Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jordi H. Badia | -- | 6158 | 2023-03-29 16:37:57 | | | |
| 2 | Catherine Yang | -3 word(s) | 6155 | 2023-03-30 04:16:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Badia, J.H.; Soto, R.; Ramírez, E.; Bringué, R.; Fité, C.; Iborra, M.; Tejero, J. Ion-Exchange Resins Application to Hydrogenation Reactions. Encyclopedia. Available online: https://encyclopedia.pub/entry/42618 (accessed on 07 February 2026).
Badia JH, Soto R, Ramírez E, Bringué R, Fité C, Iborra M, et al. Ion-Exchange Resins Application to Hydrogenation Reactions. Encyclopedia. Available at: https://encyclopedia.pub/entry/42618. Accessed February 07, 2026.
Badia, Jordi Hug, Rodrigo Soto, Eliana Ramírez, Roger Bringué, Carles Fité, Montserrat Iborra, Javier Tejero. "Ion-Exchange Resins Application to Hydrogenation Reactions" Encyclopedia, https://encyclopedia.pub/entry/42618 (accessed February 07, 2026).
Badia, J.H., Soto, R., Ramírez, E., Bringué, R., Fité, C., Iborra, M., & Tejero, J. (2023, March 29). Ion-Exchange Resins Application to Hydrogenation Reactions. In Encyclopedia. https://encyclopedia.pub/entry/42618
Badia, Jordi Hug, et al. "Ion-Exchange Resins Application to Hydrogenation Reactions." Encyclopedia. Web. 29 March, 2023.
Copy Citation
The role of ion-exchange resins (IERs) as catalysts or catalysts supports, in hydrogenation reactions is revised and their potential application is presented. Both gel-type and macroreticular, basic or acid, IERs have been used for manifold metal-catalyzed hydrogenation processes in gas and liquid phase, including hydrogenation of alkenes, alkynes, carbonyls, arenes, nitroaromatics, and more. Hydrogenation can be defined as the incorporation of hydrogen atoms, either from gaseous H2 or from other sources, to unsaturated compounds, that is compounds with double (C═C) or triple (C≡C) bonds, including those with carbonyl (C═O) or nitrile (C≡N) groups. The application of metal-doped IERs in hydrogenation reactions has been the focus of continuous attention for the research community with many relevant works published in the field.
ion-exchange resin
gel-type
macroreticular
hydrogenation
one-pot process
bifunctional catalyst
1. Hydrogenation of Alkenes
Four Pd-doped (0.25–0.45 wt.%), sulfonated, synthetic IERs were tested in the liquid-phase hydrogenation of cyclohexene to cyclohexane and cyclohexen-1-one to cyclohexanone by Zecca and coworkers [1]. All resins were exchanged with palladium acetate, and PdII was reduced to Pd0 with NaBH4 in ethanol. Depending on their composition, the solvent affinities of the resins spanned from moderately hydrophobic to clearly hydrophilic—the lower the ST amount in the resin, the higher its hydrophilicity. No effect of the hydrophobic/hydrophilic character of the resin was observed on the reported catalytic activity. Interestingly, resins with more nitrogen atoms in their structures (i.e., those based on N,N′-methylene(bis)acrylamide and N,N-dimethylacrylamide) were found to be the most active ones, with rates increasing linearly with the nitrogen/Pd molar ratio, and no metal leaching was observed in any case.
The role of the gel-type IERs properties in the hydrogenation of the C═C bonds of maleic and fumaric acids, viz. the cis and trans isomers of butenedioic acid, was investigated in Drelinkiewicz et al. (2008) [2]. They synthesized Pd-doped (0.25–2.0 wt.%, 2–3 nm) acrylic resins with crosslinking degrees of 3 and 10%. The observed swelling of the polymer mass during the hydrogenation was linked to the catalytic activity: the same hydrogenation rates were observed for both maleic and fumaric acids in the absence of swelling, while the hydrogenation rate of maleic acid was much higher than that of fumaric acid in the swollen state. Interestingly, a “bell-like” dependence between initial rates and metal content was found, with maximum activity at 0.5–1.0 wt.% Pd content.
Among the reactions tested in Moreno Marrodan et al. (2012), hydrogenation of the C═C bond in methyl 2-acetamidoacrylate and in trans-4-phenyl-3-buten-2-one was studied over Pd-doped, gel-type, cationic and anionic resins [3]. Reported conversions were 91.7% and 98.2%, respectively, with selectivities of 100% and 83.7%, over lithiated Pd/DowexTM 50W × 2 resin without any separated pre-reduction step of the ionic PdII to Pd0. Remarkably, neither activity loss upon recycling of the catalyst nor Pd leaching was observed and selectivity was unaffected after 6 cycles using methanol as the reaction solvent.
Madureira and associates hydrogenated fatty acid methyl esters (i.e., methyl undecenoate, methyl oleate, methyl ricinoleate, and methyl linoleate) and vegetable oil-based compounds (i.e., glycerol trioleate, castor oil, jojoba oil, olive oil, and very high-oleic sunflower oil) over Ru/DowexTM 50W × 2 under mild conditions (30 °C and 10 bar H2) [4]. Regarding the metal impregnation procedure (consisting of ion exchange with an aqueous metal salt solution followed by NaBH4 reduction), the authors indicate that the resin must be in the Na+ form to improve metalation yield and to avoid Ru leaching due to the presence of acidic species. According to their results, the catalytic activity of the catalysts was higher for shorter hydrocarbon chain length: hydrogenation of methyl undecenoate (C12) was faster than methyl linoleate (C19), showing 100% conversion in 60 min in front of about 50% conversion in 210 min, respectively. Moreover, the double bond in the larger unsaturated esters was located in an internal position, which hindered the reaction rate [4].
In a different approach, a lithiated DowexTM 50W × 2 resin was used to immobilize phosphine–rhodium complexes to catalyze the asymmetric hydrogenation of prochiral olefins, such as methyl 2-acetamidoacrylate, under mild conditions (room temperature, under 1–5 bar H2) [5][6][7]. Reported yields mount up to 99.9%, even in the second recycle, with a detected Rh leaching of 2.0% [5].
Hydrogenation of cyclohexene in supercritical CO2 over metal-doped AmberlystTM 15 was studied by Seki and associates [8]. The metals employed (Pd, Pt, Ru, and Rh) were incorporated into AmberlystTM 15 in different amounts from 0.5 to 2 wt.% to obtain monometallic catalysts. A bimetallic Pd-Pt catalyst was also tested. The highest conversions (i.e., up to 99%) and selectivity towards cyclohexane (i.e., >99%) were reported for Pd-based catalysts. Results obtained with Pt, Ru, and Rh catalysts were clearly less favorable with, conversion values in the range of 1–4%.
Some relevant hydrogenation reactions of alkenes found in the covered literature are shown in Scheme 1. Table 1 lists resin types, tested reactions, and obtained results of some of the reviewed works.
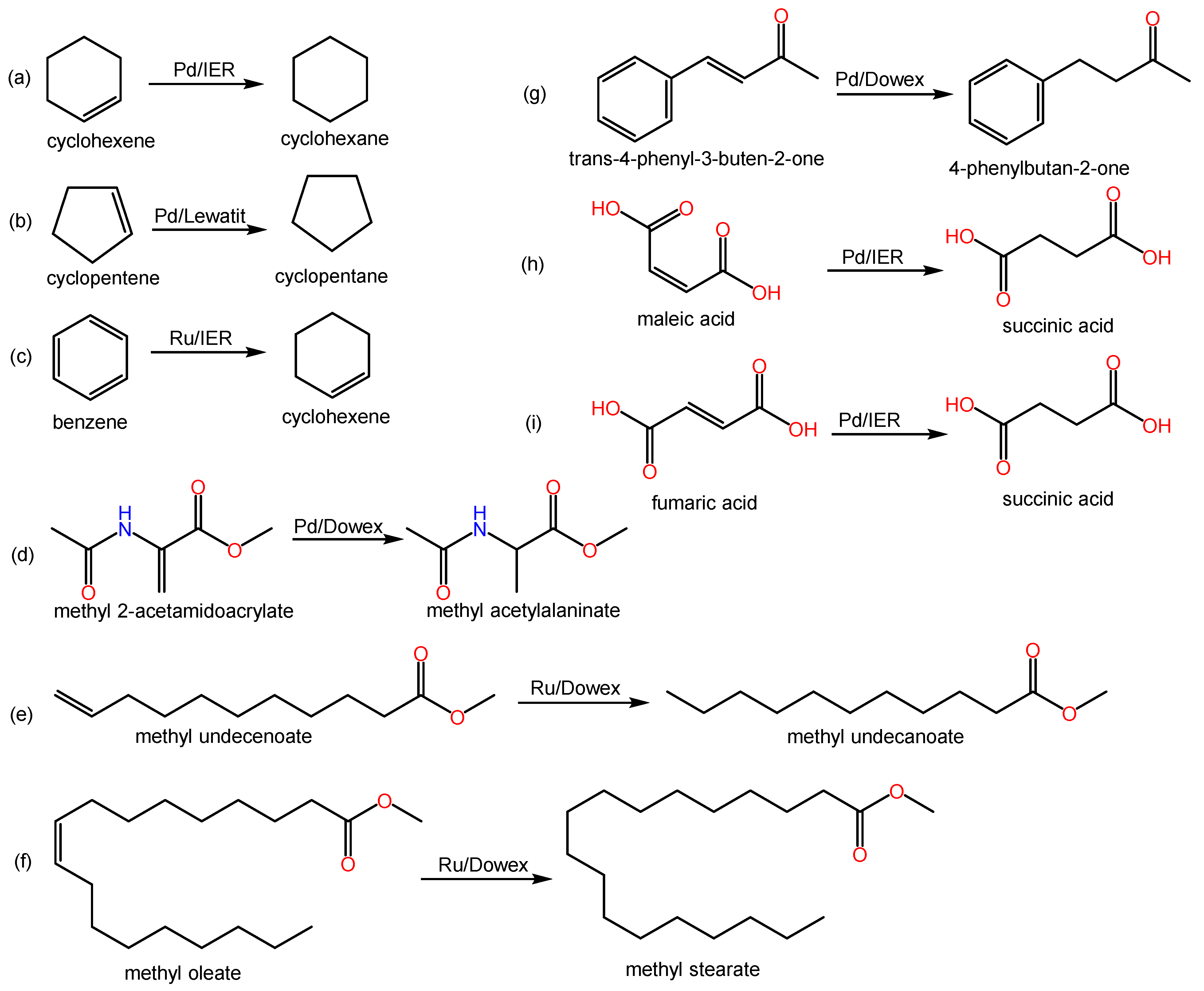
Scheme 1. Examples of hydrogenation reactions of alkenes over metal-doped IERs in the revised literature.
