent on its physical stability and the propensity of the phospholipids to undergo chemical degradation upon storage. Leakage of the encapsulated antigens due to enhanced permeability of the lipid bilayer, change in particle size distribution and hydrolytic or oxidative cleavage of the phospholipids are the instability problems that need to be taken care of. However, recently, several approaches have been utilized to overcome the stability issues of liposomes. The use of saturated lipids instead of unsaturated ones reduces the level of oxidizable lipid groups in the membrane, thereby conferring protection to the vesicles against oxidation. Storage of the liposomal dispersion in its dry form, by freeze-drying (lyophilization), enables us to overcome the issues related to oxidation and hydrolysis of the product during its shelf-life. Incorporation of cryoprotectants such as sucrose, lactose and trehalose protect the vesicles against membrane rupture/fracture, thereby preventing any loss of encapsulated material or changes in the size distribution [6]. The biological stability of liposomes can be enhanced by reducing the propensity of liposomes to undergo rapid uptake by the cells of the mononuclear phagocytic system. Several approaches such as complexation between the liposomal vesicles and polymers and grafting of hydrophilic polymers such as poly (ethylene glycol) have been investigated to produce sterically stabilized liposomes [7]. Over time, the newer generation of lipid-derived nanocarriers is emerging at a rapid pace which has overcome the limitations of conventional liposomes and has demonstrated enhanced characteristics. The section below discusses the potential role of such nanocarriers in effective vaccine delivery.
2. Virosomes
Virosomes are immunological adjuvants that were first produced by the relocation of the protein projections of the influenza virus, namely haemagglutinin and neuraminidase on the surface of unilamellar liposomes. The subunit hemagglutinin promotes virus binding, membrane fusion, and subsequent internalization into the host cells [8], whereas the enzyme neuraminidase promotes the cleavage of α-ketosidic linkage between sialic acid and the adjacent sugar residue in mucins, thereby lowering the viscosity of the mucous. This aids the virus to access the epithelial cells and further facilitates its mobility to and from the site of infection. Neuraminidase is also found to induce the production of antibodies [9]. On the isolation of the protein projections from the virus particles and subsequent purification, attachment of the subunits to the liposomes was found to occur through intercalation of the hydrophobic regions of the subunit into the lipid layer. The resulting structures were stabilized by van der Waal’s forces, and further, upon the addition of anti-influenza serum, aggregation of the viral liposomes indicated the availability of most of the binding sites for antibody attachment [10]. The virosomes thereby serve as a promising adjuvant in overcoming certain limitations associated with inactivated influenza virus vaccines such as pyrogenicity. Being both non-pyrogenic and immunogenic, virosomes have carved a niche in vaccine research with several commercial vaccines based on virosomal systems available today. Epaxal®, an aluminum-free virosomal vaccine, is based on the adsorption of formalin-inactivated hepatitis A virus (HAV) on to the surface of virosomes [11]. Epaxal® is highly immunogenic where the administration of two doses 12 months apart gives real-time protection of at least 11 years and is predicted to last for at least 30 years in more than 95% of individuals. The ability to elicit strong antibody titers, within several days following immunization and the long-term protection conferred by Epaxal®, are the major factors responsible for its commercial success [12]. Inflexal® V, a virosomal adjuvanted influenza vaccine has shown an excellent tolerability profile during the past two decades on the market owing to its biocompatibility and purity. The vaccine does not contain thiomersal or formaldehyde making it completely biodegradable. The low ovalbumin content which is an indication of the amount of residual egg protein reflects the purity of the vaccine. Further, it has demonstrated a remarkable humoral immune response as per the European Medicines Agency’s immunogenicity criteria that is based on seroprotection, geometric mean titer fold increase, and seroconversion [13]. Strategies such as the immune stimulating complex technology have been investigated widely for their dose-sparing potential. The majority of humans are immunologically naïve to avian influenza viruses such as H5N1. Therefore, two doses of vaccine are required to generate an acceptable antibody response. Formulating virosomal H5N1 vaccines with effective adjuvants enables the stimulation of immune responses at an antigen dose that is comparatively lower than that of the currently available seasonal influenza vaccines. Matrix MTM is one such adjuvant formed through the binding of cholesterol to saponins. It contains a mixture of two saponins, Matrix C, and Matrix A. The former is a highly reactogenic saponin whilst the latter is a well-tolerated but weaker adjuvant. Phase-1 clinical trial in healthy adults showed that the virosomal vaccine formulated with the adjuvant Matrix MTM enhanced the antibody response enabling a significant dose-sparing down to 1.5 μg of haemagglutinin, as compared to 30 μg of haemagglutinin in the non-adjuvanted virosomal vaccine alone [14]. The simplicity in production and industrial scalability of virosomes allows the manufacturing of adequately large batch sizes containing up to 500,000 doses as standard. Hence, virosomes can be considered one of the most dynamic and successful lipid-based nanocarriers in the field of modern vaccinology [15]. Figure 1 represents the image of an influenza virosome.
