
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elio Santacesaria | -- | 1657 | 2023-03-29 03:06:48 | | | |
| 2 | Catherine Yang | -168 word(s) | 1489 | 2023-03-29 03:17:51 | | | | |
| 3 | Catherine Yang | -8 word(s) | 1481 | 2023-03-30 02:29:18 | | | | |
| 4 | Catherine Yang | Meta information modification | 1481 | 2023-03-30 02:40:47 | | |
Video Upload Options
Ethyl acetate hydrogenation is the reverse reaction of ethanol dehydrogenation. Tuthenium complexes acting in homogeneous phase, at very low temperatures, promote both ethanol dehydrogenation and ethylacetate hydrogenation.
1. Thermodynamics of the Occurring Reactions
As mentioned, ethyl acetate hydrogenation is the reverse reaction of ethanol dehydrogenation. Therefore, it can be written for the overall reaction:
CH3COOC2H5+2 H2 2 C2H5OH (1)
ΔH°500K = - 33.64 kJ/mol; ΔG°500K = 6.44 kJ/mol; Kp-500 = 0.21
This reaction very probably occurs in two steps that are:
CH3COOCH2CH3 + H2 CH3CHO + CH3CH2OH (2)
ΔH°500K = 37.660 kJ /mol; ΔG°500K = 17.03 kJ /mol
CH3CHO + H2 CH3CH2OH (3)
ΔH°500K = -71.30 kJ/mol; ΔG°500K = -10.59 kJ/mol
We have then Kp1-500 = 0.0166 and Kp2-500 = 12.76.
Calculations have been made on thermodynamic data reported by Stull et al. [1] .
2. Catalysts Normally Employed for P.romoting the Ethyl Acetate Hydrogenation Reaction
The interest in ethyl-acetate hydrogenation to ethanol is due to the possibility to use ethanol as an alternative fuel and many papers have been published on the subject. Adkins and Folker firstly described the reaction of esters hydrogenation promoted by a copper-chromium catalyst, being copper with chromium additive often used for fatty esters hydrogenation [2][3][4][5][6]. In fact, copper catalysts are highly active for C=O bond hydrogenation but much less active for C-C bond cleavage and this explains the choice of copper-based catalysts for the ester hydrogenation. More recently, as chromium poses environmental problems, research is focused on the development of chromium-free catalysts. For this purpose, the addition to copper of other metal oxides, such as ZrO2, Fe2O3, or ZnO has been investigated with the aim of promoting the activity of copper in the hydrogenation of esters to alcohols [7]. These works are of great interest for the LOHC process because catalysts very active in the hydrogenation of esters are probably also active in the reverse reaction of alcohol dehydrogenation. However, the hydrogenation of ethyl acetate to ethanol has received poor attention in the past and according to the few published works before 2014 a conversion of 40% of ethyl-acetate with an ethanol selectivity of 80% has been reported [8]. In this article a bimetallic Cu-Zn/SiO2 catalyst has been employed. The catalyst was obtained by calcination of the synthesized compound CuZn(OH)4(H2SiO3)2.4H2O at 473 K in air followed by a reduction step with hydrogen at 573 K. The Cu/Zn molar ratio of 1:1 was the optimal one leading to ethyl acetate conversion of 82 % and ethanol selectivity of 94%. In another work [9], a Zn-promoted Cu-Al2O3 catalyst has been positively tested in a fixed bed reactor carried out at 523 K and 20atm. The catalyst was prepared by impregnating alumina with a mixture of copper nitrate and zinc nitrate in a solution by incipient wetness. By opportunely regulating calcination and reduction conditions the best results were ethyl acetate conversion of 71.5% and ethanol selectivity of 95%. An activity test was prolonged for 90 hours without any apparent catalyst deactivation. Interesting results have been obtained also by Di et al [10] by using two different Cu/SiO2 catalysts. In the first case, an acidic copper nitrate solution was added to a basic sodium silicate solution obtaining a precipitate that was then calcined and reduced, thus providing the Cu/SiO2 catalyst. The other one was prepared by using the urea hydrolysis deposition-precipitation method. Catalytic runs was performed in a fixed bed reactor operating in the temperature range 483-553 K at 30 atm using 1 g of catalyst and an LHSV =.1.24 h-1. The reactor was fed with a molar ratio H2/EA= 29. The best data obtained were 99.7% of ethyl acetate conversion and 99.07 % of selectivity to ethanol. These data seem to be optimistic, probably because of the high H2/EA ratio. Good results have been obtained by Huang et al. [11] by using a Cu/SiO2 catalyst for hydrogenating methyl acetate to methanol+ethanol, obtaining a conversion of 94% and a selectivity of 94%. The catalyst was prepared by the precipitation-gel method by reacting copper nitrate with NaOH and then supporting the gel on silica. On the other hand, Lu et al. [12] prepared by coprecipitation and tested three different catalysts of the type: Cu/ZnO/MOx where MOx could be the supports SiO2, Al2O3, and ZrO2. The best catalyst results were from Cu/ZnO/Al2O3 that, in a fixed bed reactor (filled with 3 g of catalyst) kept at 553 K and 20 atm, gave a conversion of 80% with a selectivity of 95%. A value of the activation energy equal to 115 kJ/mol was estimated for this catalyst. This work can be reinterpreted for a kinetic approach to this reaction. A confirmation of the good performances of the CuZn-SiO2 catalyst has been furnished by Zhao et al [13]. who studied the hydrogenation of the methyl-acetate on the same catalyst. In conclusion, a catalyst active in the hydrogenation of ethyl acetate and probably also in the reverse reaction of ethanol dehydrogenation is a copper-based catalyst with copper extremely dispersed and hindered from sintering for the presence of other oxides. In this regard, the role of Zinc as a promoter seems very important, while the support cannot be acidic to avoid the formation of by-products for the reactions of acetaldehyde condensation.
