Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qi, Q.; Wang, Q.; Li, Y.; Silva, D.Z.; Ruiz, M.E.L.; Ouyang, R.; Liu, B.; Miao, Y. Rhenium in Biological Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/42617 (accessed on 03 February 2026).
Qi Q, Wang Q, Li Y, Silva DZ, Ruiz MEL, Ouyang R, et al. Rhenium in Biological Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/42617. Accessed February 03, 2026.
Qi, Qingwen, Qian Wang, Yuhao Li, Dionisio Zaldivar Silva, Maria Eliana Lanio Ruiz, Ruizhuo Ouyang, Baolin Liu, Yuqing Miao. "Rhenium in Biological Imaging" Encyclopedia, https://encyclopedia.pub/entry/42617 (accessed February 03, 2026).
Qi, Q., Wang, Q., Li, Y., Silva, D.Z., Ruiz, M.E.L., Ouyang, R., Liu, B., & Miao, Y. (2023, March 29). Rhenium in Biological Imaging. In Encyclopedia. https://encyclopedia.pub/entry/42617
Qi, Qingwen, et al. "Rhenium in Biological Imaging." Encyclopedia. Web. 29 March, 2023.
Copy Citation
Rhenium (Re) is widely used in the diagnosis and treatment of cancer due to its unique physical and chemical properties. Re has more valence electrons in its outer shell, allowing it to exist in a variety of oxidation states and to form different geometric configurations with many different ligands. The luminescence properties, lipophilicity, and cytotoxicity of complexes can be adjusted by changing the ligand of Re.
rhenium
complexes
imaging
1. Introduction
With the rapid development of modern science and technology, people’s living conditions have been greatly improved in all aspects, so the life span of human beings has been significantly extended. Many studies have shown that the incidence of cancer often increases with age [1][2][3], which may be due to long-term exposure to carcinogens [4], in vivo environments being more conducive to malignant cell proliferation [5], cumulative mutation [6], etc. In any case, cancer has gradually become one of the most threatening and deadly diseases to human health. Therefore, researchers have invested a lot of energy and material resources into the development of cancer treatment, and have made some achievements. In the diagnosis of cancer, several imaging techniques [7] such as computed tomography (CT) [8][9], magnetic resonance imaging (MRI) [10], fluorescence imaging [11][12][13][14], photoacoustic (PA) imaging [15], infrared thermal imaging [16], ultrasound (US) imaging [17][18][19], positron emission tomography (PET) [20][21][22], and single photon emission computed tomography (SPECT) [23][24] have been developed. Cancer detection has become more sensitive and accurate. In the treatment of cancer, radiotherapy (RT) [25][26], chemotherapy [27][28][29][30], photodynamic therapy (PDT) [31][32][33][34][35][36], photothermal therapy (PTT) [37][38][39], sonodynamic therapy (SDT) [40][41][42], immunotherapy [43][44][45], starvation therapy (ST) [33][46][47], magnetothermal therapy (MHT) [48][49], and other therapies have also shown good anticancer effects. As a remarkable diagnostics reagent candidate, the Re element has attracted the attention of many researchers because of its unique physical and chemical properties.
The atomic number of Re is 75 and its electron arrangement is [Xe]4f145d56s2. There are two natural isotopes 185Re (37.4%) and 187Re (62.6%) that exist in nature, which are potential candidates for medical applications [50]. However, 188Re is often used in cancer radiotherapy because of the relatively weak radiation emission of 187Re. Based on the fact that there are many valence electrons in the outer layer of rhenium, it has a variety of oxidation states, which makes it possible to form different geometries with many different ligands. Therefore, Re complexes have been widely studied in the field of anticancer for a long time. At present, the photoluminescence, lipophilicity, cytotoxicity, cell uptake, biological distribution, pharmacology, and toxicology of Re complexes can be regulated by changing their ligands [51]. The structural formulas of rhenium complexes mainly involved in this paper are shown in Figure 1, Figure 2 and Figure 3. Meanwhile, some inorganic nanomaterials of Re have strong absorption capacity in the near-infrared (NIR) region and a high Z property. Therefore, studies on multimodal imaging-guided synergistic cancer therapy have been popular in recent years.
