Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Rhenium (Re) is widely used in the diagnosis and treatment of cancer due to its unique physical and chemical properties. Re has more valence electrons in its outer shell, allowing it to exist in a variety of oxidation states and to form different geometric configurations with many different ligands. The luminescence properties, lipophilicity, and cytotoxicity of complexes can be adjusted by changing the ligand of Re.
- rhenium
- complexes
- imaging
1. Introduction
With the rapid development of modern science and technology, people’s living conditions have been greatly improved in all aspects, so the life span of human beings has been significantly extended. Many studies have shown that the incidence of cancer often increases with age [1,2,3], which may be due to long-term exposure to carcinogens [4], in vivo environments being more conducive to malignant cell proliferation [5], cumulative mutation [6], etc. In any case, cancer has gradually become one of the most threatening and deadly diseases to human health. Therefore, researchers have invested a lot of energy and material resources into the development of cancer treatment, and have made some achievements. In the diagnosis of cancer, several imaging techniques [7] such as computed tomography (CT) [8,9], magnetic resonance imaging (MRI) [10], fluorescence imaging [11,12,13,14], photoacoustic (PA) imaging [15], infrared thermal imaging [16], ultrasound (US) imaging [17,18,19], positron emission tomography (PET) [20,21,22], and single photon emission computed tomography (SPECT) [23,24] have been developed. Cancer detection has become more sensitive and accurate. In the treatment of cancer, radiotherapy (RT) [25,26], chemotherapy [27,28,29,30], photodynamic therapy (PDT) [31,32,33,34,35,36], photothermal therapy (PTT) [37,38,39], sonodynamic therapy (SDT) [40,41,42], immunotherapy [43,44,45], starvation therapy (ST) [33,46,47], magnetothermal therapy (MHT) [48,49], and other therapies have also shown good anticancer effects. As a remarkable diagnostics reagent candidate, the Re element has attracted the attention of many researchers because of its unique physical and chemical properties.
The atomic number of Re is 75 and its electron arrangement is [Xe]4f145d56s2. There are two natural isotopes 185Re (37.4%) and 187Re (62.6%) that exist in nature, which are potential candidates for medical applications [50]. However, 188Re is often used in cancer radiotherapy because of the relatively weak radiation emission of 187Re. Based on the fact that there are many valence electrons in the outer layer of rhenium, it has a variety of oxidation states, which makes it possible to form different geometries with many different ligands. Therefore, Re complexes have been widely studied in the field of anticancer for a long time. At present, the photoluminescence, lipophilicity, cytotoxicity, cell uptake, biological distribution, pharmacology, and toxicology of Re complexes can be regulated by changing their ligands [51]. The structural formulas of rhenium complexes mainly involved in this paper are shown in Figure 1, Figure 2 and Figure 3. Meanwhile, some inorganic nanomaterials of Re have strong absorption capacity in the near-infrared (NIR) region and a high Z property. Therefore, studies on multimodal imaging-guided synergistic cancer therapy have been popular in recent years.
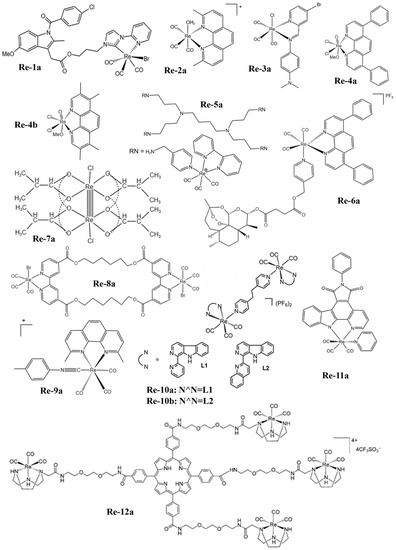
Figure 1. The structures of some Re complexes (Re-1a−Re-12a).
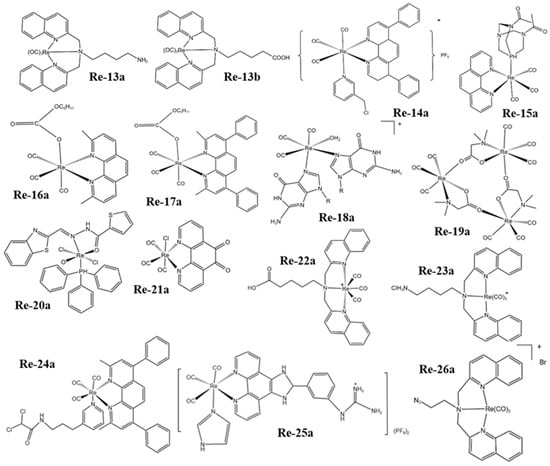
Figure 2. The structures of some Re complexes (Re-13a−Re-26a).
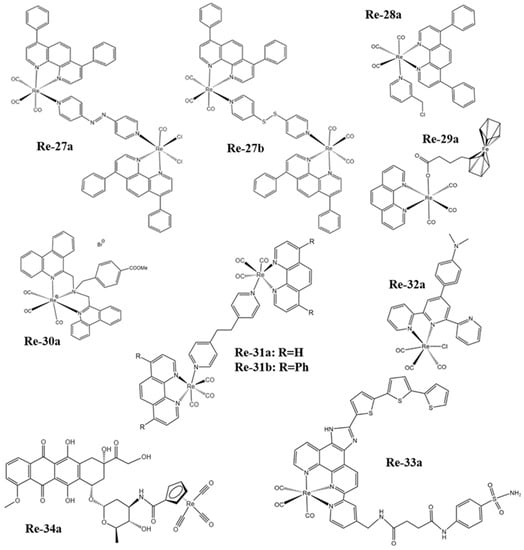
Figure 3. The structures of some Re complexes (Re-27a−Re-34a).
