Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pow Seng Yap | -- | 2262 | 2023-03-27 05:14:51 | | | |
| 2 | Catherine Yang | Meta information modification | 2262 | 2023-03-27 07:51:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, Z.; Osman, A.I.; Rooney, D.W.; Oh, W.; Yap, P. Immobilized Algae Bioremediation Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/42546 (accessed on 07 February 2026).
Chen Z, Osman AI, Rooney DW, Oh W, Yap P. Immobilized Algae Bioremediation Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/42546. Accessed February 07, 2026.
Chen, Zhonghao, Ahmed I. Osman, David W. Rooney, Wen-Da Oh, Pow-Seng Yap. "Immobilized Algae Bioremediation Technology" Encyclopedia, https://encyclopedia.pub/entry/42546 (accessed February 07, 2026).
Chen, Z., Osman, A.I., Rooney, D.W., Oh, W., & Yap, P. (2023, March 27). Immobilized Algae Bioremediation Technology. In Encyclopedia. https://encyclopedia.pub/entry/42546
Chen, Zhonghao, et al. "Immobilized Algae Bioremediation Technology." Encyclopedia. Web. 27 March, 2023.
Copy Citation
A green technology that immobilizes algae through a carrier to improve biosorbent’s stability and adsorption performance is immobilization technology. An environmentally friendly technology is bioremediation, which uses the metabolic potential of microorganisms to remove heavy metals through a series of physicochemical interactions which occur between the functional groups of microorganisms and the heavy metals.
water remediation
immobilized algae
biosorption
heavy metals
1. Mechanisms of Heavy Metal Removal by Algae
Heavy metals are metallic elements with densities greater than 5 g/cm3 and are toxic at lower concentrations [1]. Heavy metals are classified into three groups [2][3], (i) transition elements, which contain certain minor amphoteric oxides (titanium (Ti), zirconium (Zr), hafnium (Hf), rutherfordium (Rf), vanadium (V), Niobium (Nb), tantalum (Ta), chromium (Cr), molybdenum (Mo), tungsten (W), manganese (Mn), technetium (Tc), rhenium (Re), ferrum (Fe), ruthenium (Ru), osmium (Os), and zinc (Zn)); (ii) rare earth elements, including lanthanides (with lanthanum (La)) and actinides (with actinium (Ac)); and (iii) elements of the p-group dominated by gallium (Ga), indium (In), thallium (Tl), stannum (Sn), plumbum (Pb), antimony (Sb), bismuth (Bi), and polonium (Po) as the main elements of the p-group. The p-group elements are the elements of the third main group to the seventh main group plus the zero group.
Without changing their own activity, algae are capable of forming cellular protein-heavy metal complexes [4]. Organometallic complexes are further divided inside the vesicles to control the amount of heavy metal ions in the cytoplasm and lessen their hazardous effects [5]. A three-stage mechanism, involving the extracellular precipitation/accumulation of heavy metals by living cells, complexation or cellular adsorption in living and dead cells, and intracellular internalization requiring microbial activity or metabolic processes, allows algae to remove heavy metals from the environment [6][7].
