| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Arihant Ahuja | -- | 2186 | 2023-03-29 20:27:21 | | | |
| 2 | Arihant Ahuja | Meta information modification | 2186 | 2023-03-29 20:29:39 | | | | |
| 3 | Jessie Wu | Meta information modification | 2186 | 2023-03-30 05:30:09 | | | | |
| 4 | Jessie Wu | Meta information modification | 2186 | 2023-04-06 08:35:19 | | | | |
| 5 | Jessie Wu | Meta information modification | 2186 | 2023-04-06 08:36:06 | | | | |
| 6 | Jessie Wu | -8 word(s) | 2178 | 2023-04-06 09:30:30 | | | | |
| 7 | Jason Zhu | Meta information modification | 2178 | 2023-04-21 08:07:58 | | |
Video Upload Options
Shellac, an insect-derived material, has received the least attention due to its scarcity in south Asia. Currently, Shellac is used in various applications, such as furniture polish, glazing agent for candies and pharmaceutical pills, coating on fruits to increase shelf life, primers, smart sensor, 3D printing, and green electronic. However, the limitations of Shellac such as: brittleness with time, self-esterification, low transparency, solubility in alkaline medium and in most organic solvents have limited its usage in the packaging application. Many of these problems can be improved by physical blending or chemical reaction with other materials to make Shellac more durable, impede self-esterification, and facilitate the film-forming ability, which suggests the potential usage of Shellac in packaging applications.
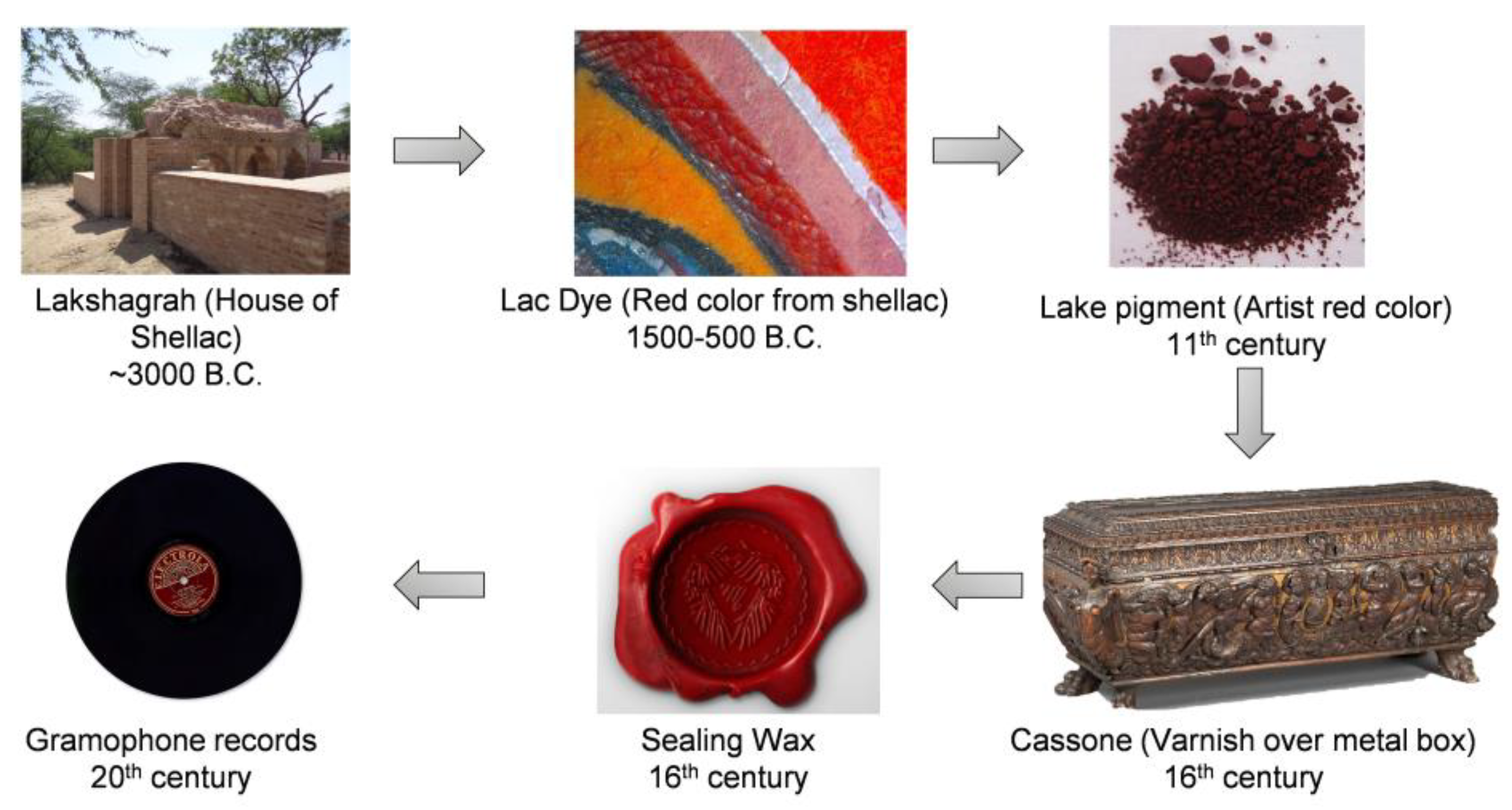
1. History of Shellac
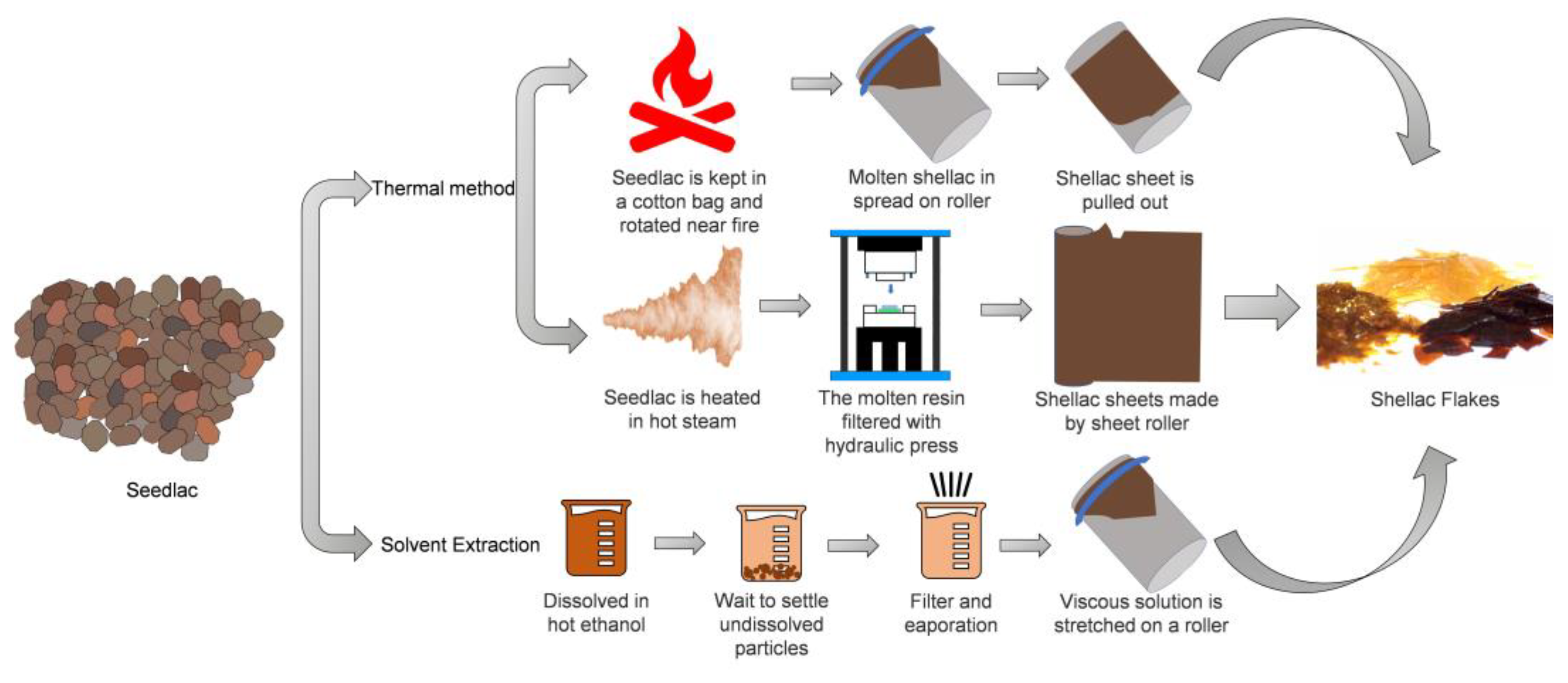
2. Source, Extraction, and Production of Shellac
2.1. Production of Resin by Lac Bug on the Tree (Sticklac)
2.2. Processing of Sticklac to Seedlac
2.3. Production of Shellac by Purification of Seedlac