Table 1. Hydrogenation reactions of alkenes over metal-doped IERs in the literature.
| Resin | Metal | Reduction Protocol | Tested Reaction | Reaction Conditions | Conversion (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Polymer | Functional Group | |||||||
| Macroreticular | Lewatit® OC 1038, ST-DVB | –SO3H | Pd (0.3 wt.%) | In situ | Hydrogenation of cyclopentene | 2 mL cyclopentene, T = 30 °C; P = 1 MPa H2; t = 2 h | 30 | - | [9] |
| 1 MPa H2 in cyclopentene at 30 °C for 2 h | 28.7 | - | [9] | ||||||
| 1 MPa H2 in water at 30 °C for 2 h | 100 | - | [9] | ||||||
| 40% aq. formaldehyde at 50 °C for 1 h | 77.8 | - | [9] | ||||||
| Gel-type | ST-DVB (4 mol%) | –SO3H | Ru (4 wt.%) | NaBH4 in EtOH | Partial hydrogenation of benzene to cyclohexene | 2 mL benzene, 0.75 mL water; T = 100 °C; P = 1.5 MPa | 43.1 | 3.0 | [10] |
| ST-DVB (8 mol%) | Ru (4 wt.%) | 44.6 | 4.4 | [10] | |||||
| DMAA–styrene sodium sulphonate–MBAA | Ru (4 wt.%) | 45.4 | 5.6 | [10] | |||||
| Ru (8 wt.%) | 45.3 | 5.4 | [10] | ||||||
| DMAA–potassium 1-methacryoyl ethylene 2-sulphonate–MBAA | Ru (4 wt.%) | 47.2 | 8.1 | [10] | |||||
| Ru (8 wt.%) | 42.3 | 7.0 | [10] | ||||||
| Gel-type | 2-Hydroxyethyl methacrylate (HEMA), ST, diethylene glycol dimethacrylate (DEGDMA, 3–10 mol%) | –COOH and C═O | Pd (0.25–2.0 wt.%) | NaBH4 in THF:MeOH (9:1) | Hydrogenation of maleic/fumaric acid | Csubstrate = 0.043 M in THF; T = 22 °C; atmospheric pressure | - | 100 | [2] |
| Macroreticular | AmberlystTM 15, ST-DVB | –SO3H | Pd (0.5 wt.%) | 100 °C for 1 h under H2/N2 flow | Hydrogenation of cyclohexene | Supercritical CO2, T = 60 °C, total P = 160 bar, cyclohexene:H2 = 1:1.8 | 86 | 95 | [8] |
| Pd (1 wt.%) | 99 | >99 | [8] | ||||||
| Pd (2 wt.%) | 56 | 99 | [8] | ||||||
| Pt (1 wt.%) | 2 | 52 | [8] | ||||||
| Pd (0.5 wt.%), Pt (0.5 wt.%) | 59 | 96 | [8] | ||||||
| Ru (1 wt.%) | 4 | 94 | [8] | ||||||
| Rh (1 wt.%) | 1 | 24 | [8] | ||||||
| Gel-type | DowexTM 1 × 2, ST-DVB | –C10H16N(Cl) | Pd (1.1 wt.%) | NaBH4 in water | Several C═C, C≡C, and C═O hydrogenation reactions | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, 20 min | no observed catalytic activity | [3] | |
| Gel-type | DowexTM 1 × 2, ST-DVB | –SO3(Li) | Pd (1.2 wt.%) | NaBH4 in water | Hydrogenation of methyl 2-acetamidoacrylate | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, 20 min | 56.3 | 100 | [3] |
| –SO3(Li) | Pd (1.2 wt.%) | 2 bar H2 in MeOH |
87.4 | 100 | [3] | ||||
| –SO3(Li) | Pd (1.3 wt.%) | In situ | 91.7 | 100 | [3] | ||||
| Gel-type | DowexTM 1 × 2, ST-DVB | –SO3(Li) | Pd (1.3 wt.%) | In situ | Hydrogenation of trans-4-phenyl-3-buten-2-one | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, 20 min | 98.2 | 83.7 | [3] |
| Gel-type | DowexTM 1 × 2, ST-DVB | –SO3(Na) | Ru (0.9 wt.%) | NaBH4 in water | Hydrogenation of methyl undecenoate, methyl oleate, methyl ricinoleate, or methyl linoleate | nsubstrate = 1.6 mmol in water (0.25 mL) and n-heptane (6 mL), T = 30 °C, P = 10 bar H2, t = 60–210 min | 50–100 | - | [4] |
| Gel-type | DowexTM 1 × 2, ST-DVB | –SO3(Na) | Ru (0.9 wt.%) | NaBH4 in water | Hydrogenation of glycerol trioleate, castor oil, jojoba oil, olive oil, or very high-oleic sunflower oil | nsubstrate = 1.6 mmol in water (0.25 mL) and n-heptane (6 mL), T = 30 °C, P = 10 bar H2, t = 60–210 min | ≤45 | - | [4] |
As Scheme 1 and Table 1 reveal, Pd is the preferred metal to hydrogenate C═C bonds, as described in previous works [11][12], but Ru-based catalysts have also been used to hydrogenate stronger C═C bonds, such as the conjugated ones in benzene rings. Regarding the IERs, both synthetic and commercial resins have been used, but mostly sulfonated and gel-type ones. Noteworthy, the anion exchange resin tested by Moreno Marrodan and coworkers yielded no significant catalytic activity in several hydrogenation reactions [3]. Different strategies regarding the activation step of the metal catalyst emerge from the revised literature, including chemical reduction of the ionic metal with NaBH4 and in situ reduction during reaction conditions (with no separate pre-reduction step). Depending on the author and explored reaction, the reported results differ substantially. For instance, Jeřábek reported much higher conversions when pre-reducing the catalyst at 1 MPa H2 in water at 30 °C for 2 h compared to in situ reduction during cyclopentene hydrogenation to cyclopentane (Table 1), whereas Moreno Marrodan reported the opposite behavior in the hydrogenation reaction of methyl 2-acetamidoacrylate to methyl acetylalaninate [3][9].
2. Hydrogenation of Alkynes
In Drelinkiewicz et al. (2009), self-made gel-type resins functionalized by amine were used, which were obtained by suspension polymerization of a mixture of glycidyl methacrylate, ST, and diethylene glycol dimethacrylate (DEGDMA). As metal nanoparticles, Pd (0.125–0.50 wt.%) of considerably large sizes (30–50 nm) were obtained when Pd was incorporated on dry resin beads using an aqueous solution of PdCl2 as the precursor, which contrasts with the nano-scaled Pd particles obtained when Pd(OAc)2 was used. However, it was the 30–50 nm Pd catalyst the one exhibiting better results for the hydrogenations of 2-butyne-1,4-diol and phenylacetylene to their respective alkenes, with selectivity values ca. 92–95% at 90% conversion of the alkyne. The better performance of the large Pd nanoparticles-containing IER is related to its metal distribution (i.e., mostly, in the outer shell of the polymer beads, forming a thin layer) [13].
3-Hexyn-1-ol was hydrogenated to 3-hexen-1-ol by Moreno Marrodan and coworkers over the already mentioned lithiated Pd/DowexTM 50W × 2 IER [3]. Very high values of both conversion (98.5%) and selectivity (>99.8%) were reported in their work.
Four monometallic and six bimetallic catalysts, combining Cu, Ag, Ni, with Pd over the commercial acidic AmberliteTM IR-120 resin in Na+ form, were synthesized and tested for the hydrogenation of 4-nitrophenol and phenylacetylene in Silva et al. (2019). Regarding the monometallic catalysts, Pd, Ag, Ni, or Cu were ion-exchanged with the resin and subsequently reduced with NaBH4. With regards to the bimetallic catalysts, two types are distinguished in their work: Pd-doped catalysts onto which a second metal was introduced by the same method (i.e., ion-exchange followed by NaBH4 reduction) and M-doped catalysts (with M being either Cu, Ag or Ni) onto which Pd was introduced as the second metal [14]. Among the monometallic catalysts, only Pd was active and selective towards ethylbenzene. Interestingly, the simultaneous presence of Ag completely inhibited the catalytic activity of Pd, while the presence of Cu enabled the selective production of styrene over ethylbenzene (i.e., >90% selectivity at high conversion values). On the other hand, Ni-containing catalysts favored ethylbenzene production. The authors attributed the superior behavior of bimetallic Cu catalysts to a geometric effect, rather than to electronic causes.
Sulman et al. (2012) and Nikoshvili et al. (2015) studied the use of hyper-crosslinked polystyrene as support for Pd nanoclusters to selectively hydrogenate C≡C bonds in acetylene alcohols (dimethylethynylcarbinol, dehydrolinalool, and dehydroisophytol) [15][16]. The effect of the solvent nature on the observed activity was investigated, revealing that catalyst activity decreases in the order: alcohols > cyclohexane > water/ethanol mixture > octane ≥ hexane ≥ xylene > toluene > heptane, which apparently correlates to the solvent polarity. According to the authors, neither solvent–substrate interactions or hydrogen solubility in each solvent can explain the reported difference in catalytic activity and selectivity, but the strength of the solvent–catalyst interactions can successfully account for the observed activity pattern [16].
As in the previous section, relevant alkynes hydrogenation reactions can be found in both Scheme 2 and Table 2, which also contain relevant information from the literature.
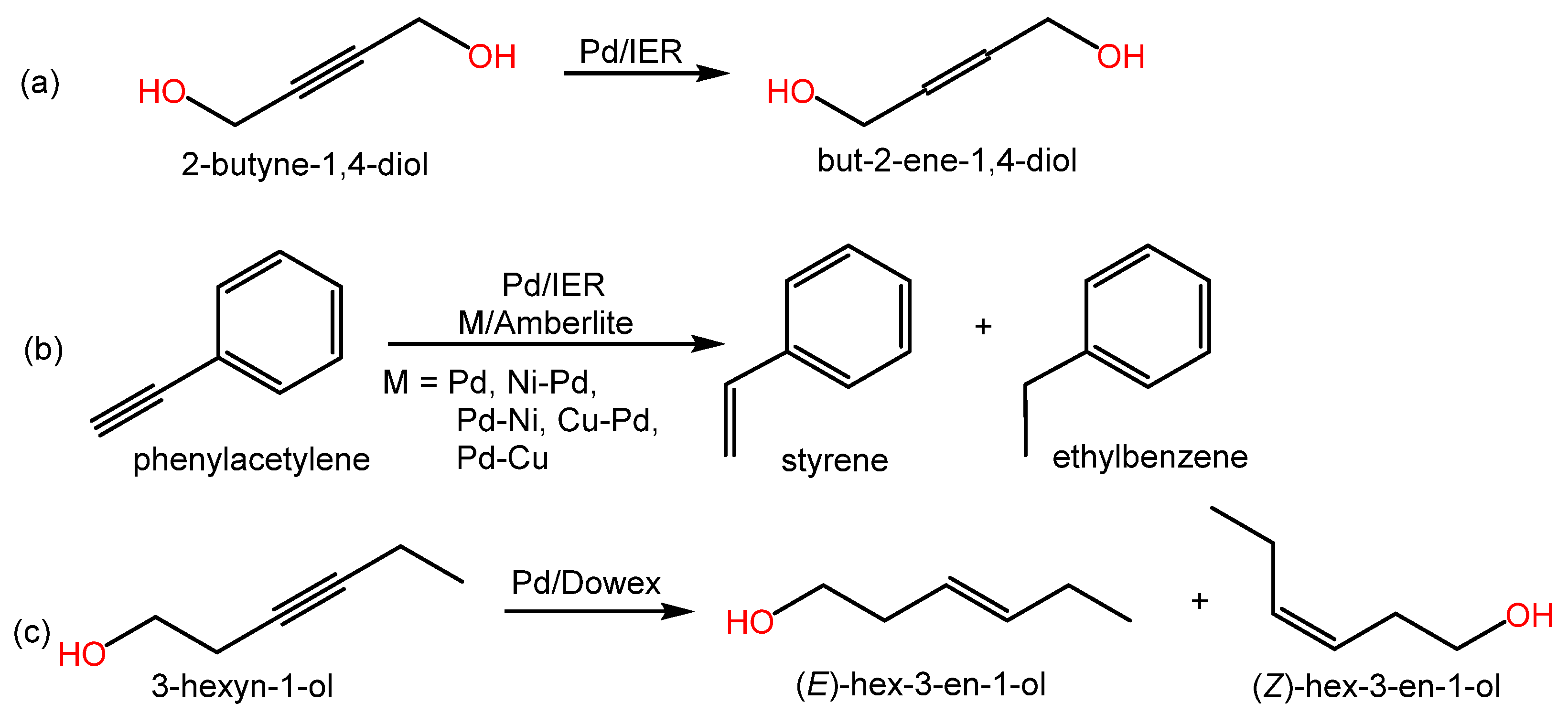
Scheme 2. Examples of hydrogenation reactions of alkenes over metal-doped IERs in the revised literature.