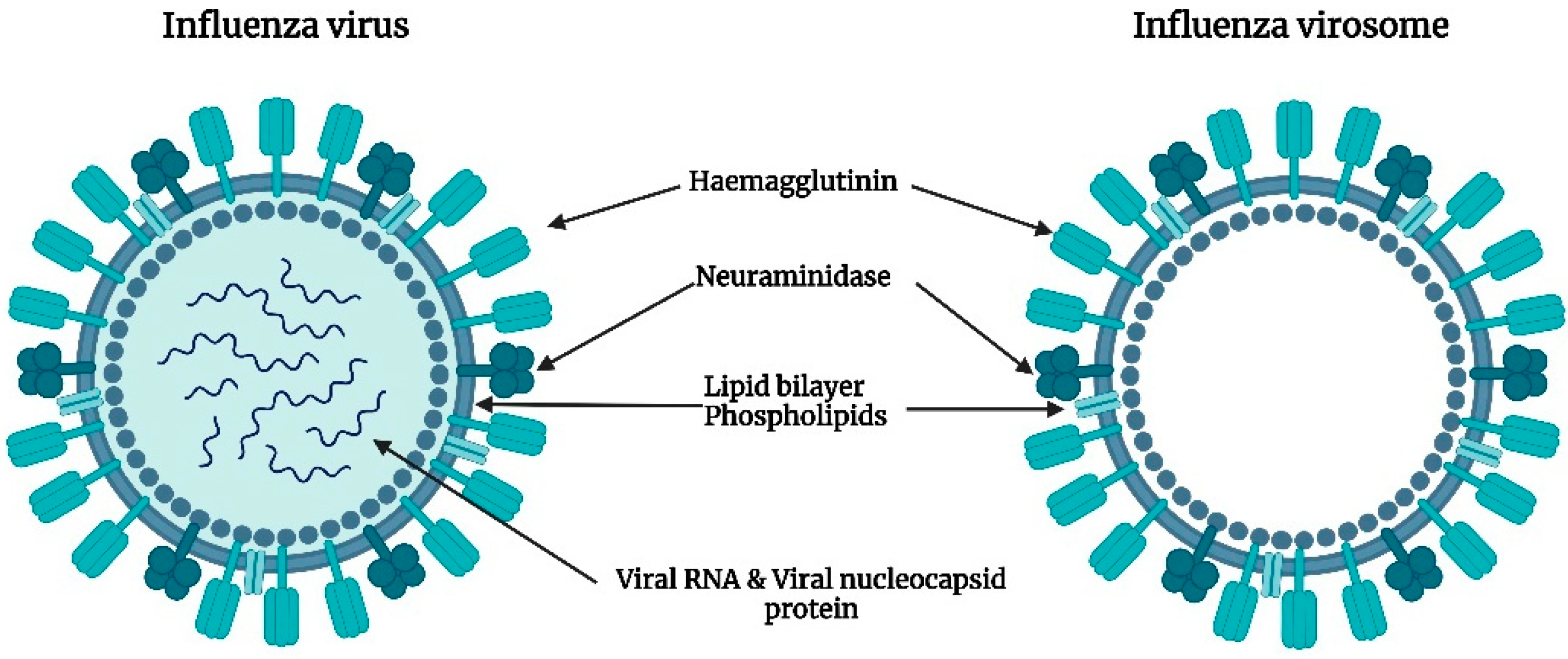
Figure 1. Representative image of an influenza virosome devoid of genetic information that mimics native influenza viruses in terms of cellular uptake and membrane fusion.