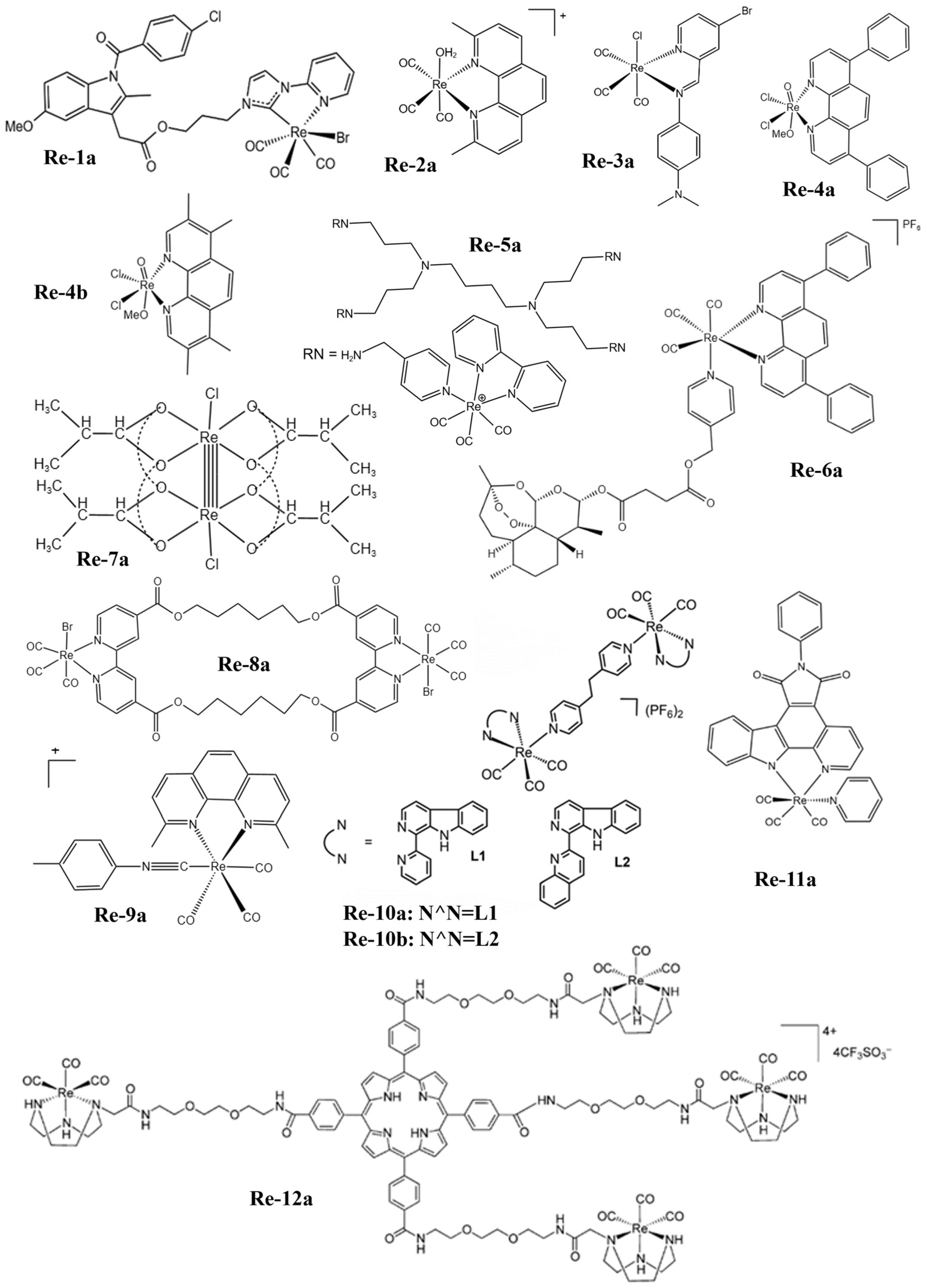
Figure 1. The structures of some Re complexes (Re-1a−Re-12a).
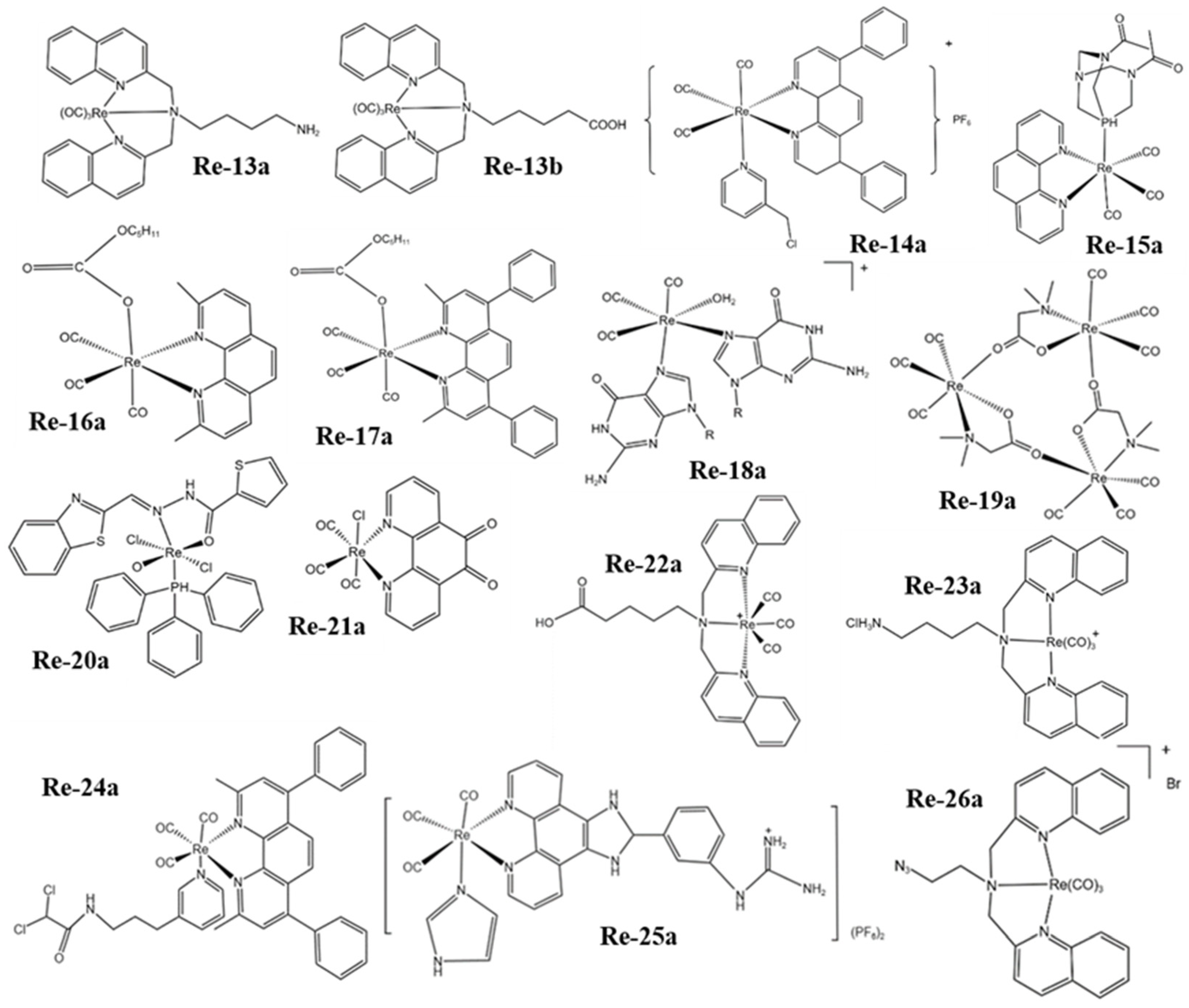
Figure 2. The structures of some Re complexes (Re-13a−Re-26a).