Table 2. Hydrogenation reactions of alkynes over metal-doped IERs in the literature.
| Resin | Metal | Reduction Protocol | Tested Reaction | Reaction Conditions | Conversion (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Polymer | Functional Group | |||||||
| Gel-type | Glycidyl methacrylate (GMA), ST, DEGDMA | –NH–CH2CH2–NH2 | Pd (0.125–0.50 wt.%) | THF:H2O solution of N2H4 × H2O | Hydrogenation of 2-butyne-1,4-diol to 2-butene-1,4-diol | Csubstrate = 0.052 M in THF; T = 22 °C; atmospheric pressure | 90 | 91.3–94.7 | [13] |
| Gel-type | GMA, ST, DEGDMA | –NH–CH2CH2–NH2 | Pd (0.125–0.50 wt.%) | THF:H2O solution of N2H4 × H2O | Hydrogenation of phenylacetylene to styrene | Csubstrate = 0.052 M in THF; T = 22 °C; atmospheric pressure | 90 | 91.4–93.6 | [13] |
| Gel-type | DowexTM 50W × 2, ST-DVB | –SO3(Li) | Pd (1.3 wt.%) | none | Hydrogenation of 3-hexyn-1-ol to 3-hexen-1-ol | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, t = 20 min | 98.5 | >99.8 | [3] |
| Hyper-crosslinked | Macronet™ MN270, ST-DVB | none | Pd (0.1–5 wt.%) | Saturation with H2 for 1 h | Hydrogenation of 2-methyl-3-butyn-2-ol to 2-methyl-3-butene-2-ol | V = 30 mL toluene, T = 90 °C, atmospheric pressure | 100 | 95.3–98.5 | [15] |
| Hyper-crosslinked | Macronet™ MN270, ST-DVB | none | Pd (0.1–5 wt.%) | Saturation with H2 for 1 h | Hydrogenation of 3,7-dimethyloct-6-en-1-yn-3-ol to 3,7-dimethyl-1,6-octadien-3-ol | V = 30 mL toluene, T = 90 °C, atmospheric pressure | 100 | 96.5–98.5 | [15] |
| Hyper-crosslinked | Macronet™ MN270, ST-DVB | none | Pd (0.1–5 wt.%) | Saturation with H2 for 1 h | Hydrogenation of 3,7,11,15-tetramethylhexadec-1-yn-3-ol to 3,7,11,15-tetramethyl-1-hexadecene-3-ol | V = 30 mL toluene, T = 90 °C, atmospheric pressure | 100 | 95.2–97.5 | [15] |
| Hyper-crosslinked | Macronet™ MN270, ST-DVB | none | Pd (0.2 wt.%) | H2 at 300 °C for 2 h | Hydrogenation of 2-methyl-3-butyn-2-ol to 2-methyl-3-butene-2-ol | T = 60 °C, P = 3 bar H2, 1500 rpm in EtOH or toluene as solvents | 95 | 93.2–99.6 | [16] |
| Hyper-crosslinked | DowexTM OPTIPORE, ST | none | Pd (0.5 wt.%) | H2 at 300 °C for 2 h | 95 | 83.0–93.5 | [16] | ||
| Gel-type | AmberLite™ IRC120, ST-DVB | –SO3(Na) | Pd (0.08 wt.%) | NaBH4 | Hydrogenation of phenylacetylene to styrene | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | >99 | 0 | [14] |
| Pd (0.09 wt.%), Ag (0.07 wt.%) | NaBH4 | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | 12 | - | [14] | ||||
| Ni (0.08 wt.%), Pd (0.10 wt.%) | NaBH4 | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | >99 | 7 | [14] | ||||
| Pd (0.06 wt.%), Ni (0.05 wt.%) | NaBH4 | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | >99 | 0 | [14] | ||||
| Cu (0.084 wt.%), Pd (0.06 wt.%) | NaBH4 | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | 67 | 91 | [14] | ||||
| Pd (0.06 wt.%), Cu (0.10 wt.%) | NaBH4 | nsubstrate = 0.5 mmol in 1 mL EtOH, T = 60 °C, P = 4 bar H2, t = 1.1 h | 83 | 93 | [14] | ||||
Only gel-type IERs and hyper-crosslinked unfunctionalized polymers have been used as support for alkynes hydrogenations in the revised literature, with Pd being the most used metal. Interestingly, Ag, Ni, nor Cu showed any catalytic activity when used as monometallic catalysts supported on an AmberliteTM resin for the hydrogenation of phenylacetylene [14]. Again, different reduction protocols have been reported with no clear data elucidating the most favorable ones towards the hydrogenation of alkynes.
3. Hydrogenation of Carbonyl Compounds
Pd-doped, highly lipophilic gel-type IERs, based on styrene or dodecyl methacrylate, were used as catalysts for the hydrogenation of 2-ethylanthraquinone to 2-ethylanthrahydroquinone, which involves the consecutive hydrogenations of C═O and C═C bonds, in both Biffis et al. (2002) and Bombi et al. (2003). Reported selectivity for the tested ST-DVB supported catalyst was 50 and 65%, depending on the reducing step procedure (H2 or NaBH4, respectively), and catalytic activity was clearly lower than a benchmark industrial catalyst (Pd/silicoaluminate). On the other hand, while activity was also low, the resins based on dodecyl methacrylate reached selectivity values as high as 98%, which were slightly above the commercial alternative at the time [17][18].
Hydrogenation of citral (3,7-dimethyl-2,6-octadienal) to geraniol (trans-3,7-dimethyl-2,6-octadienol) and nerol (cis-3,7-dimethyl-2,6-octadienol) was accomplished by Centomo and coworkers over Pt (0.43–2.5 wt.%) catalysts supported on two sets of functional gel-type resins synthesized by the authors, as well as a commercial 4-vinylpyridine (VP) polymer crosslinked with 2 mol% of DVB [19]. The first set of resins contained N,N-dimethyl-2-aminoethylmethacrylate (DMAEMA), cyanoethyl-acrylate (CEA) or methacrylic acid (MAA) as the functional monomer, and DVB as the crosslinker, while the second one contained either CEA or MAA, as the functional monomer, together with N,N-dimethylacrylamide (DMAA) and DVB. In addition, two different approaches to incorporate Pt into the resins were adopted: one based on the impregnation of the resin with mesitylene solutions of colloidal platinum, and another one involving immobilization of Pt precursors in pre-swollen resins, followed by chemical reduction. In addition, some of the tested resins were further modified by incorporation of a second metal, acting as Lewis acids (i.e., the introduction of 6.2–15.2 wt.% FeII, CoII, or ZnII in Pt-containing resins with –COOH groups). The authors state that improved selectivity can be achieved thanks to the presence of a more electropositive metal than the noble one because it would release electronic density to the noble active metal, which ultimately hinders C═C hydrogenation and favors that of C═O bonds. Reported conversions span from relatively low values (20–30%) to ≥80–90%, depending on the tested catalyst, with selectivity values reaching values above 90%, particularly with the bimetallic catalysts.
The C═O bond in methyl benzoylformate (methyl 2-oxo-2-phenylacetate) and 2,2,2-trifluoroacetophenone (2,2,2-trifluoro-1-phenylethan-1-one) was hydrogenated by Moreno Marrodan and coworkers over a lithiated Pd/DowexTM 50W × 2 IER, yielding selectivity values over 99.5% at acceptably high conversion levels (ca. 90% and 62%) when no pre-reduction of the PdII species supported on the IER was conducted [3].
Another noteworthy application of IERs in the field of carbonyl hydrogenation is that of Barbaro and associates, using DowexTM 50W × 2 resin as support for immobilizing an Ir complex. Their catalyst was used for the hydrogenation reactions of a number of substrates, including several imines, the α-keto ester dihydro-4,4-dimethyl-2,3-furandione, and terpenes like (R)-carvone, with similar results to homogeneous catalysts [20]. On the other hand, Pt-doped hyper-crosslinked polystyrene modified with cinchonidine was used as the catalyst for enantioselective hydrogenation of the C═O bond in ethylpyruvate into (R)-ethyllactate and (S)-ethyllactate in Bykov et al. (2009) [21]. The authors found that enantioselectivity of activated ketone hydrogenation with platinated hyper-crosslinked polystyrene depends on the solvent, reducing agent, temperature, substrate, catalyst, and the modifier concentrations.
Examples of C═O hydrogenation reactions and relevant data are shown in Scheme 3 and Table 3.