Biosorption activities known as rapid extracellular passive processes can be carried out by both living and non-living biomass. The primary mechanism of heavy metal adsorption by active or passive algal biomass is biosorption, which has been demonstrated to be a practical method for removing heavy metals from industrial effluent [8]. Within a few seconds, heavy metal ions are passively absorbed after interacting with negatively charged functional groups found on the algae cell surface. Heavy metals bind to cell walls that include sulfate, carboxyl, amino, and hydroxyl groups, and the attachment of heavy metal ions occurs via chelation/complexation, adsorption, electrostatic interactions, surface precipitation, and ion exchange to functional groups on the cell surface [9]. Positively or negatively charged ions will bind to the surface of the biosorbent that has been negatively charged in the ion exchange process and has grown to be the predominant mechanism [10]. Electrostatic repulsion between positively charged surfaces and metal cations may be influenced by the protonation of the functional groups of algal biomass particles and the amino and hydroxyl groups of carriers [11]. Cd(II) is transferred from aqueous solutions to algal cell surfaces by membrane flow or boundary layer diffusion, and the immobilized algal cells have more carboxylate groups, resulting in the faster transfer of Cd(II) [12]. Additionally, biosorption can create complexes with functional groups found on the surface of cells [13]. A diverse variety of biopolymers, such as humic compounds, lipids, nucleic acids, polysaccharides, proteins, and glyoxylates, are also found in cyanobacterial extracellular polymer components in algae [14]. Cyanobacterial extracellular polymers play a crucial function in the biosorption of heavy metals and serve as a barrier against hostile external conditions [15]. Polysaccharides enable heavy metals to readily bind to algae surfaces, lipids, and proteins. Moreover, heavy metals have a tendency to precipitate and accumulate on the cell surface when the pH of the solution changes rapidly during biosorption or when the concentration of the metals rises to saturation. This process is another way that algae bind to heavy metals. The heavy metals adsorbed on the surface erode the algal cell surface, while the immobilization process results in a smoother algal cell surface, and some carriers preferentially bind to metal ions, reducing the solution metal concentration, thus making it possible to protect algal cells from adsorption [16]. Algae will produce more extracellular polymeric substances (EPS) rich in negatively charged groups in response to heavy metal ions [17]. These EPS appear to be able to generate an extracellular protective barrier on the surface of the cell wall to prevent the harmful effects of heavy metals in the intracellular environment because they feature a lot of charged hydrophobic groups that are suited for the active binding of heavy metals [18][19].
Active bioaccumulation is the transport of heavy metals across the cell membrane to the cytoplasm or other organelles and requires energy to accumulate intracellular heavy metals; however, the process is a slow intracellular active accumulation of compartmentalization [20]. Depending on the kind of biomass, chemicals are absorbed, and nutrients are taken up through the surface of the biomass, which either accumulates or metabolizes substances. Ion-selective transport proteins found in the cell membrane are necessary for the whole process, which, from the absorption of metal ions to the movement of these ions throughout the cell or any organelle, takes a long time [21].
Algae must safeguard cells against non-essential metals and maintain intracellular ion concentrations at appropriate levels. As a result of structural/binding proteins, such as metallothioneins, binding to the adsorbed ions, the host cell is spared the inhibitory effects of a high concentrations of metal ions [22]. The sulfhydryl groups in phytochelatin peptides synthesized by microalgae through enzymatic synthesis are responsible for metal binding as organometallic complexes stored in the organelles of microalgal cells [23]. Additionally, acidic calcifiers and polyps promote the accumulation and storage of heavy metals [24].
Biotransformation in algae is mainly applied to the enzymatic and biochemical transformation of heavy metals but has also been used for detoxification pathways in algae. Enzymatic biotransformation is due to the non-degradable nature of heavy metals, converting them into less harmful inorganic complex forms [25]. In contrast, biotransformation is the use of electron transfer to reduce highly valued heavy metals and which will then be converted into organic heavy metal compounds [26].
Furthermore, the mechanisms of algal adsorption can differ due to the different properties of heavy metal ions [27]. The primary mechanism of adsorption of cadmium cations by algal biomass is apparently chelation, and the adsorption of nickel ions is mainly ion exchange [28]. The binding processes of lead cations, in contrast, combine ion exchange, chelation, and reduction events with the precipitation of metallic lead on algal biomass. Lead cations have a greater affinity for algal biomass [29]. Sarojini et al. [27] verified that algae adsorb Cr ions mainly through electrostatic interactions and ion exchange. To combat arsenic toxicity, microalgae oxidize As(III) to As(V), which then undergo methylation, volatilization, and extracellular excretion as they are transformed into less toxic forms [30]. Higher Cd concentrations have a considerable impact on cellular processes linked to energy consumption, DNA replication, cell cycle, and signal transduction [31]. The process by which algal cells remove heavy metal ions is shown in Figure 1.