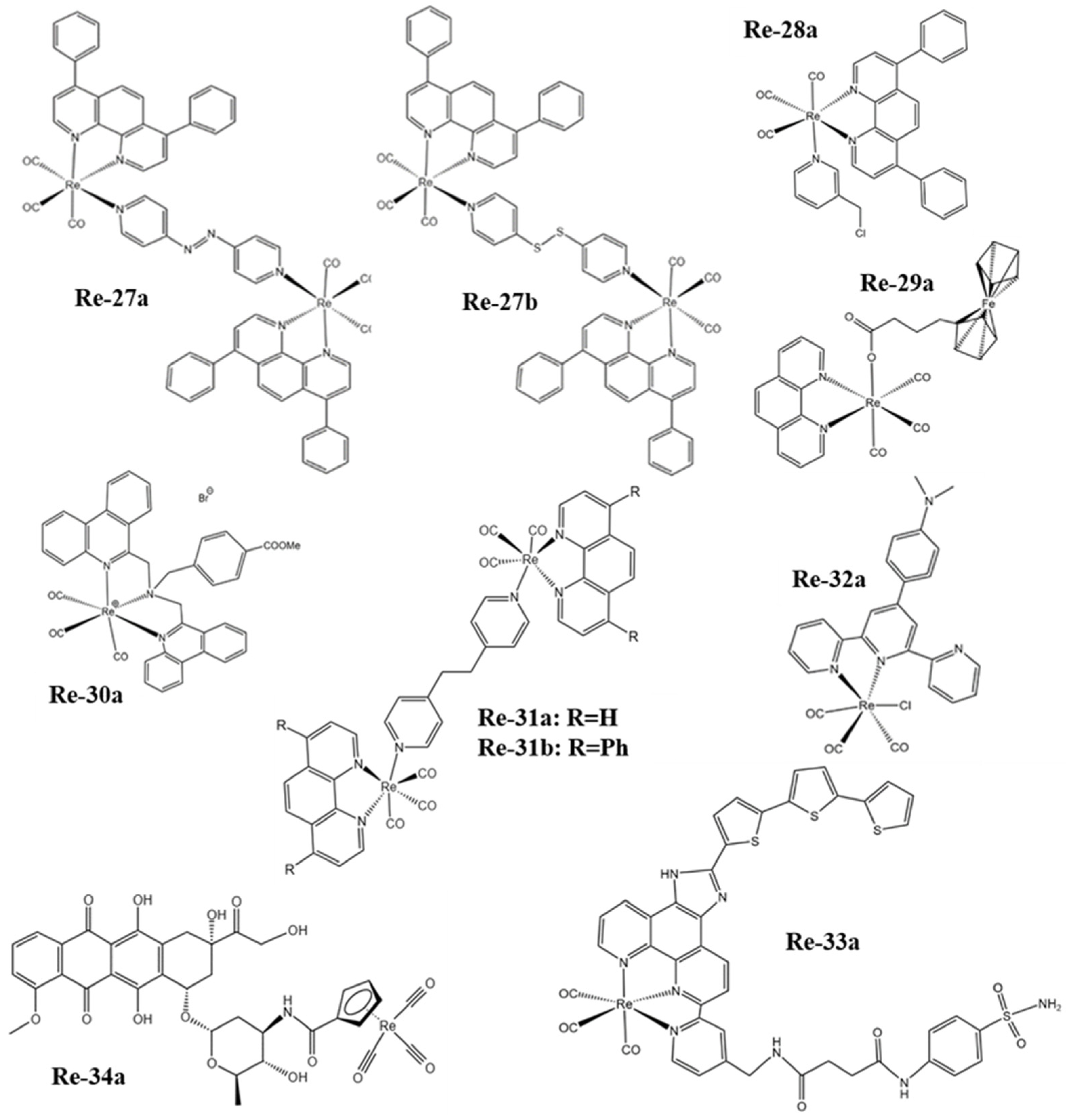
Figure 3. The structures of some Re complexes (Re-27a−Re-34a).
2. Application of Re in Biological Imaging
Many Re-related compounds have potential in biological imaging. Most Re complexes can emit phosphorescence, so they are suitable for bio-optical imaging. Optical imaging is widely used from the cell to the in vivo level, but it is limited by the wavelength of excitation. The limited depth of light penetration at the in vivo level makes the imaging resolution low. Based on the high atomic number characteristics of Re, Re complexes or nanomaterials can also be used as CT sensitizers to increase the imaging signal-to-noise ratio. Thus, rhenium compounds have biomedical imaging ability for the substances.
2.1. Optical Imaging
Re(I) tricarbonyl complexes are widely used in biological imaging because of their unique luminescent characteristics, such as long luminescent lifetime, large Stokes shift, high quantum yield, and strong absorption in the infrared transparent window (1800–2200 cm−1) of biological media [52][53][54][55][56][57].
Yang et al. [58] found that the phosphorescence intensity and lifetime of the Re-14a complex (Figure 1) showed excellent oxygen sensitivity. Specifically, the higher the O2 concentration, the weaker the phosphorescence intensity and the shorter the lifetime. Therefore, they monitored the O2 consumption in cancer cells using phosphorescence lifetime imaging, and further studied the changes in mitochondrial metabolism (Figure 4a). In addition, they found that the Re complex Re-24a (Figure 2) also had the same properties in later research [59].