3. Ethyl Acetate Hydrogenation, Kinetic Methodology in Conventional Reactors
Very few papers have been considered the kinetics of the ethyl-acetate hydrogenation to ethanol on copper-based catalysts [14]. The first one determined the intrinsic kinetics of ethyl-acetate hydrogenolysis to ethanol over a Cu-Zn/Al2O3 catalyst operating in a tubular reactor in the temperature range of 453-503 K, pressure 10-15 atm,, LHSV 0.7-1.9 h-1and molar ratio H2/EA=20-50. A power law kinetic model was applied for interpreting the experimental data and the optimal kinetic parameters were determined by mathematical regression. The second paper studied the kinetic behavior in the ethyl-acetate hydrogenation promoted by a Cu/ZrO2 catalyst prepared by coprecipitation from copper nitrate and zirconium oxynitrate with NaOH as precipitating agent. The kinetics of hydrogenation were studied at atmospheric pressure in the temperature range of 293-513 K. The hydrogenation reaction was followed by gradually increasing the temperature from 293 to 513 K stopping the temperature level for a determined time for determining conversion and yield at intermediate temperatures. The conversion varied therefore from 8 to 45%. The first ethyl acetate hydrogenation reaction was always far from the equilibrium, while acetaldehyde quickly reached the thermodynamic equilibrium. As a matter of fact, acetaldehyde hydrogenation is 6000 times more active than ethyl-acetate hydrogenation. Data were collected on conversion as a function of the residence time, and hydrogenation rates as a function of the ratio H2/Ester at different temperatures. The authors assumed the reaction mechanism suggested by Natal Santiago et al. [15] which is characterized by dissociative adsorption of ethyl acetate yielding acyl and alkoxy species:
CH3COOCH2CH3 + 2 * CH3CO* + CH3CH2O* (4)
The alkoxy fragment is rapidly hydrogenated to ethanol, whilst the acyl fragment is less reactive and, more slowly, can be partially hydrogenated to acetaldehyde or fully hydrogenated to ethanol. A moderate deactivation was observed due to coke deposition occurring at higher temperatures. This phenomenon is hindered by operating in the presence of an excess of hydrogen. A kinetic model is mentioned in the work by using, for the overall reaction, a power law kinetic expression. Reaction orders resulted in 0.1-0.3 for hydrogen and -0.4 to 0.1 for ethyl acetate, the apparent activation energy was 74 kJ/mol.
An example of a laboratory scale plant (Figure 1) is reported by the already cited Zhu et al. [9].
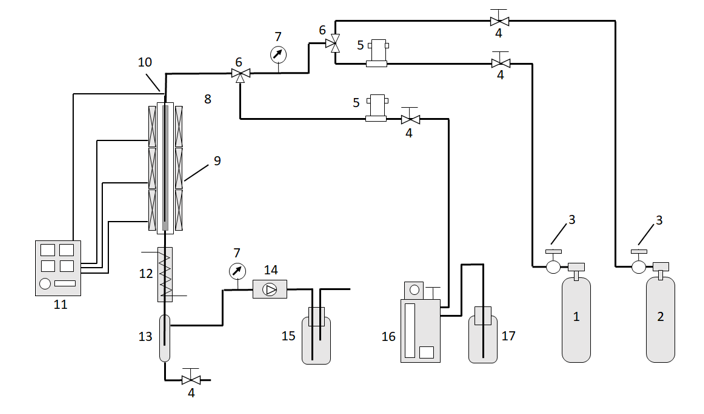
Figure 1 - Fixed-bed reaction system. 1 – H2; 2 – N2; 3 – reducing valve; 4 – valve; 5 – mass flow meter; 6 – triple valve; 7 – pressure gauge; 8 – reactor; 9 – heater; 10 – thermocouple; 11 – temperature controller; 12 – condenser; 13 –gas-liquid separator; 14 – back pressure valve; 15 – gas washing bottle; 16 – microsyringe pump; 17 – raw materials. See ref. [9]. Reported with the permission of Elsevier.
Another example is the one reported by Jiang et al. (see Figure 2 ) .