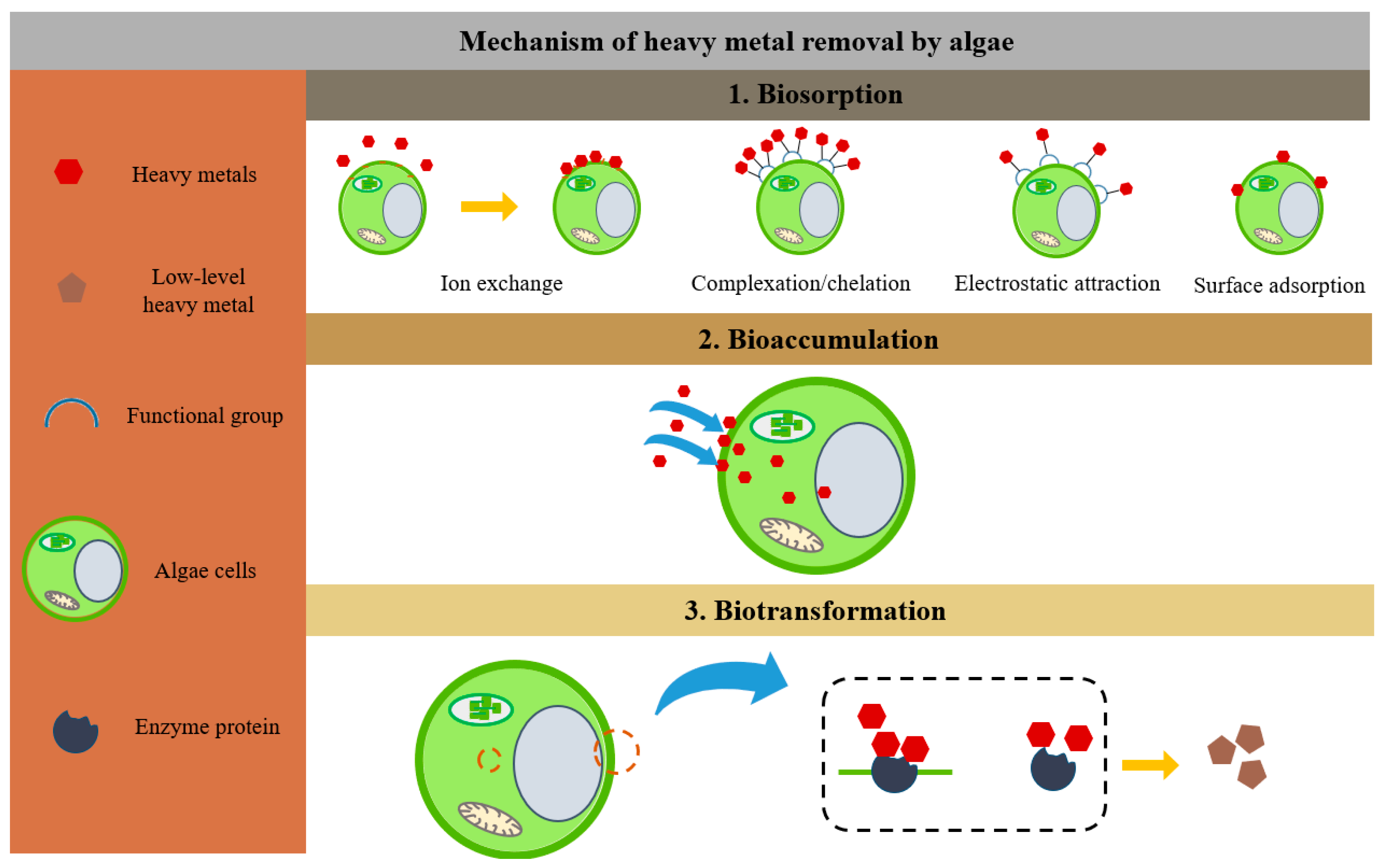
Figure 1. Removal of heavy metals by algae through biosorption, bioaccumulation, and biotransformation mechanisms.