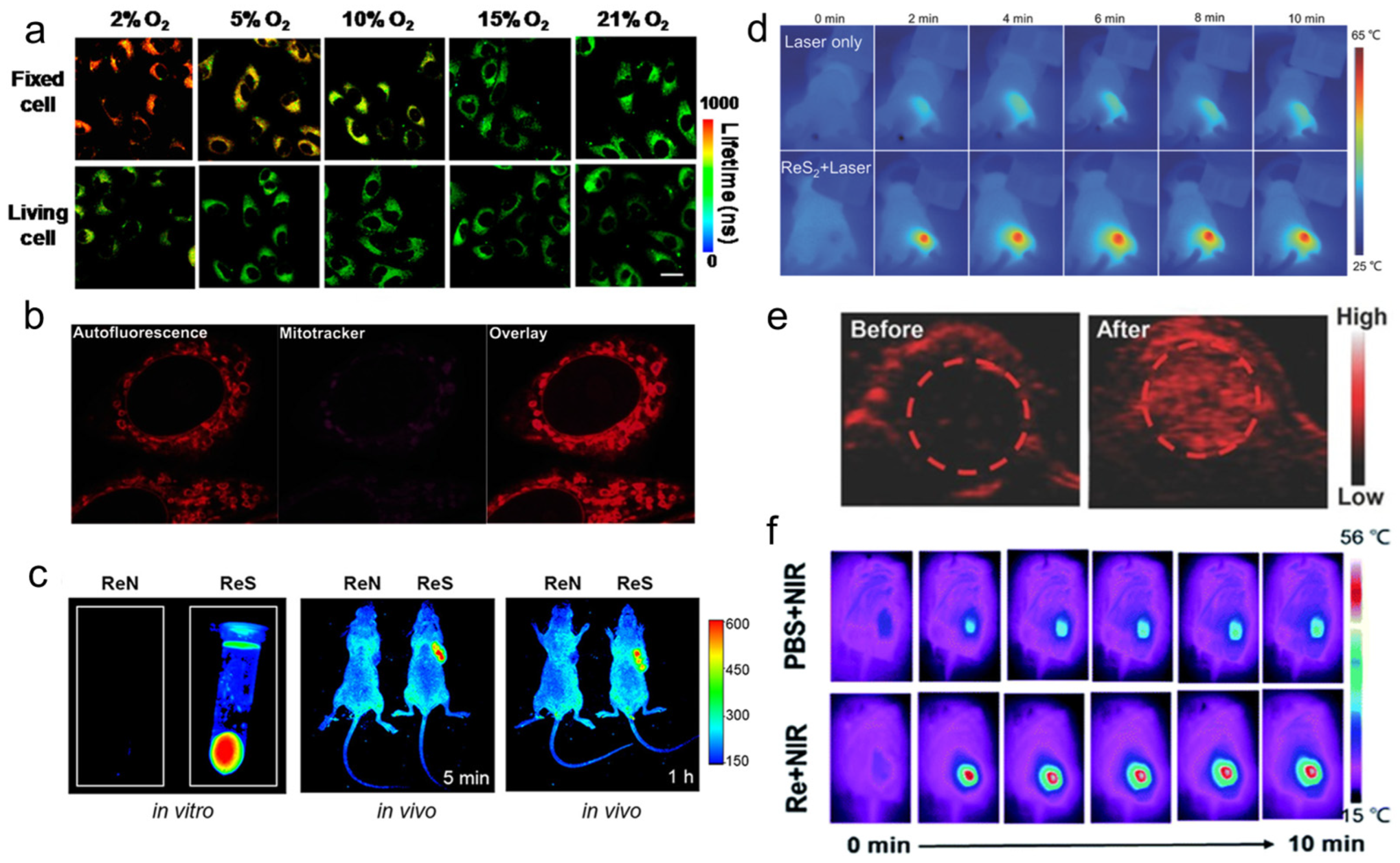
Figure 4. (a) PLIM images of Re-14a-treated fixed and living A549 cells under different oxygen partial pressures at 37 °C; (b) magnified images showing an overlay of Cp-Dox autofluorescence and MitoTracker fluorescence in HeLa cells, suggesting an accumulation of the Dox conjugate in the mitochondrial membrane; (c) fluorescence emission of Re-27a and Re-27b in vitro and in vivo; (d) thermal images of PVP capped ReS2 in in vivo PTT; (e) PA images of tumors in mice before and 24 h after i.v. injection of ReS2-PEG; (f) thermal imaging of tumor-bearing mice after injection of PBS or Re NCs. PLIM, phosphorescence lifetime imaging; PVP, polyvinyl pyrrolidone; PTT, photothermal therapy; PA, photoacoustic; PEG, polyethylene glycol; PBS, phosphate buffer saline; NCs, nanoclusters.
Imstepf et al. [60] observed that Re-34a (Figure 3) could selectively stain the mitochondrial membrane using a fluorescence microscope in the study of transferring doxorubicin to mitochondria via the rhenium complex, which indicated that Re-34a interacted with the membrane through lipophilicity, and finally proved that the Re complex could relocate doxorubicin from the nucleus to mitochondria (Figure 4b). Wang et al. [61] studied the optical imaging performance of intratumoral injection of Re-27a and Re-27b (Figure 2), and the results showed that Re-27b had better emission ability in vivo (Figure 4c).
Because infrared imaging does not involve electronic transition, photobleaching does not occur, but the sensitivity is usually not satisfactory. Most Re inorganic nano-materials used in tumor therapy have made use of their excellent photothermal conversion ability, so that they have the function of infrared thermal imaging, and usually have the ability of PA imaging. In the process of tumor treatment, infrared thermal imaging, PA, and CT imaging are usually combined to help guide cancer treatment more accurately [62][63][64]. Miao et al. [62] and Shen et al. [63] have studied the infrared thermal imaging (Figure 4d) and PA imaging (Figure 4e) properties of different ReS2 nanomaterials. In addition, Miao et al. [65] later studied the infrared thermal imaging performance of PEGylated Re NCs (Figure 4f). These results show that Re compounds have better optical imaging ability.