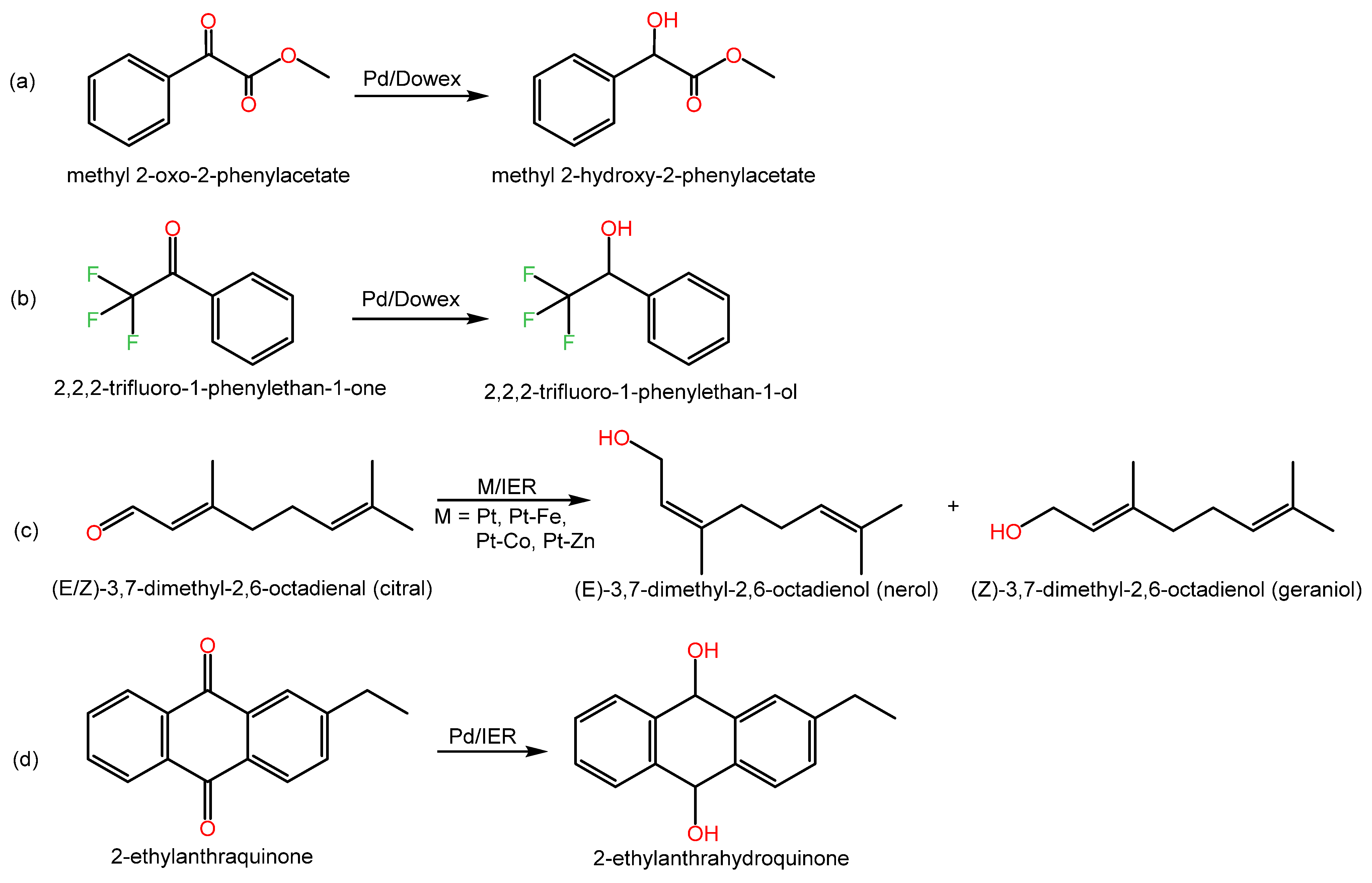
Scheme 3. Examples of hydrogenation reactions of carbonyl groups over metal-doped IERs in the revised literature.
Table 3. Hydrogenation reactions of carbonyl groups over metal-doped IERs in the literature.
| Resin | Metal | Reduction Protocol | Tested Reaction | Reaction Conditions | Conversion (%) | Selectivity (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | Polymer | Functional Group | |||||||
| Gel-type | DMA, MMA, SEMA, EDMA | –SO3H | Pd (1 wt.%) | NaBH4 | Hydrogenation of 2-ethylanthraquinone | T = 20 °C, P = 100 kPa, t = 1800 s | - | 93 | [17] |
| H2 | T = 20 °C, P = 100 kPa, t = 5100 s | - | 94 | [17] | |||||
| Gel-type | DMA, VP, EDMA | –CH2CHC5H4N | Pd (1 wt.%) | NaBH4 | T = 20 °C, P = 100 kPa, t = 1800 s | - | 93 | [17] | |
| H2 | T = 20 °C, P = 100 kPa, t = 3600 s | - | 83 | [17] | |||||
| Gel-type | DMA, MAA, EDMA | –COOH | Pd (1 wt.%) | NaBH4 | T = 20 °C, P = 100 kPa, t = 2400 s | - | 96 | [17] | |
| H2 | T = 20 °C, P = 100 kPa, t = 16,200 s | - | 98 | [17] | |||||
| Gel-type | ST, SEMA, DVB | –SO3H | Pd (1 wt.%) | NaBH4 | T = 20 °C, P = 100 kPa, t = 8400 s | - | 65 | [17] | |
| H2 | T = 20 °C, P = 100 kPa, t = 8400 s | - | 50 | [17] | |||||
| Gel-type | N,N-dimethyl-2-aminoethyl-methacrylate (DMAEMA), DVB | –C(O)O CH2CH2N (CH3)2 | Pt (0.9 wt.) | In situ at 70 °C for 1 h | Hydrogenation of 3,7-dimethyl-2,6-octadienal | T = 60 °C, atmospheric pressure under H2 flow, 25 mL EtOH | 20–30 | ≤15 | [19] |
| Gel-type | Cyanoethyl-acrylate (CEA), DVB | –CN | Pt (1.0 wt.) | ≥80–90 | 46–47 | [19] | |||
| Gel-type | MAA, DVB | –COOH | Pt (0.8 wt.) | ≥80–90 | 46–47 | [19] | |||
| Gel-type | VP, DVB | –4-C5H4N | Pt (1.0 wt.) | ≤20–30 | 54 | [19] | |||
| Gel-type | MAA, DVB | –COOH | Pt (0.71 wt.), Fe (8.2 wt.%) | 20–30 | ≥80–90 | [19] | |||
| Pt (0.89 wt.), Co (14.1 wt.%) | 80–90 | ≤80 | [19] | ||||||
| Pt (0.43 wt.), Zn (15.2 wt.%) | 20–30 | ≥80–90 | [19] | ||||||
| Gel-type | VP, DVB | –4-C5H4N | Pt (2.5 wt.), Co (6.2 wt.%) | 80–90 | ≥80–90 | [19] | |||
| Gel-type | DowexTM 50W × 2, ST-DVB | –SO3(Li) | Pd (1.3 wt.%) | In situ | Hydrogenation of methyl 2-oxo-2-phenylacetate | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, t = 20 min. | 89.8 | >99.5 | [3] |
| Pd (1.2 wt.%) | 2 bar H2 in MeOH | 32.4 | >99.5 | [3] | |||||
| –SO3H | Pd (1.5 wt.%) | In situ | 52.6 | >99.5 | [3] | ||||
| Pd (1.3 wt.%) | H2 flow | 36.9 | >99.5 | [3] | |||||
| Gel-type | DowexTM 50W × 2, ST-DVB | –SO3(Li) | Pd (1.3 wt.%) | In situ | Hydrogenation of 2,2,2-trifluoro-1-phenylethan-1-one | Csubstrate = 0.17 M in MeOH, room temperature, P = 0.8 bar H2, t = 20 min. | 65.4 | >99.8 | [3] |
Interestingly, Pd/IER catalysts have been mostly used in the revised literature to hydrogenate C═O bonds, despite Pd being more often related to C═C hydrogenations [12]. On the contrary, even though Ru-based catalysts are typically considered among the most favored ones for the hydrogenation of C═O bonds [22], scarce references report on the use of Ru/IER for this type of reaction. To date, the only references available correspond to works conducted by Barbaro and coworkers (2014, 2016), which will be further addressed, since they are focused on multistep processes involving sequential C═O hydrogenation, followed by dehydration or lactonization reactions to obtain biomass-derived compounds [23][24]. Consequently, further investigation into the potential use of Ru-doped IER for C═O hydrogenations is advised.
4. Hydrogenation of Substituted Arenes
In the cited work by Moreno Marrodan et al. (2012), hydrogenation of methyl benzoylformate and 2,2,2-trifluoroacetophenone actually constitute examples of C═O hydrogenation reactions in substituted arenes [3]. Further on, in Moreno Marrodan et al. (2015), the number of substrates belonging to the family of substituted arenes was extended, and selective C═C hydrogenation reactions over a 1.3 wt.% Rh catalyst (3 nm) supported on DowexTM 50W × 2, either in lithiated or protonated form, was investigated [25]. In their study, the effect of the bead size (i.e., beads of 38–75 µm and of 150–300 µm were used as support), the activation protocol to reduce RhI to Rh0 (including no pre-reduction step, reduction with H2, and chemical reduction with NaBH4), and catalyst recyclability was evaluated. Results showed that as-prepared lithiated Rh-doped IERs are efficient C═C hydrogenation catalysts under undemanding conditions (room temperature and 1 bar H2), yielding conversion and selectivity values as high as 100%, depending on the substrate.
In the already mentioned reference by Silva and collaborators [14], besides the hydrogenation of phenylacetylene to styrene, further hydrogenation to ethylbenzene was also studied. Over 99% conversion is reported for the monometallic Pd-doped IER and the bimetallic catalysts containing Ni. Contrarily, Ag- and Cu-containing catalysts exhibited virtually nil catalytic activity. Seki and coworkers also studied the hydrogenation of benzaldehyde in supercritical CO2 over a Pd catalyst supported on AmberlystTM 15 [8]. Modest results were reported in terms of both conversion (16%) and selectivity (33%).
Regarding the use of Pd-containing, hyper-crosslinked ST, the kinetics of the gas-phase phenol hydrogenation to cyclohexanone were evaluated by Sulman and coworkers [26]. Selectivity greater than 95% is reported at 99% conversion, using Macronet™ MN270 as the Pd support.
Relevant literature examples regarding the hydrogenation of substituted arenes using IERs and related data are compiled in Scheme 4.