3. Bilosomes
Bilosomes are vesicular structures composed of various lipids and non-ionic surfactants that are enriched by bile salts to prevent membrane destabilization thereby protecting the bioactive from degradation in the gut microenvironment. Bilosomes are prepared by incorporating lipids such as cholesterol, distearyl phosphatidyl ethanolamine (DSPE), soybean phosphatidylcholine (SPC), dipalmitoyl phosphatidylethanolamine (DPPE), and surfactants such as sorbitan monooleate and sodium tetradecyl sulfate in a combination of varying ratios. Further, stabilization of the vesicles is achieved through the addition of different bile salts such as sodium taurocholate, sodium taurodeoxycholate, chenodeoxycholic acid, and sodium glycocholate [16]. In recent years, bilosomes have been extensively explored as ideal carriers for effective oral vaccine delivery owing to their numerous advantages over conventional liposomes. Mostly, the marketed vaccines available are given through the parenteral route of administration thereby eliciting a systemic immune response. However, a systemic immune response fails to confer protection at the mucosal level which acts as the major site of entry of infectious pathogens [17]. The antigens encapsulated in bilosomes escape degradation on exposure to the harsh pH conditions of the gastrointestinal environment (Figure 2). Further, bilosomes facilitate the transport of poorly permeable proteins and polypeptides across the mucosal epithelium and efficiently deliver the antigens to macrophages and dendritic cells thereby stimulating both mucosal and systemic immunity [18]. Premanand et al. investigated the potential of recombinant baculovirus-VP1-loaded bilosomes as an oral vaccine against human enterovirus 71 (HEV71), a pathogen that causes hand, foot, and mouth disease [19]. Oral delivery of the bilosomes encapsulating tetanus toxoid demonstrated the induction of both mucosal and systemic immunity against a bacterial protein antigen [20]. Bilosomes loaded with Hepatitis B antigen (HBsAg) were developed by Shukla et al. by utilizing sorbitan tristearate, cholesterol, and diacetyl phosphate in the ratio of 7:3:1 enriched by 100 mg of sodium deoxycholate (SDC) which demonstrated an entrapment efficiency of 18–22%. The images of fluorescence microscopy revealed the selective localization and subsequent uptake of bilosomes by the gut-associated lymphoid tissues (GALT) specifically through the Payer’s patches. Further, high-dose HBsAg bilosomes containing 50 μg of the antigen demonstrated comparable anti-HBsAg IgG levels in the serum to those observed following the intramuscular administration of alum-adsorbed HBsAg (10 μg). Although a five times higher dose is required for the bilosomes to produce comparable IgG serum titers to that of intramuscular administration, it is noteworthy that intramuscular immunization failed to elicit immune responses in mucosal secretions. However, the bilosomes elicited measurable IgA levels in the mucosal secretions thereby establishing the ability of bilosomes to induce both systemic and mucosal immune responses [21]. Similar studies have demonstrated the elicitation of the mucosal immune response characterized by enhancement in the production of IgA antibodies with a range of antigens including tetanus toxoid [20], diphtheria toxoid [18], and A/Panama influenza haemagglutinin antigen [22]. Therefore, vaccine delivery systems such as the bilosomes can be a needle-free and painless immunization approach that could consequently increase patient compliance and vaccine coverage.
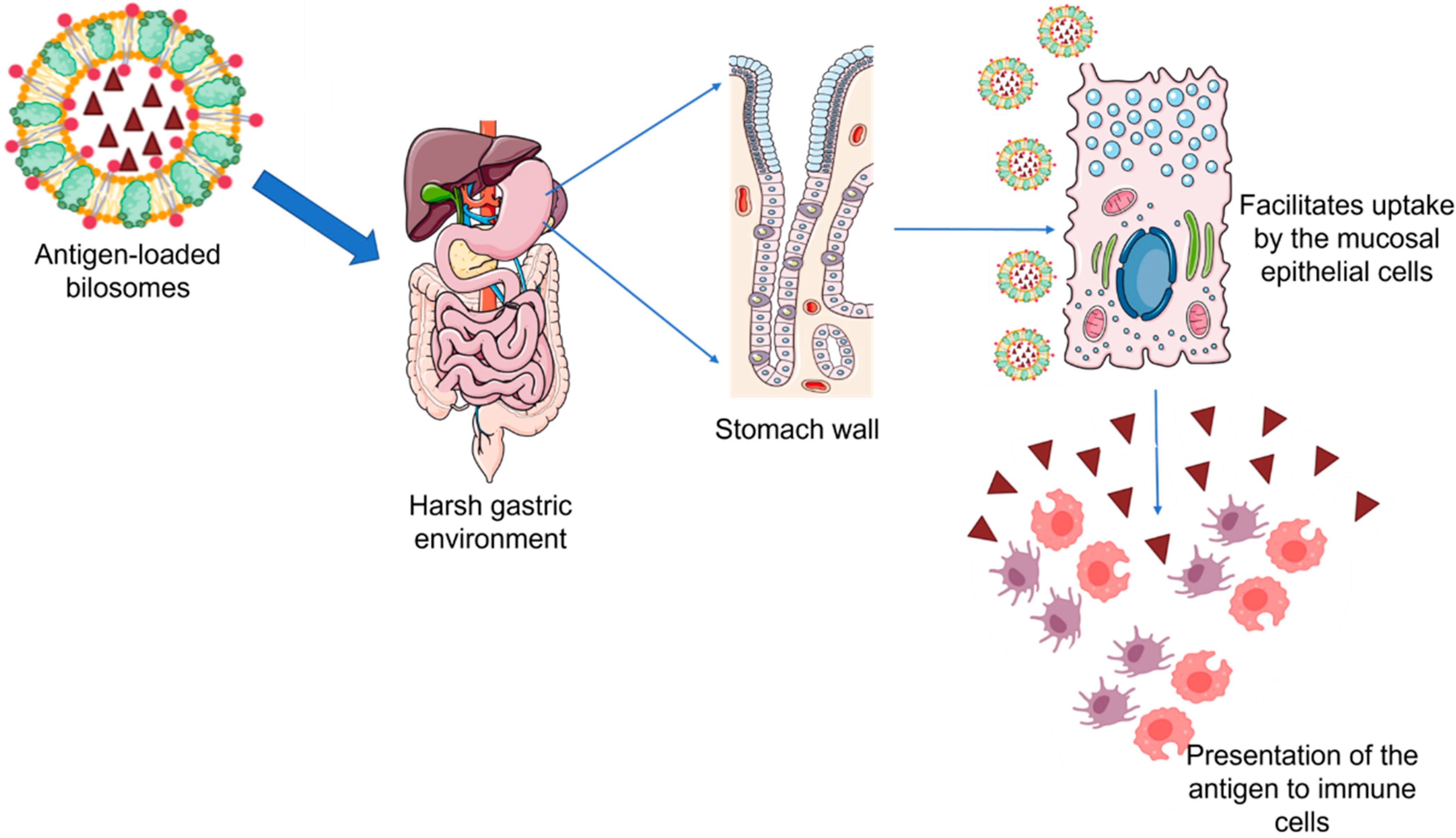
Figure 2. Antigen-loaded bilosomes escape degradation when exposed to the harsh gastric environment facilitating the uptake of antigen through the mucosal cells and its subsequent presentation to the macrophages and dendritic cells.
4. Vesosomes