2.2. CT Imaging
Because of the high atomic number property of Re itself (Z = 75), Re compounds usually have strong X-ray attenuation ability and can be used as CT contrast agents to enhance CT signals (Figure 5), which has been proven by many research results [63][64][65].
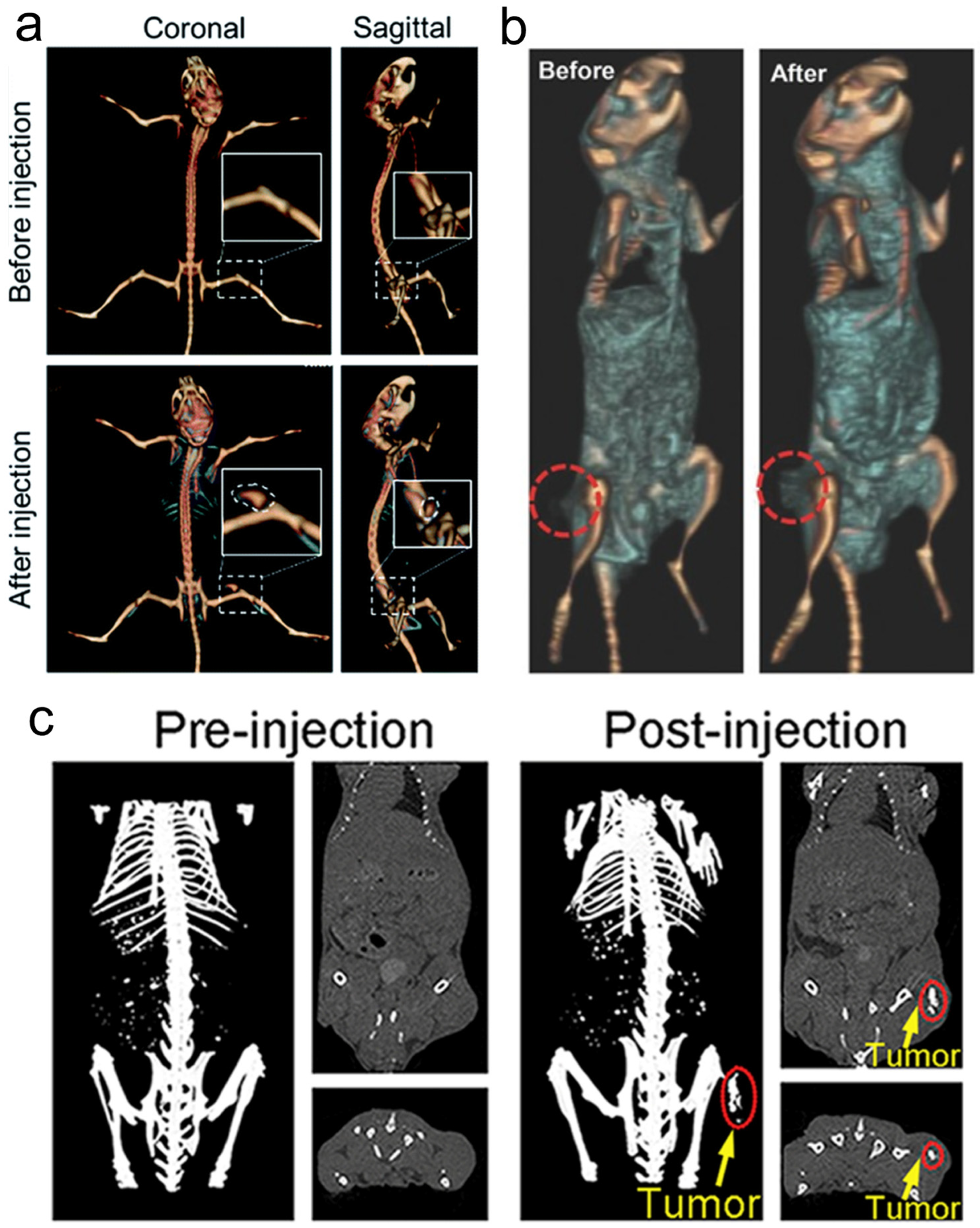
Figure 5. (a) CT imaging in vivo of 4T1 tumor-bearing BALB/c mice before and after injection of Re NCs; (b) CT images of mice before and 24 h after i.v. injection of ReS2-PEG; (c) in vivo CT imaging before (pre) and after (post) i.t. injection with ReO3 NCs. CT, computed tomography; PEG, polyethylene glycol; NCs, nanoclusters.
References
- Williams, G.M.; Baker, G.T. The potential relationships between aging and cancer. Exp. Gerontol. 1992, 27, 469–476.
- Anisimov, V.N.; Sikora, E.; Pawelec, G. Relationships between cancer and aging: A multilevel approach. Biogerontology 2009, 10, 323–338.
- Dimri, G.P. What has senescence got to do with cancer? Cancer Cell 2005, 7, 505–512.
- Xiaowei, L. Exposure to carcinogens and its effects in the formation of cancer. J. Cancer Res. Immunooncol. 2020, 6, 123.
- Anisimov, V.N. The relationship between aging and carcinogenesis: A critical appraisal. Crit. Rev. Oncol. Hematol. 2003, 45, 277–304.
- Smith, A.L.M.; Whitehall, J.C.; Greaves, L.C. Mitochondrial DNA mutations in ageing and cancer. Mol. Oncol. 2020, 16, 3276–3294.