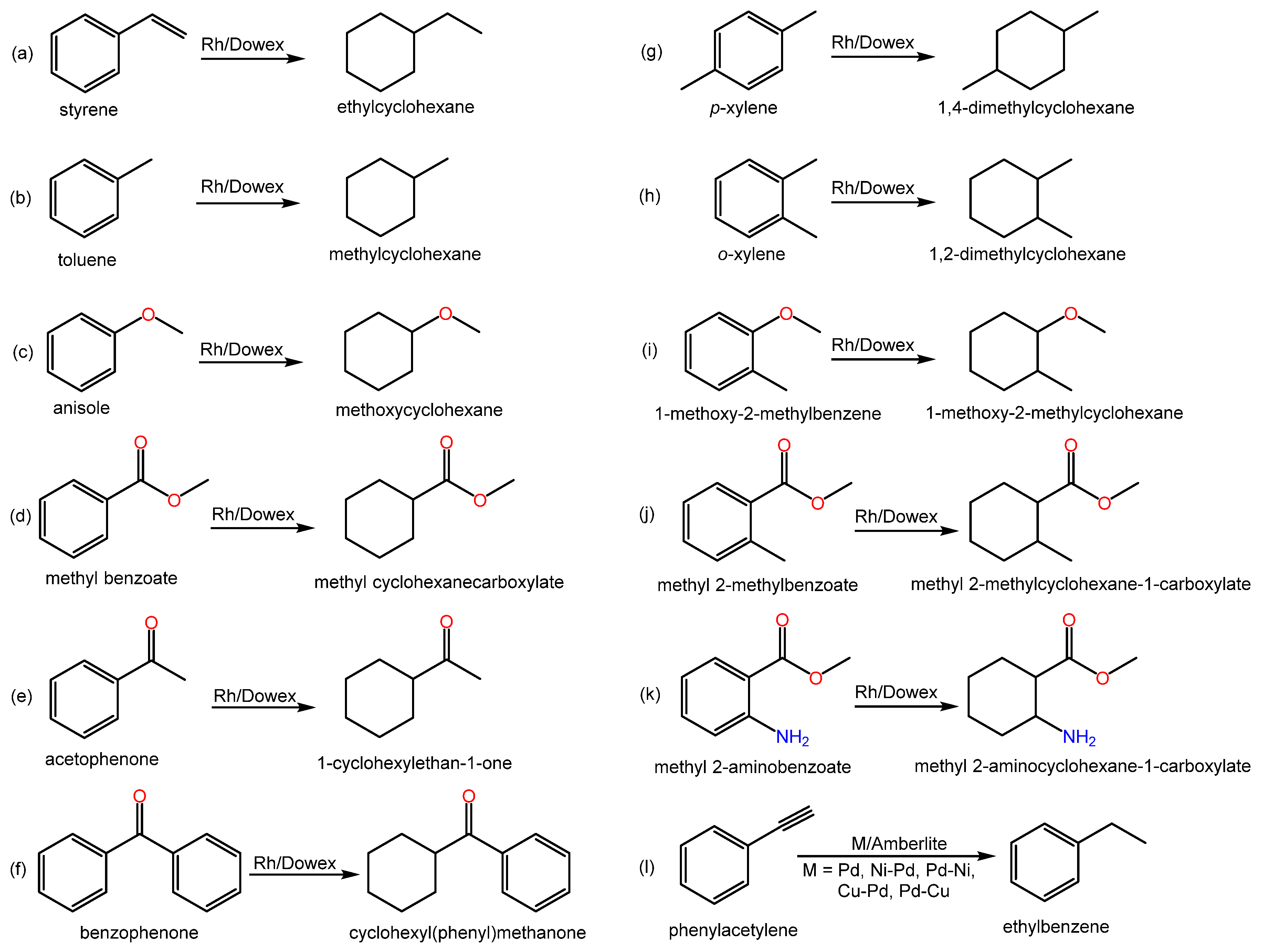
Scheme 4. Examples of hydrogenation reactions of substituted arenes over metal-doped IERs in the revised literature.
5. Hydrogenation of Nitroaromatic Compounds
Regarding the hydrogenation of nitroaromatics to their corresponding amines, some mentions can be retrieved from Corain et al. (2003, 2010) regarding Pd-doped gel-type IER, but with no specific data besides references contained therein [27][28]. In Gelbard (2005), the nitrobenzene reduction to phenylhydroxylamine, with hydrazine as the reducing agent, over Pt supported on chlorinated AmberliteTM IRA-410 is reported to achieve a 95% yield [29].
The hydrogenation of 4-nitrophenol to 4-aminophenol was evaluated with the mentioned mono- and bimetallic catalysts supported on AmberliteTM IR-120 resin. In general, bimetallic catalysts showed faster reaction rates than monometallic ones, except for monometallic Cu, which was the most active one. This is explained in terms of increased catalytically active sites and better availability [14].
6. Hydrogenation of Nitrates
The aqueous catalytic reduction of nitrates to molecular nitrogen with hydrogen was studied by Gašparovičová and coworkers (2006, 2007) over bimetallic Pd-Cu catalysts (4 wt.% Pd, 1 wt.% Cu) supported on DowexTM 1 × 4 in the chloride form [30][31]. DowexTM 1 × 4 is a gel-type ST-DVB resin functionalized with –N(CH3)3+ groups. In the first study, five reduction protocols were adopted (C1: NaBH4 in ethanol; C2: NaBH4 in water; C3: 0.1 MPa H2, Na2CO3 in H2O; C4: 0.5 MPa H2, Na2CO3 in H2O; and C5: 0.5 MPa H2, Na2CO3 in methanol) and results were compared. Distinct Pd nanoparticles sizes were observed for each reduction protocol, with C1 being undetectable (which suggests either an amorphous metal phase or with very small particles), C2 presenting the largest particles (i.e., 6.7 nm), and C5 the smallest size (i.e., 2.9 nm). As for the catalytic outcome, the highest selectivity to N2 was achieved by C1 (96%) at 71% nitrates conversion but with the largest Cu leaching detected. Overall, the best catalyst, according to the authors, was C5 since it yielded 73% conversion, with 77% selectivity towards N2, and the lowest Cu leaching. For all catalysts, Pd leaching was below detection limits [30].
In Gašparovičová et al. (2007), the Pd-Cu/DowexTM catalyst was compared to Pd-Cu/Al2O3. Two reduction protocols were applied to either type of catalyst. For the resin-based catalyst, the protocols were as follows: H2 in 2.5% water solution of Na2CO3 for 1 h at 25 °C and 0.5 MPa (catalyst 1), or 0.066 M solution of NaBH4 in ethanol for 1 h at ambient temperature under occasional stirring (catalyst 2). Interestingly, catalyst 2 presented a very homogeneous distribution of Pd and Cu, with Pd- and Cu-containing phases too small to be detected by XRPD, or amorphous, whereas Pd and Cu were mainly located in a shallow external layer of catalyst 1, with Pd nanoparticles of 3.8 nm. This suggests a clear effect of the reduction protocol in the metal distribution within the resin beads. Noticeably, expected byproducts, namely nitrites (produced by partial reduction) and ammonia (over reduction), were almost avoided with resin-based catalysts (i.e., selectivity towards N2 was 93.8% at 44% conversion with catalyst 2). On the other hand, Pd-Cu/Al2O3 catalysts were more active, yet less selective to N2 (below 60%) than resin catalysts. Furthermore, while Pd-Cu/Al2O3 catalysts showed some leaching of Cu (no leaching of Pd was detected), Cu and Pd in solution were below detection limits for resin-based catalysts [31].
Another Pd-Cu catalyst was investigated by Mendow and coworkers for the same reaction system, with the emphasis being made on the performance of two processes for nitrates removal [32]. In their work, Mendow used a macroporous, ST-DVB anion exchange resin (i.e., DiaionTM WA30), containing tertiary amine functional groups. Pd incorporation in the resin was accomplished by contact with PdCl2 solution dissolved in NaCl 0.01 M and HCl 0.01 M while bubbling N2. After reduction of the PdII to Pd0 with 35 wt.% hydrazine solution, Cu was incorporated by means of the controlled surface reduction method. In this procedure, the reduced Pd/WA30 catalyst was suspended in water and H2 was bubbled for 2 h; then, a CuNO3·3H2O solution was added while maintaining the hydrogen bubbling for 2 h. After several additional steps involving filtration, reduction, and washing operations, the final metal contents were 2 wt.% Pd and 0.5 wt.% Cu, as verified by energy-dispersive X-ray fluorescence (XRF). Depending on the operating conditions, conversion, and selectivity to N2 values as high as 100% are reported [32].
The simultaneous catalytic reduction of nitrate ions and reductive dehalogenation of organochlorinated pollutants from water was studied by Bradu and associates, who prepared bimetallic Pd-Cu catalysts over Purolite® A520E, which is a strong base anion exchange resin of the macroreticular type, presenting quaternary ammonium functional groups on an ST-DVB polymer matrix [33]. Four catalysts were prepared and compared: (i) a monometallic Pd (2.01 wt.%) over A520E was prepared by ion exchange; (ii) a bimetallic Pd-Cu (1.97 wt.% and 0.45 wt.%, respectively) catalyst sample was prepared by simultaneous ion-exchange of both metal salts with the resin; (iii) a second bimetallic Pd-Cu (1.98 wt.%, 0.48 wt.%) catalyst was prepared by consecutive stages of ion-exchange of each metal salt with the resin, with Pd being exchanged in the first place; and (iv), finally, a third bimetallic Pd-Cu (2.01 wt.%, 0.49 wt.%) catalyst was prepared by Pd ion-exchange, followed by Cu deposition by controlled surface reaction. The latter catalyst sample was able to achieve selective nitrate reduction, with a 95% conversion and 92% selectivity towards N2, with almost quantitative hydrodechlorination of 4-chlorophenol [33].
7. One-Pot Multistep Reaction Processes Involving Hydrogenation
Regarding multistep processes, several relevant examples can be found in the literature, signaling that this specific field is of great interest since it deals directly with the integration and optimization of processes enabling the one-pot synthesis of valuable chemicals. For instance, the simultaneous hydrogenation and isomerization of diisobutylenes (2,4,4-trimethylpent-1-ene, TMP1, and 2,4,4-trimethylpent-2-ene, TMP2) over a Pd-doped IER was investigated by Talwalkar and coworkers [34]. Interestingly, the commercial AmberlystTM CH28 bifunctional catalyst, which contains Pd nanoparticles and –SO3H functional groups in a macroreticular matrix, was found to favor isomerization of TMP2 to TMP1, which is more prone to hydrogenation towards isooctane than TMP2 due to the terminal position of the double bond. From this, it follows that bifunctionalization of a resin with acid and metallic groups can offer selectivity changes in isomerization reactions, making the subsequent hydrogenation step more profitable. The effects of operating parameters (i.e., temperature, catalyst loading, hydrogen pressure, impurities associated with TMPs, and catalyst reusability) were evaluated and a kinetic model was proposed.
Seki et al. (2007, 2008) reported on the one-pot synthesis of 2-ethylhexanal from crotonaldehyde, which involves hydrogenation and aldol condensation in supercritical CO2, over a bifunctional acidic resin-supported palladium catalyst [8][35]. They incorporated Pd (1 wt.%) onto AmberlystTM 15 and obtained 13–98% conversions and ~0–67% selectivity values, depending on the assayed conditions.
The one-pot synthesis of a potential analgesic over Pd/AmberlystTM 15 was studied by Wissler and coworkers. In their work, two reaction steps are involved: dehydration of the starting tertiary alcohol, followed by hydrogenation of the obtained olefin. At the proposed optimized conditions, the reported conversion was 98%, and the selectivity towards the target product was 64%. However, prompt deactivation of the catalyst in the first reuse was observed, which the authors link to possible coke formation on the resin, since they argue that the selected temperature (i.e., 150 °C) is not high enough to promote thermal instability and no Pd leaching was observed [36].