The unilamellar liposomes tend to cause premature release of contents in the physiological environment due to enzymatic degradation. To overcome the aforementioned limitation, multivesicular bodies termed ‘vesosomes’ were developed by Boyer et al. that involved encapsulating unilamellar liposomes within a second bilayer for maximizing the retention of the vesicular contents. The exterior membrane serves as a physical barrier thereby protecting the contents of the internal compartment from the action of lipases. Further, upon exposure to the complex environment of the serum, a reduction in the drug release by two orders of magnitude was observed thus enhancing the circulation time of the antigen [23]. Vesosomes have been explored as effective carriers for transcutaneous immunization, a novel strategy that involves the application of adjuvanted antigens onto the hydrated bare skin. The dense distribution of antigen-presenting cells and the immunocompetent Langerhans cells in the skin enables the production of robust immune responses upon topical administration of the vaccine formulation. Mishra et al. developed novel fusogenic vesosomes for the potential delivery of tetanus toxoid through the topical route. The developed system comprised inner cationic liposomes with a lipid composition of phosphatidylcholine, dioleoyl phosphatidylethanolamine, and dioleoyl trimethyl ammonium propane in the molar ratio of 2:1:0.5 that was, in turn, encapsulated in an outer liposomal bilayer containing dipalmitoyl phosphatidylcholine and cholesterol in the ratio of 97.5:2.5. It was concluded from the study that topical immunization with the developed vesosomes leads to a significant increase in IgG levels (p < 0.05) as compared to the conventional liposomal formulations given topically. However, the method of preparation of vesosomes at the laboratory scale is complex leading to uncertainty in its industrial scalability [24].
5. pH Fusogenic Liposomes
The pH-sensitive liposomes have evolved as a promising strategy in the intracellular delivery of drugs/antigens. Liposomes of a specific composition, typically containing either a weakly acidic or weakly basic amphiphile carry a net neutral charge and are found to be stable at physiological pH (7.4). However, following cellular internalization through receptor-mediated endocytosis, the liposomes encounter acidic conditions, and thereby undergo a phase transition owing to partial protonation of the weakly acidic groups. This in turn results in the destabilization of the membranes followed by the release of the payload into the cytoplasm. Dioleoyl phosphatidylethanolamine (DOPE) and cholesteryl hemisuccinate (CHEMS) are the most commonly used lipids that confer fusogenic properties to liposomes [25]. The selection of amphiphilic stabilizers and their molar percentage with regard to the phosphatidyl ethanolamine content determine the extent of internalization, fusogenic ability, and stability in biological fluids [26]. In certain cases, the liposomes made of pH-insensitive phospholipids tend to undergo pH-dependent fusion in the presence of toxins, viral particles, or proteins. Any variation in the pH causes conformational rearrangement in the viral proteins and subsequent destabilization of the membrane [27]. The pH-sensitive liposomes are promising vehicles for the delivery of peptides enabling their use as prophylactic or therapeutic vaccines. Watarai et al. conducted a study in which liposomes were modified using a pH-sensitive polymer with fusogenic properties, named succinylated poly(glycidol), and further tested its effectiveness as an antigen carrier. The immunization of mice with ovalbumin-containing modified liposomes subsequently resulted in the induction of a significantly higher amount of ovalbumin-specific IgG3, IgG2a, and IgG1 antibody titers in comparison to the unmodified liposomal groups [28]. Chang et al. developed pH-sensitive liposomes encapsulating V3-loop peptide, a neutralizing determinant of the human immunodeficiency virus in order to stimulate virus-specific cell-mediated and humoral immune responses. V3-loop peptide contains T-cell epitopes of the HIV glycoprotein making it an important sequence for the formulation of vaccines. The study concluded that the developed system was able to elicit the production of cytotoxic T-lymphocytes (CTL) and virus-specific neutralization antibodies whereas no response was elicited with the immunogen in the absence of liposome encapsulation [29]. Lee et al. studied the immunization potential of fluorescein isothiocyanate-conjugated H-2Kb CTL epitope encapsulated in pH-sensitive liposomes. Following three days of immunization, significant activation of CTL responses