- Guobo, G.; Yuhao, L.; Baolin, L.; Yuqing, M. Recent developments in bismuth oxyhalide-based functional nanomaterials for biomedical applications. Biomater. Sci. 2022, 10, 5809–5830.
- Shi, H.; Wang, Z.; Huang, C.; Gu, X.; Jia, T.; Zhang, A.; Wu, Z.; Zhu, L.; Luo, X.; Zhao, X.; et al. A functional CT contrast agent for in vivo imaging of tumor hypoxia. Small 2016, 12, 3995–4006.
- Yeh, B.M.; FitzGerald, P.F.; Edic, P.M.; Lambert, J.W.; Colborn, R.E.; Marino, M.E.; Evans, P.M.; Roberts, J.C.; Wang, Z.J.; Wong, M.J.; et al. Opportunities for new CT contrast agents to maximize the diagnostic potential of emerging spectral CT technologies. Adv. Drug Deliv. Rev. 2017, 113, 201–222.
- Peng, Y.-K.; Tsang, S.C.E.; Chou, P.-T. Chemical design of nanoprobes for T1-weighted magnetic resonance imaging. Mater. Today 2016, 19, 336–348.
- Diao, S.; Blackburn, J.L.; Hong, G.; Antaris, A.L.; Chang, J.; Wu, J.Z.; Zhang, B.; Cheng, K.; Kuo, C.J.; Dai, H. Fluorescence imaging in vivo at wavelengths beyond 1500 nm. Angew. Chem. Int. Ed. 2015, 54, 14758–14762.
- Ji, Y.; Jones, C.; Baek, Y.; Park, G.K.; Kashiwagi, S.; Choi, H.S. Near-infrared fluorescence imaging in immunotherapy. Adv. Drug Deliver. Rev. 2020, 167, 121–134.
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-specific near-infrared fluorescence imaging. Acc. Chem. Res. 2016, 49, 1731–1740.
- Deng, Y.; Huang, F.; Zhang, J.; Liu, J.; Li, B.; Ouyang, R.; Miao, Y.; Sun, Y.; Li, Y. PEGylated iridium-based nano-micelle: Self-assembly, selective tumor fluorescence imaging and photodynamic therapy. Dyes Pigments 2020, 182, 108651.
- Cui, L.; Rao, J. Semiconducting polymer nanoparticles as photoacoustic molecular imaging probes. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1418.
- McManus, C.; Tanure, C.B.; Peripolli, V.; Seixas, L.; Fischer, V.; Gabbi, A.M.; Menegassi, S.R.O.; Stumpf, M.T.; Kolling, G.J.; Dias, E.; et al. Infrared thermography in animal production: An overview. Comput. Electron. Agric. 2016, 123, 10–16.
- Averkiou, M.A.; Bruce, M.F.; Powers, J.E.; Sheeran, P.S.; Burns, P.N. Imaging methods for ultrasound contrast agents. Ultrasound Med. Biol. 2020, 46, 498–517.
- Borden, M.A.; Song, K.-H. Reverse engineering the ultrasound contrast agent. Adv. Colloid Interface Sci. 2018, 262, 39–49.
- Lin, P.-L.; Eckersley, R.J.; Hall, E.A.H. Ultrabubble: A laminated ultrasound contrast agent with narrow size range. Adv. Mater. 2009, 21, 3949–3952.
- Chen, F.; Goel, S.; Hernandez, R.; Graves, S.A.; Shi, S.; Nickles, R.J.; Cai, W. Dynamic positron emission tomography imaging of renal clearable gold nanoparticles. Small 2016, 12, 2775–2782.
- Bongarzone, S.; Sementa, T.; Dunn, J.; Bordoloi, J.; Sunassee, K.; Blower, P.J.; Gee, A. Imaging biotin trafficking in vivo with positron emission tomography. J. Med. Chem. 2020, 63, 8265–8275.
- Hou, S.; Choi, J.S.; Garcia, M.A.; Xing, Y.; Chen, K.J.; Chen, Y.M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted positron emission tomography imaging that employs supramolecular nanoparticles with in vivo bioorthogonal chemistry. ACS Nano 2016, 10, 1417–1424.
- Black, K.C.; Akers, W.J.; Sudlow, G.; Xu, B.; Laforest, R.; Achilefu, S. Dual-radiolabeled nanoparticle SPECT probes for bioimaging. Nanoscale 2015, 7, 440–444.
- Ding, L.; Lyu, Z.; Tintaru, A.; Laurini, E.; Marson, D.; Louis, B.; Bouhlel, A.; Balasse, L.; Fernandez, S.; Garrigue, P.; et al. A self-assembling amphiphilic dendrimer nanotracer for SPECT imaging. Chem. Commun. 2019, 56, 301–304.
- Aslan, T.N.; Aşık, E.; Volkan, M. Preparation and labeling of surface-modified magnetoferritin protein cages with a rhenium(I) carbonyl complex for magnetically targeted radiotherapy. RSC Adv. 2016, 6, 8860–8869.
- Nguyen, K.A.; Lee, A.; Patel, S.A.; Chakravorty, A.; Yu, J.B.; Kishan, A.U.; Chang, A.J. Trends in use and comparison of stereotactic body radiation therapy, brachytherapy, and dose-escalated external beam radiation therapy for the management of localized, intermediate-risk prostate cancer. JAMA Netw. Open 2020, 3, e2017144.
- Yang, J.; Yao, H.; Guo, Y.; Yang, B.; Shi, J. Enhancing tumor catalytic therapy by co-catalysis. Angew. Chem. Int. Ed. 2022, 61, e202200480.
- Wu, S.; Wang, P.; Qin, J.; Pei, Y.; Wang, Y. GSH-Depleted nanozymes with dual-radicals enzyme activities for tumor synergic therapy. Adv. Funct. Mater. 2021, 31, 2102160.