Besides the examples mentioned so far, the specific, yet broad, field of biomass transformation into high-value-added products seems to be particularly suited for using metal-doped IER. For instance, Barbaro et al. (2016) focused on the direct conversion of glucose and xylose to isosorbide and anhydroxylitol, respectively, involving hydrogenation followed by dehydration. DowexTM 50W × 2 in its H+ form was used as support for Ru, with reported yields of 84.9% for isosorbide and 94.9% for anhydroxylitol at 190 °C with 30 bar H2 in water in batch experiments [23]. Such high yields are attributed to a combined effect of (i) a favorable microporous structure of IER and a narrow size distribution of Ru nanoparticles, which would enhance hydrogenation selectivity, and, (ii) a proper balance of density and strength of Brønsted acid sites, which would enhance dehydration.
In a different approach, a mixture of Pt/C catalyst and the thermostable, ST-DVB macroreticular resin AmberlystTM 70 was used for the cellulose conversion into isosorbide, which involves three consecutive reaction steps: hydration, hydrogenation, and dehydration [37]. The same approach was adopted in Galletti et al. (2012) for the synthesis of γ-valerolactone (GVL) from levulinic acid [38].
With respect to GVL production, Au, Pt, Ir, Ni, Cu, Re, Rh, and Ru over different supports have been mentioned as effective catalysts to obtain GVL by hydrogenation of levulinic acid [24][39][40][41][42][43]. In this process, either hydrogenation followed by dehydration or dehydration–hydrogenation reactions take place. As for the use of IERs to produce GVL, Moreno Marrodan and Barbaro (2014) supported Ru (0.87 wt.%) on DowexTM 50W × 2 by contacting it with RuCl3 and further reduction with NaBH4, with water being used as the solvent in both metalation and reduction steps. Values of 16.2–99.8% conversions in batch runs and 89–100% conversions in continuous runs (residence time = 62–211 s, T = 70 °C, P = 4.8–7.0 bar H2, water) are reported, with selectivity to GVL always above 99%. No significant activity decay was observed after three consecutive runs of recycling the catalyst and no Ru leaching was detected [24].
In another study by Moreno Marrodan and coworkers, metal nanoparticles (Pd, Rh, or Ru) were supported on a perfluorinated, fluorosulfonic acid resin (i.e., Aquivion® PFSA), which is claimed to possess an acidity similar to sulfuric acid, a thermal stability well beyond that of conventional IER, and chemical inertness in aggressive environments. Full selectivity at high conversion levels is reported for the conversion of (+)-citronellal to (-)-menthol and levulinic acid to γ-valerolactone (GVL) under mild conditions over Pd-based catalysts [44].
Another prominent reaction system within this field is the synthesis of methyl isobutyl ketone (MIBK) from acetone, involving consecutive steps of condensation, dehydration, and hydrogenation (e.g., [45][46][47][48][49][50][51][52]). The commercial Pd-doped AmberlystTM CH28 was pinpointed as an effective catalyst to carry out this synthesis. Depending on the operating conditions, reported acetone conversion values span from 25 to >99%, with selectivity in the range 70–90% [45][46][47][48][49][50][51]. AmberlystTM CH43, a Pd-doped strong acid styrenic IER, was reported to provide higher selectivity towards MIBK at high temperatures in a continuous 8 h run [51]. Remarkably, Aquivion® polymers were also used as Pd supports (0.10–1.04 wt.%) for the synthesis of MIBK, showing conversions in the range 11.0–63.3% and selectivity values of 62.8–96.5% in batch runs (t = 2–22 h, T = 120–180 °C, P = 10–25 bar H2) [52].
Several multistep reactions over metal-doped IER are shown in Scheme 5. As can be seen, all multistep processes found in the literature combine metal sites with acid sites. Both macroreticular and gel-type resins have been used. Furthermore, the commercial AmberlystTM CH28 needs to be highlighted, since it is a readily available product that has shown good results in different multistep reaction processes.
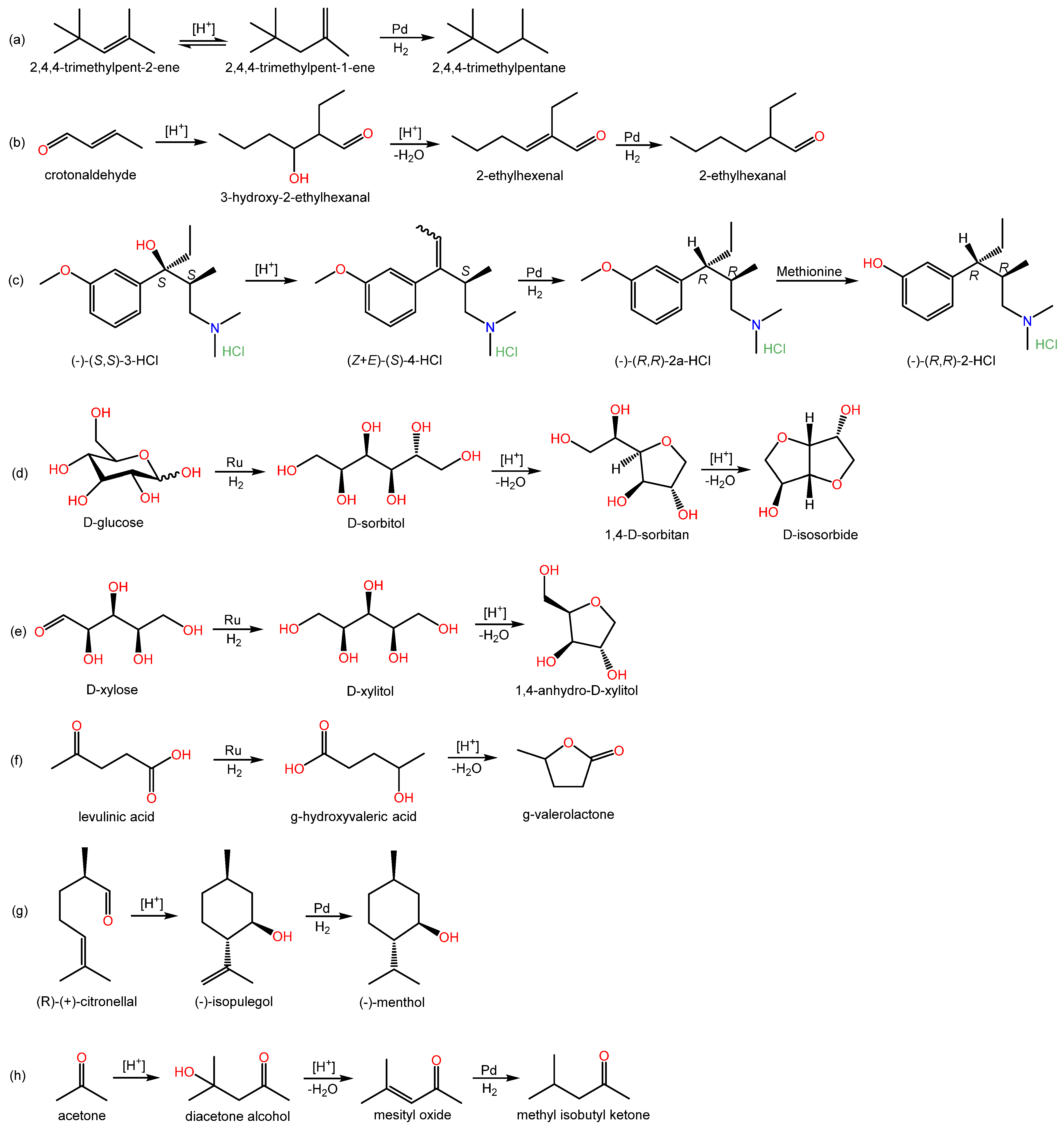
Scheme 5. Multistep reaction processes over metal-doped IERs in the revised literature.
8. Other Related Reactions
With regards to the role of IERs in other hydrogenation reactions (or related reaction systems), the direct synthesis of hydrogen peroxide from molecular oxygen and hydrogen stands out as one of the most studied processes. For instance, Sterchele and coworkers successfully prepared bimetallic Pd-Au (Pd: 1.0; Au: 0.25–1.0 wt.%) and Pd-Pt (Pd: 1.0; Pt: 0.1–1.0 wt.%) catalysts over a commercial, ST-DVB macroreticular resin, Lewatit® K2621, by simple ion-exchange in water and reduction with aqueous formaldehyde, which they tested in the direct synthesis of hydrogen peroxide [53]. They found that the presence of small amounts of either Pt or Au in Pd catalysts promoted selectivity while reducing activity in comparison with monometallic Pd catalysts, but that further addition of Pt or Au produced different effects: while activity increases with an increasing amount of Au, a maximum in hydrogen peroxide productivity was observed with 0.5 wt.% Pt addition, but also the lowest selectivity level was achieved. Other relevant examples of works dealing with this reaction system are those by Burato (2006) and Frison (2019), which have already been commented on in previous sections [54][55].
Concerning catalytic hydrodechlorination reactions, Králik et al. (2014) supported Pd and Pt over DowexTM 1 × 4, an ST-DVB gel-type resin, functionalized with trimethylammonium, and the catalysts were tested in the hydrogenation of chloronitrobenzene isomers into the corresponding chloroanilines. The resin was used either in the chloride form or in the OH− form, and about 1 wt.% metal contents were measured. From ISEC analyses before and after metal deposition, it became evident that lower concentrated polymer domains (i.e., 0.4 nmnm−3) decreased with the metal incorporation while increasing higher concentrated domains (0.8 nmnm−3) [56]. Regarding the catalytic results, x-chloronitrobenzene, x-chloroaniline, aniline, x-chloronitrosobenzene, and nitrobenzene were detected in the reaction medium after the catalytic tests, with x-chloroaniline being the target product in that work (i.e., reduction of the NO2 group, without hydrodechlorination). Significantly higher activity, but lower selectivity to x-chloroaniline, was found for the Pd-containing catalysts in the OH− form than for their chloride counterparts. As indicated by the authors, in previous works by Krátky et al. (2002, 2003), where a Pd-doped, ST-DVB sulfonated IER was compared to Pd/charcoal catalyst and the effect of different solvents was evaluated, it had already been established that condensation reactions occurring in basic environments lowered the selectivity to x-chloroaniline [57][58]. All Pt catalysts in Králik et al. (2014) were more active than the Pd ones, which the authors linked to the lower average size of Pt crystallites (2.3 nm) in comparison with the Pd ones (4.5 nm) [56].
Han and coworkers used AmberliteTM IRA-900 and IRA-958 (both in Cl− form) as support for Pd (0.1–11.2 wt.%, 3–5 nm) to catalyze the complete hydrodechlorination of triclosan (5-chloro-2-(2,4-dichlorophenoxy)phenol). Both resins are of the macroreticular type with quaternary amine as the functional group, but IRA-900 is an ST-DVB resin whereas IRA-958 is acrylic. IRA-958 showed better performance than IRA-900, which was attributed to the hydrophobicity, pore size, and surface area of its polymeric matrix, and to larger Pd particle size (4.6 and 3.7 nm for IRA-958 and IRA-900, respectively, but with some larger aggregates of 20–40 nm diameter range observed on IRA-958). Both catalysts were reused multiple times without significant loss in effectivity or leaching [59].