induced by the delivered antigen was observed. It can be concluded from these studies that pH-sensitive liposomes act as strong peptide adjuvants making them promising carriers for the development of prophylactic and therapeutic vaccines [30]. Poly(glycidol) derivatives such as 3-methylglutarylated poly(glycidol) (MGlu-PG) and succinylated poly(glycidol) (Suc-PG) have been studied for modification of liposomes owing to their ability to confer fusogenic properties at mildly acidic pH. The liposomes modified with poly(glycidol) derivatives possess carboxyl groups in the polymeric side chain that provides a negative charge to the liposome’s surface. Therefore, such liposomes are taken up preferentially by the dendritic cells resulting in efficient activation of CTLs [31].
6. Ethosomes
Lipid-nanocarriers that are composed of a relatively high amount of ethanol, approximately 25 to 40%, are termed ethosomes. The presence of ethanol in lipid vesicular structures increases the permeation at the stratum corneum enabling transdermal delivery of drugs and antigens across the skin [32]. In a comparative study on the skin penetration properties of ethosomes with other liposomal derivatives tagged with ovalbumin peptides, it was found that a strong immune response was elicited upon delivery of the peptides by ethosomes owing to enhanced permeation into the deeper regions of the skin [33]. Zhang et al. formulated an ethosomal carbomer hydrogel containing 30% ethanol that was able to trigger a proper humoral immune response on applying to the skin of mice [34]. Raghuvanshi et al. developed a single-dose HBsAg ethosomal vaccine for administration through the nasal route. The developed formulation demonstrated a higher immunological response as compared to the alum-HBsAg vaccine. A single dose of ethosomal vaccine showed effective and measurable immunoglobulin and cytokine levels eliminating the need for a booster dose of vaccine. This serves as a proof of concept for the utilization of ethosomes as a potential alternative to conventional needle-based vaccination [35].
7. Transferosomes
Owing to structural resemblance with cellular vesicles/exocytotic vesicles, transferosomes (also referred to as ultra-deformable liposomes) have been widely investigated for the transport of active drug substances and antigens. The membrane structure of transferosomes comprises phospholipids, an edge activator, and a surfactant molecule with a single chain. The presence of edge activators such as sodium deoxycholate, Span (60, 65, 80), sodium cholate, dipotassium glycyrrhizinate, and Tween (20, 60, and 80) differentiates transferosomes from the conventional liposomes. The role of edge activators in transferosomes is to cause destabilization of the phospholipid bilayer that enhances the deformability of the nanocarrier by reducing its interfacial tension [36]. This ultra-deformability of transferosomes makes it ideal for transdermal delivery and the diffusion of antigens to the APCs, thereby making it superior in comparison to conventional liposomes [37]. However, different studies have reported contradictory results regarding the ability of transferosomes in enhancing skin permeability. For instance, delivery of the Hepatitis B surface antigen by means of a plasmid DNA-cationic transferosome complex demonstrated superior levels of humoral and cellular immune responses as compared to vaccination with conventional liposomes [38], whereas Ding et al. reported that transcutaneous immunization with transferosomes alone did not enhance immunogenicity but required skin pre-treatment with microneedles [39]. Wu and his co-workers designed transferosomes with positive and negative surface charges and integrated them with hyaluronic acid microneedles for transdermal immunization. It was observed that the cationic nano vaccines were more efficient in activating the maturation of dendritic cells and inducing Th1 immunity in comparison to their anionic counterparts as suggested by a significant increase in IgG2a/IgG1 ratio and elevated cytokine secretion from Th1 cells without an enhancement in the Th2 response [40]. This enhanced Th1 antigen-specific immune response in lymph nodes through a transdermal vaccine delivery system can be used as a potential approach for immunotherapy.