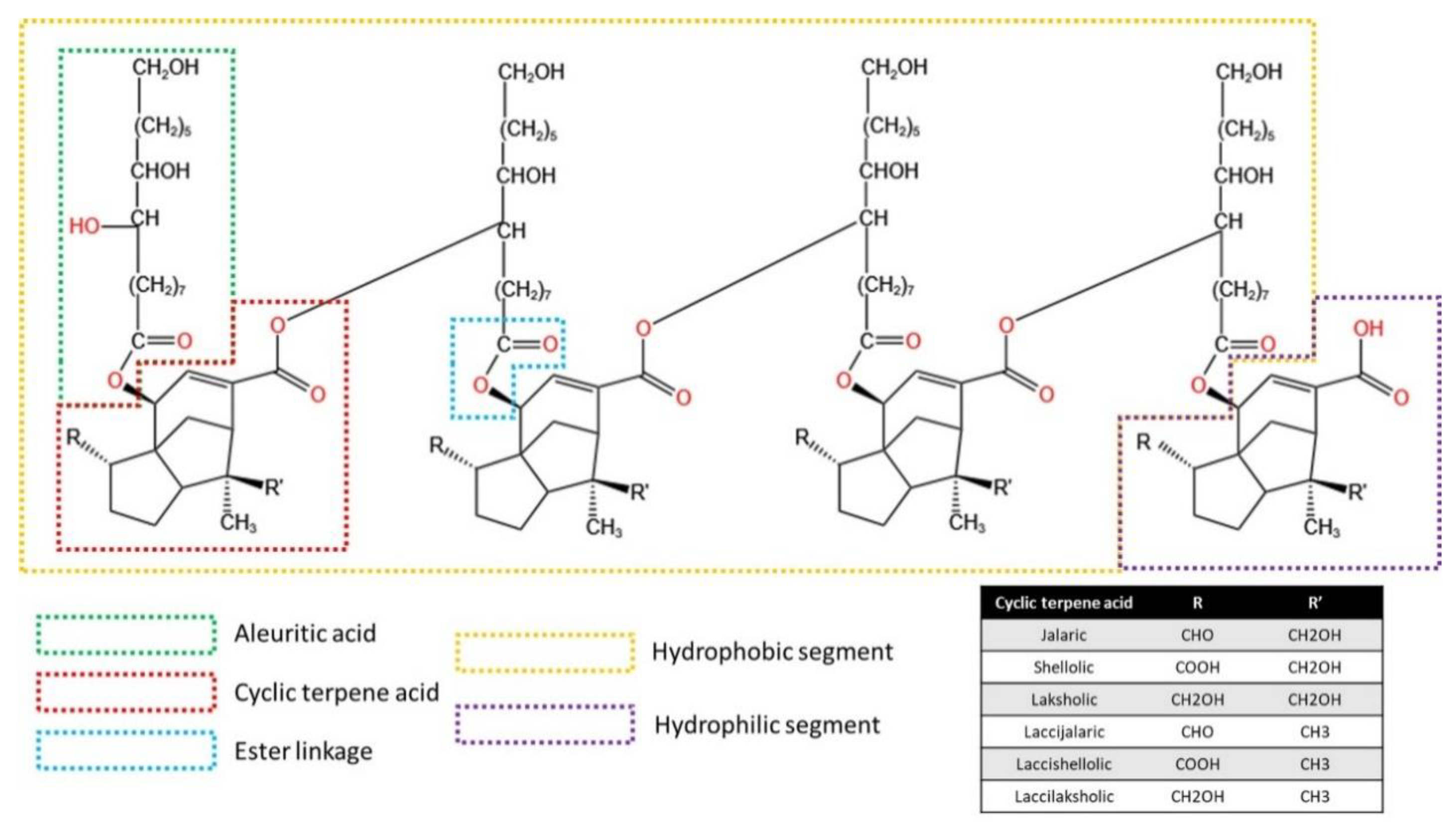
3. Structural Composition and Structure of Shellac
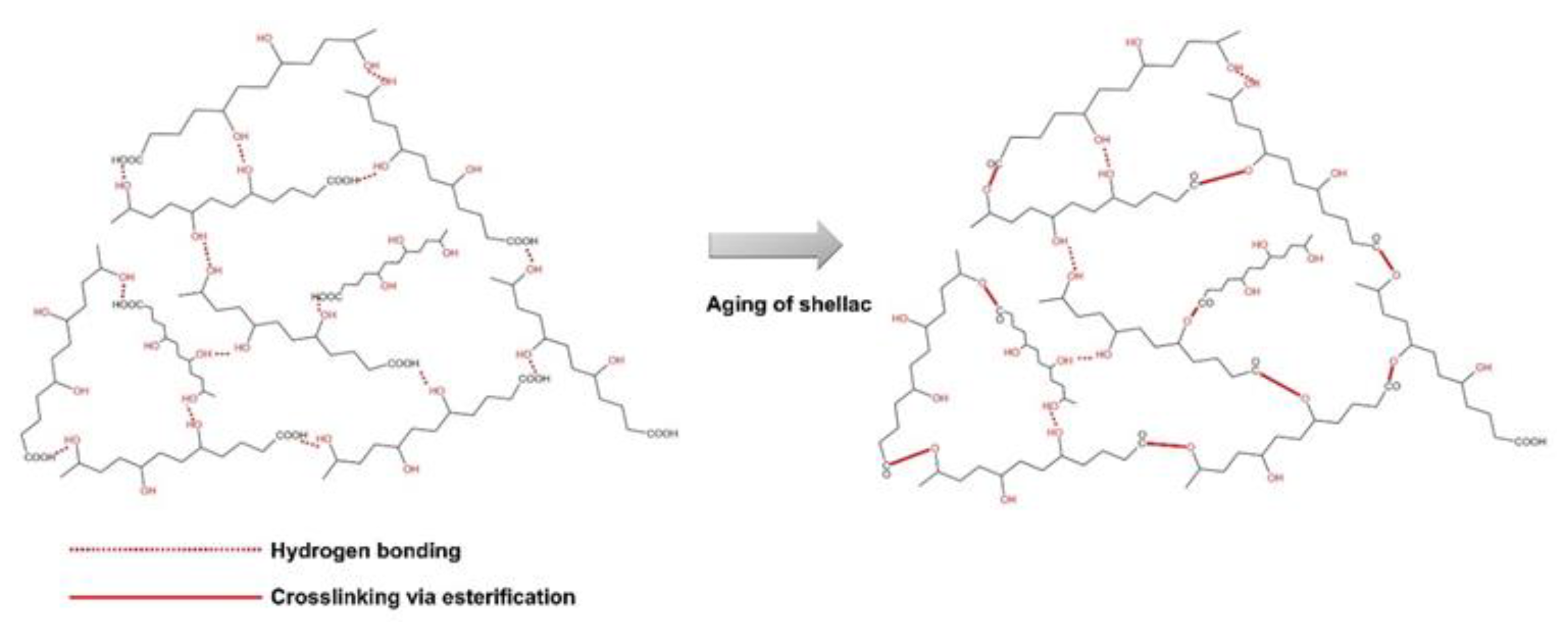
4. Physicochemical Properties of Shellac
5. Potential in the Packaging Application
| Properties | Shellac | PE | PP | PET | PVC | PCL | PLA | P3HB4HB |
|---|---|---|---|---|---|---|---|---|
| Melting Point (°C) | 75–90 [16] |
115–135 [35][36] | 170 [35] |
245–255 [35][36] |
210 [36] |
68 [37] |
155–165 [37] |
167.59 [38] |
| Tensile Strength (MPa) | 5.7–14 [39] |
7–25 [40] |
27–98 [40] |
157–177 [40] |
42–55 [40] |
38.3 [41] |
37.6 [42] |
87.4 [38] |
| Elongation (%) | 3.05 [43] |
300–900 [40] |
200–1000 [40] |
70 [40] |
20–180 [40] |
839.2 [41] |
59.2 [42] |
28.1 [38] |
| Water Contact Angle (°) | 88.07 [44] |
88 [45] |
88 [45] |
76 [45] |
90 [45] |
80 [41] |
65.2 [42] |
64.7 [38] |
| WVP × 1014 (gm-m/m2-s-Pa) | 834–1150 [46][47][48] | 6.673–8.704 [40] | 201–401 [40] | 501–1980 [40] | 18.279 [40] |