- Ju, Y.; Wang, Z.; Ali, Z.; Zhang, H.; Wang, Y.; Xu, N.; Yin, H.; Sheng, F.; Hou, Y. A pH-responsive biomimetic drug delivery nanosystem for targeted chemo-photothermal therapy of tumors. Nano Res. 2022, 15, 4274–4284.
- Abuduwaili, W.; Wang, X.; Huang, A.-T.; Sun, J.-L.; Xu, R.-C.; Zhang, G.-C.; Liu, Z.-Y.; Wang, F.; Zhu, C.-F.; Liu, T.-T.; et al. Iridium complex-loaded sorafenib nanocomposites for synergistic chemo-photodynamic therapy of hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2022, 14, 37356–37368.
- Luo, T.; Fan, Y.; Mao, J.; Yuan, E.; You, E.; Xu, Z.; Lin, W. Dimensional reduction enhances photodynamic therapy of metal-organic nanophotosensitizers. J. Am. Chem. Soc. 2022, 144, 5241–5246.
- Jia, T.; Xu, J.; Dong, S.; He, F.; Zhong, C.; Yang, G.; Bi, H.; Xu, M.; Hu, Y.; Yang, D.; et al. Mesoporous cerium oxide-coated upconversion nanoparticles for tumor-responsive chemo-photodynamic therapy and bioimaging. Chem. Sci. 2019, 10, 8618–8633.
- Gao, X.; Feng, J.; Song, S.; Liu, K.; Du, K.; Zhou, Y.; Lv, K.; Zhang, H. Tumor-targeted biocatalyst with self-accelerated cascade reactions for enhanced synergistic starvation and photodynamic therapy. Nano Today 2022, 43, 101433.
- Wang, X.; Song, K.; Deng, Y.; Liu, J.; Peng, Q.; Lao, X.; Xu, J.; Wang, D.; Shi, T.; Li, Y.; et al. Benzothiazole-decorated iridium-based nanophotosensitizers for photodynamic therapy of cancer cells. Dalton Trans. 2022, 51, 3666–3675.
- Xu, Y.; Wang, X.; Song, K.; Du, J.; Liu, J.; Miao, Y.; Li, Y. BSA-encapsulated cyclometalated iridium complexes as nano-photosensitizers for photodynamic therapy of tumor cells. RSC Adv. 2021, 11, 15323–15331.
- Deng, Y.; Pan, S.; Zheng, J.; Hong, Y.; Liu, J.; Chang, H.; Miao, Y.; Sun, Y.; Li, Y. Electrostatic self-assembled iridium(III) nano-photosensitizer for selectively disintegrated and mitochondria targeted photodynamic therapy. Dyes Pigments 2020, 175, 108105.
- Liu, B.; Jiang, F.; Sun, J.; Wang, F.; Liu, K. Biomacromolecule-based photo-thermal agents for tumor treatment. J. Mater. Chem. B 2021, 9, 7007–7022.
- Zhang, L.; Zhang, Y.; Xue, Y.; Wu, Y.; Wang, Q.; Xue, L.; Su, Z.; Zhang, C. Transforming weakness into strength: Photothermal-therapy-induced inflammation enhanced cytopharmaceutical chemotherapy as a combination anticancer treatment. Adv. Mater. 2019, 31, e1805936.
- Deng, Y.; Wang, X.; Liu, Y.; Xu, Y.; Zhang, J.; Huang, F.; Li, B.; Miao, Y.; Sun, Y.; Li, Y. Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy. Acta Biomater. 2021, 119, 323–336.
- Geng, P.; Yu, N.; Liu, X.; Zhu, Q.; Wen, M.; Ren, Q.; Qiu, P.; Zhang, H.; Li, M.; Chen, Z. Sub 5 nm Gd3+ -hemoporfin framework nanodots for augmented sonodynamic theranostics and fast renal clearance. Adv. Healthcare Mater. 2021, 10, e2100703.
- Gong, F.; Cheng, L.; Yang, N.; Betzer, O.; Feng, L.; Zhou, Q.; Li, Y.; Chen, R.; Popovtzer, R.; Liu, Z. Ultrasmall oxygen-deficient bimetallic oxide MnWOX nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv. Mater. 2019, 31, e1900730.
- Song, K.; Chen, G.; He, Z.; Shen, J.; Ping, J.; Li, Y.; Zheng, L.; Miao, Y.; Zhang, D. Protoporphyrin-sensitized degradable bismuth nanoformulations for enhanced sonodynamic oncotherapy. Acta Biomater. 2023, 158, 637–648.
- Wei, D.; Chen, Y.; Huang, Y.; Li, P.; Zhao, Y.; Zhang, X.; Wan, J.; Yin, X.; Liu, T.; Yin, J.; et al. NIR-light triggered dual-cascade targeting core-shell nanoparticles enhanced photodynamic therapy and immunotherapy. Nano Today 2021, 41, 101288.
- Ding, Y.; Sun, Z.; Gao, Y.; Zhang, S.; Yang, C.; Qian, Z.; Jin, L.; Zhang, J.; Zeng, C.; Mao, Z.; et al. Plasmon-driven catalytic chemotherapy augments cancer immunotherapy through induction of immunogenic cell death and blockage of IDO pathway. Adv. Mater. 2021, 33, e2102188.
- Zheng, L.; Fan, Y.; Wang, X.; Yang, Z.; Zhang, Y.; Liu, T.; Chen, M.; Kang, S.; Guo, S.; Shi, Z.; et al. Nanoagonist-mediated GSDME-dependent pyroptosis remodels the inflammatory microenvironment for tumor photoimmunotherapy. Adv. Funct. Mater. 2022, 33, 2200811.
- Yu, S.; Chen, Z.; Zeng, X.; Chen, X.; Gu, Z. Advances in nanomedicine for cancer starvation therapy. Theranostics 2019, 9, 8026–8047.