8. Immuno-Liposomes
Immunoliposomes also referred to as antibody-directed liposomes have been recognized as a potential tool for the site-specific delivery of drugs/diagnostic agents/antigens. The coupling of antibodies onto the surface of liposomes allows for active tissue targeting via binding to the receptors located on the cell surface [41]. Eskandari et al. prepared immunoliposomes by grafting non-immune mouse IgG onto the surface of liposomes. They further evaluated the effect of immunoliposomes on the intensity and type of generated immune response against Leishmania, and also compared their efficacy with conventional liposomes. The results of the study suggested that immunoliposomes induced strong cell-mediated immune responses against L. major challenge in BALB/c mice. Thus, it can be concluded that immunoliposomes can be used as a potential strategy for immunization against L. major [42]. In another study, LAG3 (lymphocyte activating gene 3-IgG) and P5 peptide were coupled into the surface of PEGylated liposomes for simultaneous co-delivery and were investigated for their therapeutic effectiveness in a mice model of TUBO breast cancer. A higher percentage of CD8+ and CD4+ T cells in the spleen followed by a pronounced and rapid infiltration of these effector cells into the tumor site were observed. These observations suggest that LAG3-Ig-P5-immunoliposomes can serve as a potential candidate for developing a highly targeted therapeutic cancer vaccine in the treatment of HER2/neu+ breast cancer [43]. A similar study was performed by Rodalec and his coworkers in which immunoliposomes made of synthetic lipids (ANC-2) and natural (antibody nanoconjugate-1 [ANC-1]) encapsulating docetaxel to treat HER2-positive breast cancers were developed. In vitro study results suggested significant antiproliferative efficacy and targeted drug delivery in breast cancer [44].
9. Lipid Nanoparticles
The recent development of lipid nanoparticle-based mRNA vaccines for combating COVID-19 has thrown a spotlight on the emerging role of these nanocarriers as potential vehicles for the delivery of a wide variety of therapeutics including antigens. Owing to some of the limitations associated with the early generation of lipid-based carriers, i.e., the liposomes, the next generation of lipid nanoparticles (LNPs) have emerged as frontiers in drug and vaccine delivery. LNPs mainly include solid–lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) [45].
SLNs are composed of solid lipids whereas NLCs are composed of mixtures of solid and liquid-crystalline lipids. In comparison to conventional liposomes, SLNs, and NLCs have considerably high loading capacities. Additionally, the reduced mobility of molecules in the solid state allows these carriers to control the release of their drug/antigen payloads more effectively. Structurally, these carriers are composed of lipids and stabilizing agents such as surfactants along with other coating materials. Typically, LNPs comprise four lipid components, namely, ionizable lipid, cholesterol, phospholipids, and PEGylated lipid [46]. Ionizable lipids are most commonly used in the preparation of LNPs owing to their nontoxicity in comparison to non-ionizable lipids. The ionizable lipids remain neutral at physiological pH and upon cellular uptake, they become positively charged owing to the acidic pH of the endosomes.
SLNs comprising cationic lipids can bind to negatively charged DNA molecules resulting in an SLN/DNA complex that has the potential to be used as a vaccine. Various studies have been conducted to explore the potential of SLNs as vaccine carriers [47]. Francis et al. developed an adjuvanted solid lipid nanoparticle (SLN-A) for use as a DNA vaccine carrier encoding the Urease alpha (UreA) antigen from Helicobacter pylori. SLNs were synthesized by a modified solvent emulsification technique and were composed of cationic lipids monophosphoryl lipid A as an adjuvant. The developed formulation was evaluated for zeta potential, morphology, and in vitro transfection capacity. In vitro study results showed that the developed formulation can be efficiently transfected in murine immune cells for expression of recombinant Helicobacter pylori antigen Urease A, thus demonstrating their potential for vaccine delivery [48]. A comparative study was performed to evaluate two vaccine delivery strategies namely electroporation and a cationic SLN formulation in the administration of a DNA vaccine harboring Leishmania donovani A2 antigen along with Leishmania infantum cysteine proteinases. Further, its potential against Leishmania infantum challenge was evaluated. The study results demonstrated that similar to the electroporation delivery system, SLNs as a nanoscale vehicle of Leishmania antigens could enhance immune response, hence indicating the effectiveness of these approaches against visceral leishmaniasis [49].
Gerhardt and his colleagues developed thermostable and lyophilizable NLCs for the delivery of replicating RNA-based vaccines administered through the intramuscular route. The developed NLCs were composed of liquid lipids (squalene) and solid lipids (trimyristin) surrounded by surfactants (sorbitan monostearate and polysorbate 80) and a cationic lipid (1,2-dioleoyl-3-trimethylammonium-propane) [DOTAP]. The system was complexed with mRNA and was able to retain stability at room temperature for around 8 months and at refrigerated temperature for around 21 months [50]. A live-attenuated RNA hybrid vaccine composed of an in vitro transcribed and highly attenuated CHIKV genome encapsulated in a stable NLC formulation has been developed by Voigt and his co-workers [51]. In another study, Kaur et al. prepared NLC from didodecyldimethylammonium bromide (DDAB), oleic acid and co-encapsulated a Pam 2 CS derivative (T-2, TLR2 agonist) with an imidazoquinoline derivative (T-7, TLR7 agonist) as a combination vaccine adjuvant. The developed formulation exhibited high TLR7 and TLR2 agonistic activity. The adjuvant potency of this system was also evaluated in mice with influenza hemagglutinin protein and recombinant hepatitis B surface antigen. The study results demonstrated that NLCs containing T-2 and T-7 induced good efficacy in mice challenged with a lethal dose of influenza virus [52].