1680 [41] |
4820 [42] |
359 [38] |
| Properties | Shellac | Starch | Gelatin | Pectin | Chitosan | Guar Gum |
|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | 5.7–14 [39] |
2.4 [49] |
57.16 [50] |
3.63 [51] |
14.95 [52] |
18.01 [53] |
| Elongation (%) | 3.05 [43] |
50 [49] |
2.96 [50] |
43.77 [51] |
8.26 [52] |
31.58 [53] |
| Water Contact Angle (°) | 88.07 [44] |
23.18–66.91 [54] | 72 [50] |
31.69 [51] |
52.5 [55] |
N.A. |
| WVP × 1014 (gm-m/m2-s-Pa) | 834–1150 [46][47][48] |
23,000–35,000 [49] | 8890 [50] |
55,300 [51] |
63,400 [56] |
386,100 [57] |
References
- Patel, A.R.; Rajarethinem, P.S.; Grędowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible Applications of Shellac Oleogels: Spreads, Chocolate Paste and Cakes. Food Funct. 2014, 5, 645–652.
- Chellappan, K. Multi-Disciplinary Study Confirms Mahabharat True Chronicle of History. Available online: https://www.dailypioneer.com/2022/india/multi-disciplinary-study-confirms-mahabharat-true-chronicle-of-history.html (accessed on 18 September 2022).
- Sharma, K.K. File:Laakshagrah1.Jpg. Available online: https://commons.wikimedia.org/wiki/File:Laakshagrah1.jpg (accessed on 31 January 2023).
- Berbers, S.V.J.; Tamburini, D.; van Bommel, M.R.; Dyer, J. Historical Formulations of Lake Pigments and Dyes Derived from Lac: A Study of Compositional Variability. Dye. Pigment. 2019, 170, 107579.
- Merrifield, M.P. Medieval and Renaissance Treatises on the Arts of Painting; Courier Dover Publications: New York, NY, USA, 1999; ISBN 9780486404400.
- Derrick, M.R.; Stulik, D.C.; Landry, J.M.; Bouffard, S.P. Furniture Finish Layer Identification by Infrared Linear Mapping Microspectroscopy. J. Am. Inst. Conserv. 1992, 31, 225–236.