2. Immobilized Algae Technology
Immobilization is carried out by attaching microalgae to the external surface of a supporting biological carrier. Cell immobilization techniques, metabolic processes with reduced susceptibility to senescence and significant stability over time, have been inspired by the attachment of living microorganisms to one another and to solid surfaces [32]. The target cells will be encapsulated by a porous polymer layer, thus allowing the process to diffuse the substrate into the cells [33]. The small particle size of the free particle biosorbent, the strong densification, and the uneven distribution on the reaction bed make the process less efficient and more difficult to separate [34]. The combined synergistic impact of immobilized systems can improve resistance to cell growth disruption, prevent photoinhibition and minimize cytotoxicity, and considerably aid microalgal cells in tolerating and adapting to environmental stress or toxicity [35][36]. Immobilized algae boost volumetric output, increase substrate usage, and increase resistance to harmful elements (e.g., extreme pH, temperature, and toxic compounds) [37]. Immobilized Sargassum contrasts with free Sargassum for Cu(II) ions, and immobilized adsorbents have high metal uptake, improving biosorption of nickel ions by 49% and copper ions by 36% [38].
Furthermore, during immobilization, the mobility of algal cells is affected by the limited intracapsular space, which can lead to high shear stresses from chemical forces and interactions between the support matrix and microalgal cell walls [39]. Immobilization processes prevent biomass loss from the process and improve operational flexibility, and the immobilization or sequestration of cells in small confined spaces may trigger interactions that enhance nutrient uptake [40]. Therefore, cell immobilization technology will accelerate the rate of nutrient uptake by microalgae, thus increasing the efficiency of wastewater treatment systems, which can further increase productivity and thus reduce production costs [41][42]. Additionally, compared to suspended systems, the substrate may restrict or lessen the degree of photon accessibility of algal cells, which will result in less biomass formation. However, the morphological and physicochemical characteristics of immobilized algae can be altered by homogenizing the intracapsular and extracapsular phases as well as by enhancing the substrate’s characteristics in order to improve intracapsular mobility and achieve effective mass transfer performance. The creation of improved reactor designs, as well as the provision of infrastructure and logistics, are necessary for the scale-up of immobilized algae technology to create algal beads on a commercial scale [43]. Algal immobilization techniques include adsorption, encapsulation, entrapment, and self-immobilization [44]. Figure 2 summarizes the advantages and disadvantages of immobilization techniques.