References
- Marasini, N.; Ghaffar, K.A. Liposomes as a Vaccine Delivery System. In Micro and Nanotechnology in Vaccine Development, 1st ed.; Skwarczynski, M., Toth, I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 221–239.
- Mazur, F.; Bally, M. Liposomes and lipid bilayers in biosensors. Adv. Colloid Interface Sci. 2017, 249, 88–99.
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134.
- Bulbake, U.; Doppalapudi, S. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12.
- Mirchandani, Y.; Patravale, V.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control. Release 2021, 335, 457–464.
- Bendas, G.; Wilhelm, F.; Ritcher, W.; Peter, N. Synthetic glycolipids as membrane-bound cryoprotectants in the freeze-drying process of liposomes. Eur. J. Pharm. Sci. 1996, 4, 211–222.
- Trubetskoy, V.; Torchilin, V. Use of polyoxyethylene-lipid conjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv. Drug Deliv. Rev. 1995, 16, 311–320.
- Hamilton, B.S.; Whittaker, G.R. Influenza virus-mediated membrane fusion: Determinants of hemagglutinin fusogenic activity and experimental approaches for assessing virus fusion. Viruses 2012, 4, 1144–1168.
- Air, G.M.; Laver, W.G. The neuraminidase of influenza virus. Proteins Struct. Funct. Bioinform. 1989, 6, 341–356.
- Almeida, J.D.; Edwards, D.C. Formation of Virosomes From Influenza Subunits and Liposomes. Lancet 1975, 2, 899–901.
- Bovier, P.A. Epaxal®: A virosomal vaccine to prevent hepatitis A infection. Expert Rev. Vaccines 2008, 7, 1141–1150.
- Hatz, C.; Van Der Ploeg, R. Successful memory response following a booster dose with a virosome-formulated hepatitis A vaccine delayed up to 11 years. Clin. Vaccine Immunol. 2011, 18, 885–887.
- Herzog, C.; Hartmann, K. Eleven years of Inflexal® V-a virosomal adjuvanted influenza vaccine. Vaccine 2009, 27, 4381–4387.
- Cox, R.J.; Pedersen, G. Evaluation of a virosomal H5N1 vaccine formulated with Matrix MTM adjuvant in a phase I clinical trial. Vaccine 2011, 29, 8049–8059.
- Metcalfe, I.C.; Gluck, R. Virosomes for vaccine delivery. In Immunopotentiators in Modern Vaccines; Reinhard, G., Virgil, S., Eds.; Academic Press: London, UK, 2006; pp. 179–189.
- Shukla, A.; Mishra, V. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899.
- Shukla, A.; Katare, O.P. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int. J. Pharm. 2010, 385, 47–52.
- Shukla, A.; Singh, B. Significant systemic and mucosal immune response induced on oral delivery of diphtheria toxoid using nano-bilosomes. Br. J. Pharmacol. 2011, 164, 820–827.
- Premanand, B.; Prabakaran, M. Recombinant Baculovirus Associated with Bilosomes as an Oral Vaccine Candidate against HEV71 Infection in Mice. PLoS ONE 2013, 8, e55536.
- Mann, J.F.S.; Scales, H.E. Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods 2006, 38, 90–95.
- Shukla, A.; Khatri, K. Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). J. Pharm. Pharm. Sci. 2008, 11, 59–66.
- Mann, J.F.S.; Ferro, V.A. Optimisation of a lipid based oral delivery system containing A/Panama influenza haemagglutinin. Vaccine 2004, 22, 2425–2429.
- Boyer, C.; Zasadzinski, J.A. Multiple lipid compartments slow vesicle contents release in lipases and serum. ACS Nano 2007, 1, 176–182.
- Mishra, V.; Mahor, S. Development of novel fusogenic vesosomes for transcutaneous immunization. Vaccine 2006, 24, 5559–5570.
- Shi, G.; Guo, W. Efficient intracellular drug and gene delivery using folate receptor-targeted pH-sensitive liposomes composed of cationic/anionic lipid combinations. J. Control. Release 2002, 80, 309–319.
- Simões, S.; Nuno Moreira, J. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965.