- Nolley, S. Sealing Wax–Conservation DistList. Available online: https://cool.culturalheritage.org/byform/mailing-lists/cdl/1998/1019.html (accessed on 18 September 2022).
- Rehding, A. On the Record. Cambridge Opera J. 2006, 18, 59–82.
- Nabais, P.; Melo, M.J.; Lopes, J.A.; Vieira, M.; Castro, R.; Romani, A. Organic Colorants Based on Lac Dye and Brazilwood as Markers for a Chronology and Geography of Medieval Scriptoria: A Chemometrics Approach. Herit. Sci. 2021, 9, 32.
- Mediatus File:Lilli Marlene 1939.Jpg. Available online: https://commons.wikimedia.org/wiki/File:Lilli_Marlene_1939.jpg (accessed on 31 January 2023).
- Nelika, G. File:Waxseal.Png. Available online: https://commons.wikimedia.org/wiki/File:Waxseal.png (accessed on 31 January 2023).
- Metropolitan Museum of Art File: Cassone MET SLP1939-1.Jpg. Available online: https://commons.wikimedia.org/wiki/File:Cassone_MET_SLP1939-1.jpg (accessed on 31 January 2023).
- Jimtaisong, A. Aluminium and Calcium Lake Pigments of Lac Natural Dye. Brazilian J. Pharm. Sci. 2020, 56, 1–9.
- Ahmad, A.; Kaushik, S.; Ramamurthy, V.V.; Lakhanpaul, S.; Ramani, R.; Sharma, K.K.; Vidyarthi, A.S. Mouthparts and Stylet Penetration of the Lac Insect Kerria Lacca (Kerr) (Hemiptera: Tachardiidae). Arthropod. Struct. Dev. 2012, 41, 435–441.
- Cambridge Dictionary Shellac. Available online: https://dictionary.cambridge.org/dictionary/english/shellac (accessed on 18 September 2022).
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a Multifunctional Biopolymer: A Review on Properties, Applications and Future Potential. Int. J. Biol. Macromol. 2022, 215, 203–223.
- Berenbaum, M.R. Ninety-Nine More Maggots, Mites, and Munchers; University of Illinois Press: Champaign, IL, USA, 1993.
- Sharma, K.K.; Chowdhury, A.R.; Srivastava, S. Chemistry and Applications of Lac and Its By-Product. In Natural Materials and Products from Insects: Chemistry and Applications; Springer International Publishing: Cham, Switzerland, 2020; pp. 21–37. ISBN 9783030366100.
- Pal, G. Impact of Scientific Lac Cultivation Training on Lac Economy—A Study in Jharkhand. Agric. Econ. Res. Rev. 2009, 22, 139–144.
- Mohanta, J.; Dey, D.G.; Mohanty, N. Performance of Lac Insect, Kerria Lacca Kerr In Conventional And Non-Conventional Cultivation around Similipal Biosphere Reserve, Odisha, India. Bioscan 2012, 7, 237–240.
- Derry, J. Investigating Shellac: Documenting the Process, Defining the Product: A Study on the Processing Methods of Shellac, and the Analysis of Selected Physical and Chemical Characteristics. Master’s Thesis, University of Oslo, Oslo, Norway, 2012.
- Eugster, S.A. File:Shellac Three Colours.Jpeg. Available online: https://commons.wikimedia.org/wiki/File:Shellac_three_colours.jpeg (accessed on 31 January 2023).
- Sharma, S.K.; Shukla, S.K.; Vaid, D.N. Shellac-Structure, Characteristics & Modification. Def. Sci. J. 1983, 33, 261–271.
- Farag, Y.; Leopold, C.S. Physicochemical Properties of Various Shellac Types. Dissolution Technol. 2009, 16, 33–39.