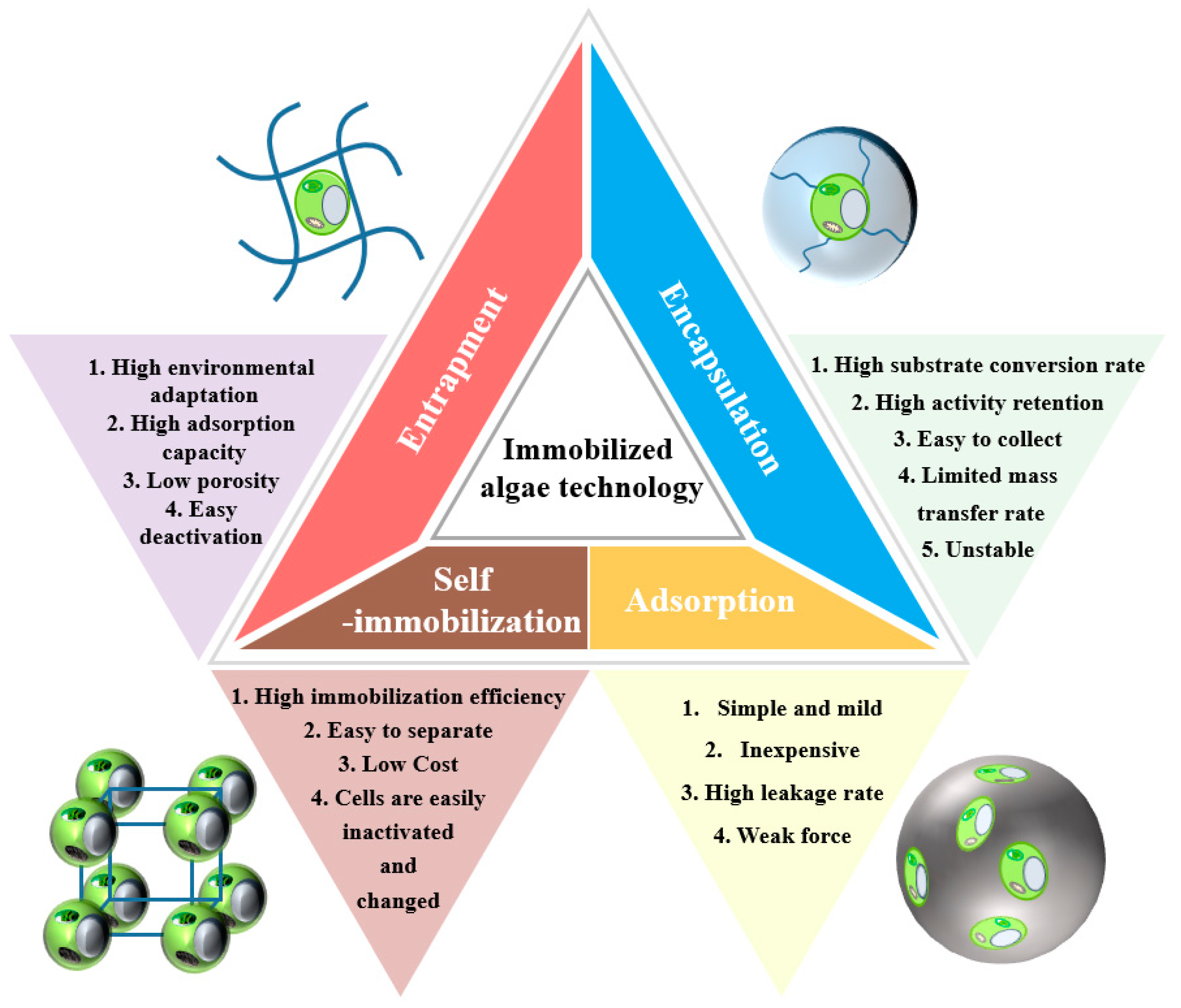
Figure 2. Advantages, disadvantages, and characterization of methods for the immobilization of algae by entrapment, encapsulation, adsorption, and self-immobilization.
2.1. Adsorption
Adsorption is a process that forms a physical bond between the surface of the water-insoluble carrier and immobilized algae through weak molecular forces like van der Waals interactions and ionic and hydrogen bonding, which are relatively gentle and quick. As a result, during use, there is a significant amount of cell leakage from the carrier due to the adsorption process [45]. Shen et al. [46] uncovered that effective adsorption of Fe2O3 on microalgal surfaces resulted in nanoscale spherical iron oxide covering the microalgae, opening the door to the potential of immobilizing microalgae using metal oxides. Through adsorption on the surface of the substance and passage through the algal cells, surface-immobilized algae lowered the heavy metal burden in the effluent. The growth of algal cells adsorbed onto the biofilm surface reduces the recovery cost because the method is easier to perform [47]. Adsorption-type immobilized algae have a lower cell concentration than encapsulated cells, and cells leak from the surface of the carrier during algal growth [48].
2.2. Encapsulation
Encapsulation is a permanent kind of immobilization in which cells are confined in a capsule space created by membrane walls. The cells can float freely in the inner space of the capsule despite being physically constrained [49]. Whitton et al. [50] investigated how light affected calcium alginate beads, encapsulating immobilized microalgae for nutrient remediation. The use of the enclosed carriers increased the substrate conversion and simplicity of collection by shielding the microbes from environmental stress/shock loading and hazardous byproducts. Alginate bead encapsulation techniques have drawbacks, such as poor swelling and mechanical qualities, which can cause damage or mass loss during adsorption [51]. Additionally, the encapsulation method limits the mass transfer rate, is unstable at a specific pH, and easily dissolves in buffers. Qin et al. [52] developed novel algae-encapsulated macro-capsules combined with membrane separation, where dual encapsulation created a restricted microaerobic environment with higher biomass harvesting and activity, the improved stability of live cells, and reduced cell leakage rates.