On the other hand, Jadbabaei et al. (2017) used nonionic polymeric resins (that is crosslinked AmberliteTM XAD-4 and hyper-crosslinked Purolite® MN200 and MN100, the latter with a small fraction of tertiary amine functional groups) and basic ST-DVB IER (i.e., AmberliteTM IRA-910 and IRA-96, with dimethyl-ethanolammonium and tertiary amine functional groups, respectively) as supports for Pd catalysts aiming at the catalytic hydrodechlorination of 4-chlorophenol. A Langmuir-Hinshelwood kinetic model was developed, with surface reaction as the rate-determining step, which suggested an enhancing effect of adsorption on the catalytic reactivity. IRA910 was found to facilitate Cl− adsorption and promote the production of PdCl3− and PdCl42− species, which are inactive for catalytic reduction [60].
Hydrogenolysis of glycerol, which involves chemical bond cleavage with the simultaneous addition of a hydrogen atom to the resulting molecular fragments [61], yields the production of 1,2- and 1,3-propanediol, together with ethylene glycol. Kusunoki et al. (2005), Miyazawa et al. (2006), and Centomo et al. (2013) studied this reaction system in connection to the use of IER. However, physical mixtures of carbon-supported metal catalysts (e.g., Ru/C, Pt/C, Pd/C, Rh/C) with IER were found to be much more selective towards 1,2-propanediol (which is the preferred target product) than tested metal-doped IER [62][63][64]. Furthermore, in the framework of the hydrogenolysis of glycerol, van Ryneveld and coworkers combined Ru/C catalysts with thermal-resistant IERs, such as AmberlystTM DT and AmberlystTM 70 [65]. Regarding hydrodeoxygenation, a particular type of hydrogenolysis involving oxygen removal from oxygen-containing compounds (which is an interesting reaction system for the valorization of biomass waste streams, e.g., [66]), has been found to be effectively achieved by monometallic Cu/C and bimetallic Mg-Cu/C obtained from the weakly acidic, acrylic IER DiaionTM WK11, which was calcinated after ion-exchanging it with the designated precursor salts in Wang et al. (2021, 2022). Particularly relevant features of those works are the reported high metal loadings (>50 wt.%) and small metal particle sizes (<15 nm) [67][68].
References
- Zecca, M.; Fišera, R.; Palma, G.; Lora, S.; Hronec, M.; Králik, M. Activity Enhancement by the Support in the Hydrogenation of C=C Bonds over Polymer-Supported Palladium Catalysts. Chem.-A Eur. J. 2000, 6, 1980–1986.
- Drelinkiewicz, A.; Knapik, A.; Waksmundzka-Góra, A.; Bukowska, A.; Bukowski, W.; Noworól, J. Functional Gel-Type Resin Based Palladium Catalysts: The Role of Polymer Properties in the Hydrogenation of the CC Bond of Maleic and Fumaric Acids, the Isomers of Dicarboxylic Acids. React. Funct. Polym. 2008, 68, 1059–1071.
- Marrodan, C.M.; Berti, D.; Liguori, F.; Barbaro, P. In Situ Generation of Resin-Supported Pd Nanoparticles under Mild Catalytic Conditions: A Green Route to Highly Efficient, Reusable Hydrogenation Catalysts. Catal. Sci. Technol. 2012, 2, 2279.
- Madureira, A.; Noël, S.; Léger, B.; Ponchel, A.; Monflier, E. Catalytic Hydrogenation of Derived Vegetable Oils Using Ion-Exchange Resin-Supported Ruthenium Nanoparticles: Scope and Limitations. ACS Sustain. Chem. Eng. 2022, 10, 16588–16597.
- Barbaro, P.; Bianchini, C.; Giambastiani, G.; Oberhauser, W.; Bonzi, L.M.; Rossi, F.; Dal Santo, V. Recycling Asymmetric Hydrogenation Catalysts by Their Immobilisation onto Ion-Exchange Resins. Dalton Trans. 2004, 12, 1783.
- Barbaro, P. Recycling Asymmetric Hydrogenation Catalysts by Their Immobilization onto Ion-Exchange Resins. Chem.-A Eur. J. 2006, 12, 5666–5675.
- Kleman, P.; Barbaro, P.; Pizzano, A. Chiral Rh Phosphine–Phosphite Catalysts Immobilized on Ionic Resins for the Enantioselective Hydrogenation of Olefins in Water. Green Chem. 2015, 17, 3826–3836.
- Seki, T.; Grunwaldt, J.D.; van Vegten, N.; Baiker, A. Palladium Supported on an Acidic Resin: A Unique Bifunctional Catalyst for the Continuous Catalytic Hydrogenation of Organic Compounds in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2008, 350, 691–705.
- Delbecq, F.; Sautet, P. Competitive C C and C O Adsorption of α-β-Unsaturated Aldehydes on Pt and Pd Surfaces in Relation with the Selectivity of Hydrogenation Reactions: A Theoretical Approach. J. Catal. 1995, 152, 217–236.
- Hronec, M.; Cvengrošová, Z.; Králik, M.; Palma, G.; Corain, B. Hydrogenation of Benzene to Cyclohexene over Polymer-Supported Ruthenium Catalysts. J. Mol. Catal. A Chem. 1996, 105, 25–30.
- Hetterley, R.D.; Mackey, R.; Jones, J.T.A.; Khimyak, Y.Z.; Fogg, A.M.; Kozhevnikov, I.V. One-Step Conversion of Acetone to Methyl Isobutyl Ketone over Pd-Mixed Oxide Catalysts Prepared from Novel Layered Double Hydroxides. J. Catal. 2008, 258, 250–255.
- Jeřábek, K. Palladium Hydrogenation Catalysts Supported on Ion-Exchange Resins. J. Mol. Catal. 1989, 55, 247–255.
- Drelinkiewicz, A.; Stanuch, W.; Knapik, A.; Ghanem, A.; Kosydar, R.; Bukowska, A.; Bukowski, W. Amine Groups Functionalized Gel-Type Resin Supported Pd Catalysts: Physicochemical and Catalytic Properties in Hydrogenation of Alkynes. J. Mol. Catal. A Chem. 2009, 300, 8–18.
- Silva, T.R.; de Oliveira, D.C.; Pal, T.; Domingos, J.B. The Catalytic Evaluation of Bimetallic Pd-Based Nanocatalysts Supported on Ion Exchange Resin in Nitro and Alkyne Reduction Reactions. New J. Chem. 2019, 43, 7083–7092.
- Sulman, E.M.; Nikoshvili, L.Z.; Matveeva, V.G.; Tyamina, I.Y.; Sidorov, A.I.; Bykov, A.V.; Demidenko, G.N.; Stein, B.D.; Bronstein, L.M. Palladium Containing Catalysts Based on Hypercrosslinked Polystyrene for Selective Hydrogenation of Acetylene Alcohols. Top. Catal. 2012, 55, 492–497.
- Nikoshvili, L.; Shimanskaya, E.; Bykov, A.; Yuranov, I.; Kiwi-Minsker, L.; Sulman, E. Selective Hydrogenation of 2-Methyl-3-Butyn-2-Ol over Pd-Nanoparticles Stabilized in Hypercrosslinked Polystyrene: Solvent Effect. Catal. Today 2015, 241, 179–188.
- Biffis, A.; Ricoveri, R.; Campestrini, S.; Kralik, M.; Jeřàbek, K.; Corain, B. Highly Chemoselective Hydrogenation of 2-Ethylanthraquinone to 2-Ethylanthrahydroquinone Catalyzed by Palladium Metal Dispersed inside Highly Lipophilic Functional Resins. Chem.-A Eur. J. 2002, 8, 2962.
- Bombi, G.; Lora, S.; Zancato, M.; D’Archivio, A.A.; Jeřábek, K.; Corain, B. Generating Palladium Nanoclusters inside Very Lipophilic Gel-Type Functional Resins: Preliminary Catalytic Tests in the Hydrogenation of 2-Ethyl-Anthraquinone to 2-Ethylanthrahydroquinone. J. Mol. Catal. A Chem. 2003, 194, 273–281.
- Centomo, P.; Zecca, M.; Lora, S.; Vitulli, G.; Caporusso, A.; Tropeano, M.; Milone, C.; Galvagnio, S.; Corain, B. Novel Pt Catalysts Supported on Functional Resins for the Chemoselective Hydrogenation of Citral to the -Unsaturated Alcohols Geraniol and Nerol. J. Catal. 2005, 229, 283–297.
- Barbaro, P.; Gonsalvi, L.; Guerriero, A.; Liguori, F. Facile Heterogeneous Catalytic Hydrogenations of C=N and C=O Bonds in Neat Water: Anchoring of Water-Soluble Metal Complexes onto Ion-Exchange Resins. Green Chem. 2012, 14, 3211.
- Bykov, A.; Matveeva, V.; Sulman, M.; Valetskiy, P.; Tkachenko, O.; Kustov, L.; Bronstein, L.; Sulman, E. Enantioselective Catalytic Hydrogenation of Activated Ketones Using Polymer-Containing Nanocomposites. Catal. Today 2009, 140, 64–69.
- Michel, C.; Gallezot, P. Why Is Ruthenium an Efficient Catalyst for the Aqueous-Phase Hydrogenation of Biosourced Carbonyl Compounds? ACS Catal. 2015, 5, 4130–4132.
- Barbaro, P.; Liguori, F.; Moreno-Marrodan, C. Selective Direct Conversion of C 5 and C 6 Sugars to High Added-Value Chemicals by a Bifunctional, Single Catalytic Body. Green Chem. 2016, 18, 2935–2940.
- Moreno-Marrodan, C.; Barbaro, P. Energy Efficient Continuous Production of γ-Valerolactone by Bifunctional Metal/Acid Catalysis in One Pot. Green Chem. 2014, 16, 3434–3438.
- Moreno-Marrodan, C.; Liguori, F.; Mercadé, E.; Godard, C.; Claver, C.; Barbaro, P. A Mild Route to Solid-Supported Rhodium Nanoparticle Catalysts and Their Application to the Selective Hydrogenation Reaction of Substituted Arenes. Catal. Sci. Technol. 2015, 5, 3762–3772.
- Sulman, E.M.; Ivanov, A.A.; Chernyavsky, V.S.; Sulman, M.G.; Bykov, A.I.; Sidorov, A.I.; Doluda, V.Y.; Matveeva, V.G.; Bronstein, L.M.; Stein, B.D.; et al. Kinetics of Phenol Hydrogenation over Pd-Containing Hypercrosslinked Polystyrene. Chem. Eng. J. 2011, 176–177, 33–41.
- Corain, B. Functional Resins as Innovative Supports for Catalytically Active Metal Nanoclusters. J. Mol. Catal. A Chem. 2003, 204–205, 755–762.
- Corain, B.; Zecca, M.; Canton, P.; Centomo, P. Synthesis and Catalytic Activity of Metal Nanoclusters inside Functional Resins: An Endeavour Lasting 15 Years. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1495–1507.