- Farag, Y. Characterization of Different Shellac Types and Development of Shellac-Coated Dosage Forms. Ph.D. Thesis, Der Fakultät für Mathematik, Informatik und Naturwissenschaften der Universität, Hamburg, Germany, 2010.
- Khairuddin; Pramono, E.; Utomo, S.B.; Wulandari, V.; A’an Zahrotul, W.; Clegg, F. The Effect of Polyethylene Glycol on Shellac Stability. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012066.
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Development of Biocomposite Films Incorporated with Different Amounts of Shellac, Emulsifier, and Surfactant. Food Hydrocoll. 2017, 72, 174–184.
- Du, Y.; Wang, L.; Mu, R.; Wang, Y.; Li, Y.; Wu, D.; Wu, C.; Pang, J. Fabrication of Novel Konjac Glucomannan/Shellac Film with Advanced Functions for Food Packaging. Int. J. Biol. Macromol. 2019, 131, 36–42.
- Mohamed, S.A.A.; El-Sakhawy, M.; Nashy, E.-S.H.A.; Othman, A.M. Novel Natural Composite Films as Packaging Materials with Enhanced Properties. Int. J. Biol. Macromol. 2019, 136, 774–784.
- Zhang, Y.; Li, T.; Zhang, H.; Zhang, H.; Chi, Y.; Zhao, X.; Li, H.; Wen, Y. Blending with Shellac to Improve Water Resistance of Soybean Protein Isolate Film. J. Food Process Eng. 2020, 43, e13515.
- Licciardello, F.; Wittenauer, J.; Saengerlaub, S.; Reinelt, M.; Stramm, C. Rapid Assessment of the Effectiveness of Antioxidant Active Packaging—Study with Grape Pomace and Olive Leaf Extracts. Food Packag. Shelf Life 2015, 6, 1–6.
- Song, G.; Sun, R.; Li, H.; Zhang, H.; Xia, N.; Guo, P.; Jiang, L.W.; Zhang, X.; Rayan, A.M. Effects of Pine Needle Essential Oil Combined with Chitosan Shellac on Physical and Antibacterial Properties of Emulsions for Egg Preservation. Food Biophys. 2022, 17, 260–272.
- Li, Y.; Dong, Q.; Chen, J.; Li, L. Effects of Coaxial Electrospun Eugenol Loaded Core-Sheath PVP/Shellac Fibrous Films on Postharvest Quality and Shelf Life of Strawberries. Postharvest Biol. Technol. 2020, 159, 111028.
- Ariyanto, H.D.; Chiba, M.; Oguma, K.; Tatsuki, M.; Yoshii, H. Release Behavior of 1-Methylcylopropene Coated Paper-Based Shellac Solution in Response to Stepwise Humidity Changes to Develop Novel Functional Packaging for Fruit. Packag. Technol. Sci. 2019, 32, 523–533.
- Kazemi Najafi, S. Use of Recycled Plastics in Wood Plastic Composites—A Review. Waste Manag. 2013, 33, 1898–1905.
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of Bio-Based Plastics on Current Recycling of Plastics. Sustainability 2018, 10, 1487.
- Maraveas, C. Production of Sustainable and Biodegradable Polymers from Agricultural Waste. Polymers 2020, 12, 1127.
- Sharma, P.; Ahuja, A.; Dilsad Izrayeel, A.M.; Samyn, P.; Rastogi, V.K. Physicochemical and Thermal Characterization of Poly (3-Hydroxybutyrate-Co-4-Hydroxybutyrate) Films Incorporating Thyme Essential Oil for Active Packaging of White Bread. Food Control 2022, 133, 108688.
- Arnautov, A.; Korhov, V.; Faitelson, E. Physicomechanical Properties of Shellac Films Grafted by Using Ultraviolet Irradiation. Mech. Compos. Mater. 2013, 49, 163–170.
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93.
- Ding, Y.; Han, A.; Zhou, H.; Zhou, Q.; Song, H.; Chen, R.; Guo, S. Preparation of Poly(ε-Caprolactone) Based Composites through Multistage Biaxial-Stretching Extrusion with Excellent Oxygen and Water Vapor Barrier Performance. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106494.