Figure 2 - 1-Ethyl acetate reservoir; 2- Hydrogen bottle; 3 Pump; 4- Pressure gauge; 5- Bho 6- Pressure reducer; 7-Non-return valve; 8-Tubular reactor; 9-Heating furnace; 10-11Thermocouples and thermoregulators; 12 Liquid condenser; 13- Liquid product container; 14- valve; 15-16 Valves; 17 Gas Chromatograph-;18- Hydrogen to the vent. See ref. [14].
Based on these data, it could be possible to elaborate a simplified scheme for the corresponding LOHC industrial plant.
4. The Industrial Ethanol-Ethylacetate LOHC process
A work published very recently by Mewavala et al. [16] has specifically been devoted to the possibility of using the ethanol-ethyl acetate system as LOHC by examining three different aspects: the thermodynamics of the chemical reaction, the energy balance of the process and the assessment of green house gas emission. This work confirmed that the energy demand for dehydrogenation is small and the authors calculated an energy efficiency of the system equal to 88%. The results obtained by the authors show that the ethanol-ethyl acetate system is very promising as LOHC and is worth further study. In particular, the same authors furnished a complete Block Flow Diagram developed in Aspen Plus 10 of the ethanol-ethyl acetate LOHC process for a production of 500 kg/h of hydrogen.
References
- Stull, D.R.; Vestrum, E.F.; Sinke, G.C. The Chemical Thermodynamics of Organic Compounds, 1st ed.; John Wiley: New York, NY, USA, 1969.
- Thakur, D.S.; Carrick, W. Copper Chromite Hydrogenation Catalysts for Production of Fatty Alcohols. WO 2012/074841, 7 June 2012.
- He, L.; Cheng, H.; Liang, G.; Yu, Y.; Zhao, F. Effect of structure of CuO/ZnO/Al2O3 composites on catalytic performance for hydrogenation of fatty acid ester. Appl. Catal. A Gen. 2013, 452, 88–93.
- Huang, H.; Cao, G.; Wang, S. An evaluation of alkylthiols and dialkyl disulfides on deactivation of Cu/Zn catalyst in hydrogenation of dodecyl methyl ester to dodecanol. J. Ind. Eng. Chem. 2014, 20, 988–993.
- Brands, D.S.; Poels, E.K.; Bliek, A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts. Appl. Catal. A Gen. 1999, 184, 279–289.
- Kim, S.M.; Lee, M.E.; Choi, J.W.; Suh, D.J.; Suh, Y.W. Role of ZnO in Cu/ZnO/Al2O3 catalyst for hydrogenolysis of butyl butyrate. Catal. Commun. 2011, 12, 1328–1332.
- Yuan, P.; Liu, Z.Y.; Zhang, W.; Sun, H.; Liu, S. Cu-Zn/Al2O3 catalyst for the hydrogenation of esters to alcohols. Chin. J. Catal. 2010, 31, 769–775.
- Zhu, Y.; Shi, X.W.L. Hydrogenation of ethyl acetate to ethanol over bimetallic Cu-Zn/SiO2 catalysts prepared by means of coprecipitation. Bull. Korean Chem. Soc. 2014, 35, 141–146.
- Zhu, Y.; Shi, L. Zn promoted Cu–Al catalyst for hydrogenation of ethyl acetate to alcohol. J. Ind. Eng. Chem. 2014, 20, 2341–2347.
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol. Appl. Catal. A Gen. 2016, 510, 244–259.
- Huang, X.; Ma, M.; Miao, S.; Zheng, Y.; Chen, M.; Shen, W. Hydrogenation of methyl acetate to ethanol over a highly stable Cu/SiO2 catalyst: Reaction mechanism and structural evolution. Appl. Catal. A Gen. 2017, 531, 79–88.
- Lu, Z.; Yin, H.; Wang, A.; Hu, J.; Xue, W.; Yin, H.; Liu, S. Hydrogenation of ethyl acetate to ethanol over Cu/ZnO/MOx (MOx = SiO2, Al2O3, and ZrO2) catalysts. J. Ind. Eng. Chem. 2016, 37, 208–215.
- Zhao, Y.; Shan, B.; Wang, Y.; Zhou, J.; Wang, S.; Ma, X. An effective CuZn-SiO2 bimetallic catalyst prepared by hydrolysis precipitation method for the hydrogenation of methyl acetate to ethanol. Ind. Eng. Chem. Res. 2018, 57, 4526–4534.
- Jiang, X.C.; Wang, Z.G.; Li, C.X. Intrinsic kinetics of ethyl acetate hydrogenolysis to ethanol over a Cu-Zn/Al2O3 catalyst. J. Beijing Univ. Chem. Technol. 2014, 41, 36–39.
- Natal Santiago, M.A.; Sánchez-Castillo, M.A.; Cortight, R.D.; Dumesic, J.A. Catalytic reduction of acetic acid, methyl acetate, and ethyl acetate over silica-supported copper. J. Catal. 2000, 193, 16–28.
- Mevawala, C.; Brooks, K.; Bowden, M.E.; Breunig, H.M.; Tran, B.L.; Gutiérrez, O.Y.; Autrey, T.; Müller, K. The ethanol–ethyl acetate system as a biogenic hydrogen carrier. Energy Technol. 2022, 11, 2200892.