- Gelbard, G. Organic Synthesis by Catalysis with Ion-Exchange Resins. Ind. Eng. Chem. Res. 2005, 44, 8468–8498.
- Gašparovičová, D.; Králik, M.; Hronec, M.; Biffis, A.; Zecca, M.; Corain, B. Reduction of Nitrates Dissolved in Water over Palladium-Copper Catalysts Supported on a Strong Cationic Resin. J. Mol. Catal. A Chem. 2006, 244, 258–266.
- Gašparovičová, D.; Králik, M.; Hronec, M.; Vallušová, Z.; Vinek, H.; Corain, B. Supported Pd–Cu Catalysts in the Water Phase Reduction of Nitrates: Functional Resin versus Alumina. J. Mol. Catal. A Chem. 2007, 264, 93–102.
- Mendow, G.; Sánchez, A.; Grosso, C.; Querini, C.A. A Novel Process for Nitrate Reduction in Water Using Bimetallic Pd-Cu Catalysts Supported on Ion Exchange Resin. J. Environ. Chem. Eng. 2017, 5, 1404–1414.
- Bradu, C.; Căpăţ, C.; Papa, F.; Frunza, L.; Olaru, E.-A.; Crini, G.; Morin-Crini, N.; Euvrard, É.; Balint, I.; Zgura, I.; et al. Pd-Cu Catalysts Supported on Anion Exchange Resin for the Simultaneous Catalytic Reduction of Nitrate Ions and Reductive Dehalogenation of Organochlorinated Pollutants from Water. Appl. Catal. A Gen. 2019, 570, 120–129.
- Talwalkar, S.; Thotla, S.; Sundmacher, K.; Mahajani, S. Simultaneous Hydrogenation and Isomerization of Diisobutylenes over Pd-Doped Ion-Exchange Resin Catalyst. Ind. Eng. Chem. Res. 2009, 48, 10857–10863.
- Seki, T.; Grunwaldt, J.-D.; Baiker, A. Continuous Catalytic “One-Pot” Multi-Step Synthesis of 2-Ethylhexanal from Crotonaldehyde. Chem. Commun. 2007, 3562–3564.
- Wissler, M.C.; Jagusch, U.P.; Sundermann, B.; Hoelderich, W.F. One-Pot Synthesis of a New Potential Analgesic over Bifunctional Palladium/Amberlyst Catalysts. Catal. Today 2007, 121, 6–12.
- Yamaguchi, A.; Sato, O.; Mimura, N.; Shirai, M. One-Pot Conversion of Cellulose to Isosorbide Using Supported Metal Catalysts and Ion-Exchange Resin. Catal. Commun. 2015, 67, 59–63.
- Galletti, A.M.R.; Antonetti, C.; de Luise, V.; Martinelli, M. A Sustainable Process for the Production of γ-Valerolactone by Hydrogenation of Biomass-Derived Levulinic Acid. Green Chem. 2012, 14, 688.
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of Levulinic Acid and Use as a Platform Chemical for Derived Products. Resour. Conserv. Recycl. 2000, 28, 227–239.
- Wright, W.R.H.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valerolactone. ChemSusChem 2012, 5, 1657–1667.
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-Valerolactone from Lignocellulosic Biomass for Sustainable Fuels and Chemicals Supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620.
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic Reactions of Gamma-Valerolactone: A Platform to Fuels and Value-Added Chemicals. Appl. Catal. B 2015, 179, 292–304.
- Piskun, A.S.; de Haan, J.E.; Wilbers, E.; van de Bovenkamp, H.H.; Tang, Z.; Heeres, H.J. Hydrogenation of Levulinic Acid to γ-Valerolactone in Water Using Millimeter Sized Supported Ru Catalysts in a Packed Bed Reactor. ACS Sustain. Chem. Eng. 2016, 4, 2939–2950.
- Moreno-Marrodan, C.; Liguori, F.; Barbaro, P.; Caporali, S.; Merlo, L.; Oldani, C. Metal Nanoparticles Supported on Perfluorinated Superacid Polymers: A Family of Bifunctional Catalysts for the Selective, One-Pot Conversion of Vegetable Substrates in Water. ChemCatChem 2017, 9, 4256–4267.
- Talwalkar, S.; Mahajani, S. Synthesis of Methyl Isobutyl Ketone from Acetone over Metal-Doped Ion Exchange Resin Catalyst. Appl. Catal. A Gen. 2006, 302, 140–148.
- Vandersall, M.T.; Weinand, R.A. Metal-Doped Sulfonated Ion Exchange Resin Catalysts. European Patent EP 1 321 450 A2, 25 June 2003.
- Vandersall, M.T.; Weinand, R.A. Metal Doped Sulfonated Ion Exchange Resin Catalysts. U.S. Patent 6,977,314, 20 December 2005.
- Nicol, W.; du Toit, E.L. One-Step Methyl Isobutyl Ketone Synthesis from Acetone and Hydrogen Using Amberlyst® CH28. Chem. Eng. Process. Process Intensif. 2004, 43, 1539–1545.
- Bombos, D.; Bombos, M.; Stanciu, I.; Bacalum, F.; Matei, V.; Juganaru, T.; Neagoe, S.; Naum, N. Bifunctional Catalysts Based on Ion-Exchangers. Analele Universităţii din Bucureşti – Chimie, Anul XIV (serie nouă), 2005; Volume I–II, pp. 417–423.
- O’Keefe, W.K.; Ng, F.T.T.; Rempel, G.L. Experimental Studies on the Syntheses of Mesityl Oxide and Methyl Isobutyl Ketone via Catalytic Distillation. Ind. Eng. Chem. Res. 2007, 46, 716–725.
- Trejo, J.A.; Tate, J.; Martenak, D.; Huby, F.; Baxter, S.M.; Schultz, A.K.; Olsen, R.J. State of the Art Bifunctional Hydrogenation Heterogeneous Polymeric Catalyst. Top. Catal. 2010, 53, 1156–1162.
- Liguori, F.; Oldani, C.; Capozzoli, L.; Calisi, N.; Barbaro, P. Liquid-Phase Synthesis of Methyl Isobutyl Ketone over Bifunctional Heterogeneous Catalysts Comprising Cross-Linked Perfluorinated Sulfonic Acid Aquivion Polymers and Supported Pd Nanoparticles. Appl. Catal. A Gen. 2021, 610, 117957.
- Sterchele, S.; Biasi, P.; Centomo, P.; Canton, P.; Campestrini, S.; Salmi, T.; Zecca, M. Pd-Au and Pd-Pt Catalysts for the Direct Synthesis of Hydrogen Peroxide in Absence of Selectivity Enhancers. Appl. Catal. A Gen. 2013, 468, 160–174.
- Burato, C.; Centomo, P.; Rizzoli, M.; Biffis, A.; Campestrini, S.; Corain, B. Functional Resins as Hydrophilic Supports for Nanoclustered Pd(0) and Pd(0)-Au(0) Catalysts Designed for the Direct Synthesis of Hydrogen Peroxide. Adv. Synth. Catal. 2006, 348, 255–259.
- Frison, F.; Dalla Valle, C.; Evangelisti, C.; Centomo, P.; Zecca, M. Direct Synthesis of Hydrogen Peroxide under Semi-Batch Conditions over Un-Promoted Palladium Catalysts Supported by Ion-Exchange Sulfonated Resins: Effects of the Support Morphology. Catalysts 2019, 9, 124.
- Králik, M.; Vallušová, Z.; Major, P.; Takáčová, A.; Hronec, M.; Gašparovičová, D. Hydrogenation of Chloronitrobenzenes over Pd and Pt Catalysts Supported on Cationic Resins. Chem. Pap. 2014, 68, 1690–1700.
- Krátky, V.; Králik, M.; Mecarova, M.; Stolcova, M.; Zalibera, L.; Hronec, M. Effect of Catalyst and Substituents on the Hydrogenation of Chloronitrobenzenes. Appl. Catal. A Gen. 2002, 235, 225–231.
- Krátky, V.; Králik, M.; Kaszonyi, A.; Stolcová, M.; Zalibera, L.; Mecárová, M.; Hronec, M. Reaction Pathways and the Role of Solvent in the Hydrogenation of Chloronitrobenzenes. Collect. Czechoslov. Chem. Commun. 2003, 68, 1819–1832.
- Han, B.; Liu, W.; Li, J.; Wang, J.; Zhao, D.; Xu, R.; Lin, Z. Catalytic Hydrodechlorination of Triclosan Using a New Class of Anion-Exchange-Resin Supported Palladium Catalysts. Water Res. 2017, 120, 199–210.
- Jadbabaei, N.; Ye, T.; Shuai, D.; Zhang, H. Development of Palladium-Resin Composites for Catalytic Hydrodechlorination of 4-Chlorophenol. Appl. Catal. B 2017, 205, 576–586.
- Yan, L.; Zhang, Q.; Deng, W.; Zhang, Q.; Wang, Y. Catalytic Valorization of Biomass and Bioplatforms to Chemicals through Deoxygenation. In Advances in Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 66, pp. 1–108. ISBN 9780128203699.
- Kusunoki, Y.; Miyazawa, T.; Kunimori, K.; Tomishige, K. Highly Active Metal–Acid Bifunctional Catalyst System for Hydrogenolysis of Glycerol under Mild Reaction Conditions. Catal. Commun. 2005, 6, 645–649.
- Miyazawa, T.; Kusunoki, Y.; Kunimori, K.; Tomishige, K. Glycerol Conversion in the Aqueous Solution under Hydrogen over Ru/C + an Ion-Exchange Resin and Its Reaction Mechanism. J. Catal. 2006, 240, 213–221.
- Centomo, P.; Nese, V.; Sterchele, S.; Zecca, M. Resin-Based Catalysts for the Hydrogenolysis of Glycerol to Propylene Glycol. Top. Catal. 2013, 56, 822–830.
- Van Ryneveld, E.; Mahomed, A.S.; van Heerden, P.S.; Friedrich, H.B. Direct Hydrogenolysis of Highly Concentrated Glycerol Solutions Over Supported Ru, Pd and Pt Catalyst Systems. Catal. Lett. 2011, 141, 958–967.
- Dierks, M.; Cao, Z.; Rinaldi, R. Design of Task-Specific Metal Phosphides for the Sustainable Manufacture of Advanced Biofuels. In Advances in Inorganic Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 77, pp. 219–239. ISBN 9780323850582.
- Wang, W.; Nakagawa, K.; Yoshikawa, T.; Masuda, T.; Fumoto, E.; Koyama, Y.; Tago, T.; Fujitsuka, H. Selective Aqueous Phase Hydrodeoxygenation of Erythritol over Carbon-Supported Cu Catalyst Prepared from Ion-Exchange Resin. Appl. Catal. A Gen. 2021, 619, 118152.
- Wang, W.; Tago, T.; Fujitsuka, H. Hydrodeoxygenation of C3–4 Polyols to C3–4 Diols over Carbon-Supported Bimetallic Catalysts Prepared from Ion Exchange Resin. Catal. Today 2022, 411–412, 29–31.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
30 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