2.3. Entrapment
The method of the entrapment of cells in a polymer matrix and self-adhesion of the cells to the surface of solid support is entrapment and is the most commonly used immobilization method [53]. This method captures algal cells into a supporting matrix, namely a fiber or natural gel polymer. Maswanna et al. [54] entrapped green alga Tetraspora sp. CU2551 in alginate substrates with 10–50 times higher hydrogen production than cyanobacteria, which was considered a promising biological system. It has a bigger specific surface area, can adsorb a higher density of bacteria and algae, can sustain a greater pollution load, and is more adaptive to environmental conditions than adsorption immobilization and self-immobilization on the carrier surface [55]. Kube et al. [56] discovered that immobilizing algal cells by enclosing them in alginate beads assisted in the beginning and sustained larger densities of algae in the reactor, which enabled the quick removal of heavy metals. Saxena et al. [57] used freshwater diatom Nitzschia palea entrapped in calcium alginate hydrogel beads by gelation method without swelling behavior and in a more stable form to consume the nitrate, phosphate, and ammonia load in the water column. The gelation reaction was also shown to be reversible. Entrapment immobilization suffers from the high inactivation of algal cells [58]. The low porosity of immobilized algal cells via natural polymers can lead to restricted nutrient diffusion and thus affect the bio-removal efficiency of immobilized cells [48].
2.4. Self-Immobilization
The filamentous fungus can serve as immobilization carriers for mycorrhizal self-immobilization since they can spontaneously cluster into spheres and immobilize various mycorrhizal species [59]. Applying multifunctional reagents and crosslinking immobilization encourages the creation of channels between functional groups on the outer cell membrane [60]. The successful use of crosslinked polyethyleneimine polymer on immobilize C. vulgaris cells was achieved [61]. Electrostatic interaction between the negatively charged microalgae surface and the positively charged adsorbent amine results in a significant improvement in immobilization efficiency [62]. Carrier-free engagement can reduce the cost of materials and replace time-consuming and expensive technologies [63][64]. Artificially induced conditions of leading to the formation of algal cell aggregates have fewer mass transfer limitations and can better enhance cell growth, resulting in higher cell density. However, the cellular makeup of microalgae can be unintentionally altered when algal cells are exposed to chemicals and severe environments that could harm the cell surface and decrease their metabolic activity [65].
References
- Sankhla, M.; Kumar, R.; Biswas, A. Dynamic nature of heavy metal toxicity in water and sediments of Ayad River with climatic change. Int. J. Hydro. 2019, 3, 339–343.
- Appenroth, K.-J. Definition of “Heavy Metals” and Their Role in Biological Systems. In Soil Heavy Metals; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–29.
- Ameri, A.; Tamjidi, S.; Dehghankhalili, F.; Farhadi, A.; Saati, M.A. Application of algae as low cost and effective bio-adsorbent for removal of heavy metals from wastewater: A review study. Environ. Technol. Rev. 2020, 9, 85–110.
- Priatni, S.; Ratnaningrum, D.; Warya, S.; Audina, E. Phycobiliproteins production and heavy metals reduction ability of Porphyridium sp. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012006.
- Balaji, S.; Kalaivani, T.; Sushma, B.; Pillai, C.V.; Shalini, M.; Rajasekaran, C. Characterization of sorption sites and differential stress response of microalgae isolates against tannery effluents from ranipet industrial area—An application towards phycoremediation. Int. J. Phytoremediat. 2016, 18, 747–753.
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886.
- Goswami, R.K.; Agrawal, K.; Shah, M.P.; Verma, P. Bioremediation of heavy metals from wastewater: A current perspective on microalgae-based future. Lett. Appl. Microbiol. 2022, 75, 701–717.
- Ahmad, S.; Pandey, A.; Pathak, V.V.; Tyagi, V.V.; Kothari, R. Phycoremediation: Algae as Eco-friendly Tools for the Removal of Heavy Metals from Wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 53–76.