- Rhim, J.-W. Preparation and Characterization of Vacuum Sputter Silver Coated PLA Film. LWT—Food Sci. Technol. 2013, 54, 477–484.
- Qussi, B.; Suess, W.G. The Influence of Different Plasticizers and Polymers on the Mechanical and Thermal Properties, Porosity and Drug Permeability of Free Shellac Films. Drug Dev. Ind. Pharm. 2006, 32, 403–412.
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Luangtana-Anan, M.; Terada, K.; Yonemochi, E.; Yoshihashi, Y. Determination of Surface Free Energy and Contact Angle for Hydrolyzed Shellac. Adv. Mater. Res. 2012, 506, 270–273.
- Hild, F. Surface Energy of Plastics. Available online: https://www.tstar.com/blog/bid/33845/surface-energy-of-plastics (accessed on 28 September 2022).
- Luangtana-anan, M.; Nunthanid, J.; Limmatvapirat, S. Effect of Molecular Weight and Concentration of Polyethylene Glycol on Physicochemical Properties and Stability of Shellac Film. J. Agric. Food Chem. 2010, 58, 12934–12940.
- Soradech, S.; Limatvapirat, S.; Luangtana-anan, M. Stability Enhancement of Shellac by Formation of Composite Film: Effect of Gelatin and Plasticizers. J. Food Eng. 2013, 116, 572–580.
- Luangtana-anan, M.; Saengsod, S.; Limmatvapirat, S. Improvement of Bleached Shellac as Enteric Coating by Composite Formation. AAPS PharmSciTech 2021, 22, 241.
- Belibi, P.C.; Daou, T.J.; Ndjaka, J.-M.B.; Michelin, L.; Brendlé, J.; Nsom, B.; Durand, B. Tensile and Water Barrier Properties of Cassava Starch Composite Films Reinforced by Synthetic Zeolite and Beidellite. J. Food Eng. 2013, 115, 339–346.
- Saranti, T.F.d.S.; Melo, P.T.S.; Cerqueira, M.A.; Aouada, F.A.; de Moura, M.R. Performance of Gelatin Films Reinforced with Cloisite Na+ and Black Pepper Essential Oil Loaded Nanoemulsion. Polymers 2021, 13, 4298.
- Younis, H.G.R.; Zhao, G. Physicochemical Properties of the Edible Films from the Blends of High Methoxyl Apple Pectin and Chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066.
- Kumar, H.; Ahuja, A.; Kadam, A.A.; Rastogi, V.K.; Negi, Y.S. Antioxidant Film Based on Chitosan and Tulsi Essential Oil for Food Packaging. Food Bioprocess Technol. 2023, 16, 342–355.
- Tripathi, J.; Ambolikar, R.; Gupta, S.; Jain, D.; Bahadur, J.; Variyar, P.S. Methylation of Guar Gum for Improving Mechanical and Barrier Properties of Biodegradable Packaging Films. Sci. Rep. 2019, 9, 14505.
- Żołek-Tryznowska, Z.; Kałuża, A. The Influence of Starch Origin on the Properties of Starch Films: Packaging Performance. Materials 2021, 14, 1146.
- Naidoo, S.; Nomadolo, N.; Matshe, W.M.R.; Cele, Z.; Balogun, M. Exploring the Potential of N-Acylated Chitosan for the Removal of Toxic Pollutants from Wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2019, 655, 012047.
- Kirtil, E.; Aydogdu, A.; Svitova, T.; Radke, C.J. Assessment of the Performance of Several Novel Approaches to Improve Physical Properties of Guar Gum Based Biopolymer Films. Food Packag. Shelf Life 2021, 29, 100687.
- Priyadarshi, R.; Sauraj; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan Films Incorporated with Apricot (Prunus Armeniaca) Kernel Essential Oil as Active Food Packaging Material. Food Hydrocoll. 2018, 85, 158–166.