- Torchilin, V.P.; Zhou, F. pH-sensitive liposomes. J. Liposome Res. 1993, 3, 201–255.
- Watarai, S.; Iwase, T. Efficiency of pH-sensitive fusogenic polymer-modified liposomes as a vaccine carrier. Sci. World J. 2013, 2013, 903234.
- Chang, J.S.; Choi, M.J. Immunogenicity of synthetic HIV-1 V3 loop peptides by MPL adjuvanted pH-sensitive liposomes. Vaccine 1999, 17, 1540–1548.
- Lee, K.Y.; Chun, E. Investigation of antigen delivery route in vivo and immune-boosting effects mediated by pH-sensitive liposomes encapsulated with Kb-restricted CTL epitope. Biochem. Biophys. Res. Commun. 2002, 292, 682–688.
- Yuba, E.; Kojima, C. pH-Sensitive fusogenic polymer-modified liposomes as a carrier of antigenic proteins for activation of cellular immunity. Biomaterials 2010, 31, 943–951.
- Touitou, E.; Dayan, N. Ethosomes-Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418.
- Rattanapak, T.; Young, K. Comparative study of liposomes, transfersomes, ethosomes and cubosomes for transcutaneous immunisation: Characterisation and in vitro skin penetration. J. Pharm. Pharmacol. 2012, 64, 1560–1569.
- Zhang, Y.; Ng, W. Formulation and in vitro stability evaluation of ethosomal carbomer hydrogel for transdermal vaccine delivery. Colloids Surf. B Biointerfaces 2018, 163, 184–191.
- Raghuvanshi, A.; Shah, K. Ethosome as antigen delivery carrier: Optimisation, evaluation and induction of immunological response via nasal route against hepatitis B. J. Microencapsul. 2022, 39, 352–363.
- Mirtaleb, M.S.; Shahraky, M.K. Advances in biological nano-phospholipid vesicles for transdermal delivery: A review on applications. J. Drug Deliv. Sci. Technol. 2021, 61, 102331.
- Pielenhofer, J.; Sohl, J. Current Progress in Particle-Based Systems for Transdermal Vaccine Delivery. Front. Immunol. 2020, 11, 266.
- Wang, J.; Hu, J.H. Strong cellular and humoral immune responses induced by transcutaneous immunization with HBsAg DNA-cationic deformable liposome complex. Exp. Dermatol. 2007, 16, 724–729.
- Ding, Z.; Bal, S.M. Transcutaneous immunization studies in mice using diphtheria toxoid-loaded vesicle formulations and a microneedle array. Pharm. Res. 2011, 28, 145–158.
- Wu, X.; Li, Y. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B 2019, 7, 4854–4866.
- Torchilin, V.P. Immunoliposomes. Drug Deliv. Oncol. From Basic Res. Cancer Ther. 2011, 2, 951–987.
- Eskandari, F.; Talesh, G.A. Immunoliposomes containing Soluble Leishmania Antigens (SLA) as a novel antigen delivery system in murine model of leishmaniasis. Exp. Parasitol. 2014, 146, 78–86.
- Mohammadian Haftcheshmeh, S.; Zamani, P. Immunoliposomes bearing lymphocyte activation gene 3 fusion protein and P5 peptide: A novel vaccine for breast cancer. Biotechnol. Prog. 2021, 37, e3095.
- Rodallec, A.; Brunel, J.M. Docetaxel–trastuzumab stealth immunoliposome: Development and in vitro proof of concept studies in breast cancer. Int. J. Nanomed. 2018, 13, 3451–3465.
- Tenchov, R.; Bird, R. Lipid Nanoparticles from Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015.
- Letao, X.; Xing, W. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109.
- Francis, J.E.; Skakic, I. Design and Preparation of Solid Lipid Nanoparticle (SLN)-Mediated DNA Vaccines. Methods Mol. Biol. 2022, 2412, 355–366.
- Francis, J.E.; Skakic, I. Solid lipid nanoparticle carrier platform containing synthetic TLR4 agonist mediates non-viral DNA vaccine delivery. Vaccines 2020, 8, 551.
- Saljoughian, N.; Zahedifard, F. Cationic solid-lipid nanoparticles are as efficient as electroporation in DNA vaccination against visceral leishmaniasis in mice. Parasite Immunol. 2013, 35, 397–408.
- Gerhardt, A.; Voigt, E. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022, 25, 205–214.
- Voigt, E.A.; Fuerte-Stone, J. Live-attenuated RNA hybrid vaccine technology provides single-dose protection against Chikungunya virus. Mol. Ther. 2021, 29, 2782–2793.
- Kaur, A.; Kanwar, R. Combined delivery of TLR2 and TLR7 agonists by Nanostructured lipid carriers induces potent vaccine adjuvant activity in mice. Int. J. Pharm. 2022, 613, 121378.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
31 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