- Yang, B.; Ding, L.; Chen, Y.; Shi, J. Augmenting tumor-starvation therapy by cancer cell autophagy inhibition. Adv. Sci. 2020, 7, 1902847.
- Beola, L.; Asin, L.; Roma-Rodrigues, C.; Fernandez-Afonso, Y.; Fratila, R.M.; Serantes, D.; Ruta, S.; Chantrell, R.W.; Fernandes, A.R.; Baptista, P.V.; et al. The intracellular number of magnetic nanoparticles modulates the apoptotic death pathway after magnetic hyperthermia treatment. ACS Appl. Mater. Interfaces 2020, 12, 43474–43487.
- Yoo, D.; Jeong, H.; Noh, S.H.; Lee, J.H.; Cheon, J. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew. Chem. Int. Ed. 2013, 52, 13047–13051.
- Tu, W.; Denizot, B. Synthesis of small-sized rhenium sulfide colloidal nanoparticles. J. Colloid Interface Sci. 2007, 310, 167–170.
- Collery, P.; Desmaele, D.; Vijaykumar, V. Design of rhenium compounds in targeted anticancer therapeutics. Curr. Pharm. Des. 2019, 25, 3306–3322.
- Hostachy, S.; Policar, C.; Delsuc, N. Re(I) carbonyl complexes: Multimodal platforms for inorganic chemical biology. Coord. Chem. Rev. 2017, 351, 172–188.
- Lo, K.K.W.; Zhang, K.Y.; Li, S.P.Y. Recent exploitation of luminescent rhenium(I) tricarbonyl polypyridine complexes as biomolecular and cellular probes. Eur. J. Inorg. Chem. 2011, 2011, 3551–3568.
- Clede, S.; Policar, C. Metal-carbonyl units for vibrational and luminescence imaging: Towards multimodality. Chem. Eur. J. 2015, 21, 942–958.
- Moherane, L.; Alexander, O.T.; Schutte-Smith, M.; Kroon, R.E.; Mokolokolo, P.P.; Biswas, S.; Prince, S.; Visser, H.G.; Manicum, A.-L.E. Polypyridyl coordinated rhenium(I) tricarbonyl complexes as model devices for cancer diagnosis and treatment. Polyhedron 2022, 228, 116178.
- Sharma, S.A.; Vaibhavi, N.; Kar, B.; Das, U.; Paira, P. Target-specific mononuclear and binuclear rhenium(I) tricarbonyl complexes as upcoming anticancer drugs. RSC Adv. 2022, 12, 20264–20295.
- Li, Y.; Luo, Z.; Song, Y.; Yuan, Y.; Peng, X.; Song, J.; Qu, J. Rhenium disulfide nanosheets as a promising probe for intracellular two-photon luminescence imaging. Sens. Actuators B Chem. 2022, 362, 131781.
- Yang, J.; Zhao, J.X.; Cao, Q.; Hao, L.; Zhou, D.; Gan, Z.; Ji, L.N.; Mao, Z.W. Simultaneously inducing and tracking cancer cell metabolism repression by mitochondria-immobilized rhenium(I) complex. ACS Appl. Mater. Interfaces 2017, 9, 13900–13912.
- Yang, J.; Cao, Q.; Zhang, H.; Hao, L.; Zhou, D.; Gan, Z.; Li, Z.; Tong, Y.X.; Ji, L.N.; Mao, Z.W. Targeted reversal and phosphorescence lifetime imaging of cancer cell metabolism via a theranostic rhenium(I)-DCA conjugate. Biomaterials 2018, 176, 94–105.
- Imstepf, S.; Pierroz, V.; Rubbiani, R.; Felber, M.; Fox, T.; Gasser, G.; Alberto, R. Organometallic rhenium complexes divert doxorubicin to the mitochondria. Angew. Chem. 2016, 128, 2842–2845.
- Wang, F.X.; Liang, J.H.; Zhang, H.; Wang, Z.H.; Wan, Q.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Mitochondria-accumulating rhenium(I) tricarbonyl complexes induce cell death via irreversible oxidative stress and glutathione metabolism disturbance. ACS Appl. Mater. Interfaces 2019, 11, 13123–13133.
- Miao, Z.H.; Lv, L.X.; Li, K.; Liu, P.Y.; Li, Z.; Yang, H.; Zhao, Q.; Chang, M.; Zhen, L.; Xu, C.Y. Liquid exfoliation of colloidal rhenium disulfide nanosheets as a multifunctional theranostic agent for in vivo photoacoustic/CT imaging and photothermal therapy. Small 2018, 14, 1703789.
- Shen, S.; Chao, Y.; Dong, Z.; Wang, G.; Yi, X.; Song, G.; Yang, K.; Liu, Z.; Cheng, L. Bottom-up preparation of uniform ultrathin rhenium disulfide nanosheets for image-guided photothermal radiotherapy. Adv. Funct. Mater. 2017, 27, 1700250.
- Zhang, W.; Deng, G.; Li, B.; Zhao, X.; Ji, T.; Song, G.; Xiao, Z.; Cao, Q.; Xiao, J.; Huang, X.; et al. Degradable rhenium trioxide nanocubes with high localized surface plasmon resonance absorbance like gold for photothermal theranostics. Biomaterials 2018, 159, 68–81.
- Miao, Z.; Chen, S.; Xu, C.Y.; Ma, Y.; Qian, H.; Xu, Y.; Chen, H.; Wang, X.; He, G.; Lu, Y.; et al. PEGylated rhenium nanoclusters: A degradable metal photothermal nanoagent for cancer therapy. Chem. Sci. 2019, 10, 5435–5443.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
827
Revisions:
2 times
(View History)
Update Date:
30 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