2. Application of Re in Biological Imaging
Many Re-related compounds have potential in biological imaging. Most Re complexes can emit phosphorescence, so they are suitable for bio-optical imaging. Optical imaging is widely used from the cell to the in vivo level, but it is limited by the wavelength of excitation. The limited depth of light penetration at the in vivo level makes the imaging resolution low. Based on the high atomic number characteristics of Re, Re complexes or nanomaterials can also be used as CT sensitizers to increase the imaging signal-to-noise ratio. Thus, rhenium compounds have biomedical imaging ability for the substances.
2.1. Optical Imaging
Re(I) tricarbonyl complexes are widely used in biological imaging because of their unique luminescent characteristics, such as long luminescent lifetime, large Stokes shift, high quantum yield, and strong absorption in the infrared transparent window (1800–2200 cm−1) of biological media [117,138,139,140,141,142].
Yang et al. [108] found that the phosphorescence intensity and lifetime of the Re-14a complex (Figure 1) showed excellent oxygen sensitivity. Specifically, the higher the O2 concentration, the weaker the phosphorescence intensity and the shorter the lifetime. Therefore, they monitored the O2 consumption in cancer cells using phosphorescence lifetime imaging, and further studied the changes in mitochondrial metabolism (Figure 4a). In addition, they found that the Re complex Re-24a (Figure 2) also had the same properties in later research [104].
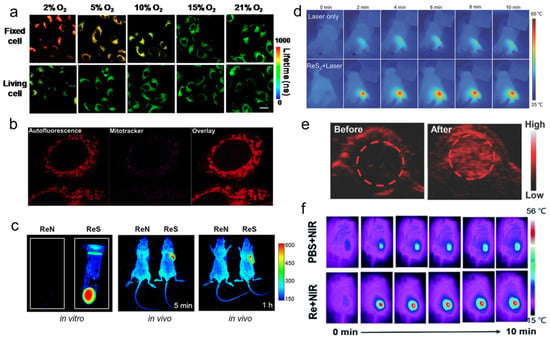
Figure 4. (a) PLIM images of Re-14a-treated fixed and living A549 cells under different oxygen partial pressures at 37 °C; (b) magnified images showing an overlay of Cp-Dox autofluorescence and MitoTracker fluorescence in HeLa cells, suggesting an accumulation of the Dox conjugate in the mitochondrial membrane; (c) fluorescence emission of Re-27a and Re-27b in vitro and in vivo; (d) thermal images of PVP capped ReS2 in in vivo PTT; (e) PA images of tumors in mice before and 24 h after i.v. injection of ReS2-PEG; (f) thermal imaging of tumor-bearing mice after injection of PBS or Re NCs. PLIM, phosphorescence lifetime imaging; PVP, polyvinyl pyrrolidone; PTT, photothermal therapy; PA, photoacoustic; PEG, polyethylene glycol; PBS, phosphate buffer saline; NCs, nanoclusters.
Imstepf et al. [114] observed that Re-34a (Figure 3) could selectively stain the mitochondrial membrane using a fluorescence microscope in the study of transferring doxorubicin to mitochondria via the rhenium complex, which indicated that Re-34a interacted with the membrane through lipophilicity, and finally proved that the Re complex could relocate doxorubicin from the nucleus to mitochondria (Figure 4b). Wang et al. [107] studied the optical imaging performance of intratumoral injection of Re-27a and Re-27b (Figure 2), and the results showed that Re-27b had better emission ability in vivo (Figure 4c).
Because infrared imaging does not involve electronic transition, photobleaching does not occur, but the sensitivity is usually not satisfactory. Most Re inorganic nano-materials used in tumor therapy have made use of their excellent photothermal conversion ability, so that they have the function of infrared thermal imaging, and usually have the ability of PA imaging. In the process of tumor treatment, infrared thermal imaging, PA, and CT imaging are usually combined to help guide cancer treatment more accurately [132,133,137]. Miao et al. [132] and Shen et al. [133] have studied the infrared thermal imaging (Figure 4d) and PA imaging (Figure 4e) properties of different ReS2 nanomaterials. In addition, Miao et al. [131] later studied the infrared thermal imaging performance of PEGylated Re NCs (Figure 4f). These results show that Re compounds have better optical imaging ability.
2.2. CT Imaging
Because of the high atomic number property of Re itself (Z = 75), Re compounds usually have strong X-ray attenuation ability and can be used as CT contrast agents to enhance CT signals (Figure 5), which has been proven by many research results [131,133,137].
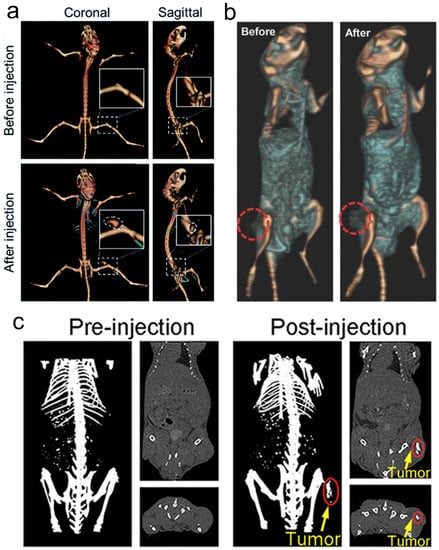
Figure 5. (a) CT imaging in vivo of 4T1 tumor-bearing BALB/c mice before and after injection of Re NCs; (b) CT images of mice before and 24 h after i.v. injection of ReS2-PEG; (c) in vivo CT imaging before (pre) and after (post) i.t. injection with ReO3 NCs. CT, computed tomography; PEG, polyethylene glycol; NCs, nanoclusters.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28062733
This entry is offline, you can click here to edit this entry!