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of Rare Earth Elements through Cell Surface Display of Lanthanide Binding Tags. Environ. Sci. Technol. 2016, 50, 2735–2742.
- Chojnacka, K. Biosorption and bioaccumulation–The prospects for practical applications. Environ. Int. 2010, 36, 299–307.
- Sargın, İ.; Arslan, G.; Kaya, M. Efficiency of chitosan–algal biomass composite microbeads at heavy metal removal. React. Funct. Polym. 2016, 98, 38–47.
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H.A. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256.
- Saba, S. Biosorption of Heavy Metals. In Biosorption; Jan, D., Branislav, V., Eds.; IntechOpen: Rijeka, Italy, 2018; p. Ch. 2.
- Aswathi Mohan, A.; Robert Antony, A.; Greeshma, K.; Yun, J.-H.; Ramanan, R.; Kim, H.-S. Algal biopolymers as sustainable resources for a net-zero carbon bioeconomy. Bioresour. Technol. 2022, 344, 126397.
- Greeshma, K.; Kim, H.-S.; Ramanan, R. The emerging potential of natural and synthetic algae-based microbiomes for heavy metal removal and recovery from wastewaters. Environ. Res. 2022, 215, 114238.
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.-H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038.
- Pereira, S.B.; Mota, R.; Vieira, C.P.; Vieira, J.; Tamagnini, P. Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci. Rep. 2015, 5, 14835.
- Hou, J.; Yang, Y.; Wang, P.; Wang, C.; Miao, L.; Wang, X.; Lv, B.; You, G.; Liu, Z. Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ. Sci. Pollut. Res. 2017, 24, 226–235.
- Zhang, J.; Zhou, F.; Liu, Y.; Huang, F.; Zhang, C. Effect of extracellular polymeric substances on arsenic accumulation in Chlorella pyrenoidosa. Sci. Total Environ. 2020, 704, 135368.
- Zohoorian, H.; Ahmadzadeh, H.; Molazadeh, M.; Shourian, M.; Lyon, S. Chapter 41–Microalgal bioremediation of heavy metals and dyes. In Handbook of Algal Science, Technology and Medicine; Konur, O., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 659–674.
- Chugh, M.; Kumar, L.; Shah, M.P.; Bharadvaja, N. Algal Bioremediation of heavy metals: An insight into removal mechanisms, recovery of by-products, challenges, and future opportunities. Energy Nexus 2022, 7, 100129.
- Tripathi, S.; Poluri, K.M. Metallothionein-and Phytochelatin-Assisted Mechanism of Heavy Metal Detoxification in Microalgae. In Approaches to the Remediation of Inorganic Pollutants; Hasanuzzaman, M., Ed.; Springer: Singapore, 2021; pp. 323–344.
- Ahmad, J.; Ali, A.A.; Baig, M.A.; Iqbal, M.; Haq, I.; Irfan Qureshi, M. Chapter 8–Role of Phytochelatins in Cadmium Stress Tolerance in Plants. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 185–212.
- García-García, J.D.; Sánchez-Thomas, R.; Moreno-Sánchez, R. Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 2016, 34, 859–873.
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.-S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal Phycoremediation: A Glimpse into a Sustainable Environment. Toxics 2022, 10, 525.
- Yen, H.-W.; Chen, P.-W.; Hsu, C.-Y.; Lee, L. The use of autotrophic Chlorella vulgaris in chromium (VI) reduction under different reduction conditions. J. Taiwan Inst. Chem. Eng. 2017, 74, 1–6.
- Sarojini, G.; Venkatesh Babu, S.; Rajamohan, N.; Senthil Kumar, P.; Rajasimman, M. Surface modified polymer-magnetic-algae nanocomposite for the removal of chromium- equilibrium and mechanism studies. Environ. Res. 2021, 201, 111626.
- Raize, O.; Argaman, Y.; Yannai, S. Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol. Bioeng. 2004, 87, 451–458.
- Abdel -Aty, A.M.; Ammar, N.S.; Abdel Ghafar, H.H.; Ali, R.K. Biosorption of cadmium and lead from aqueous solution by fresh water alga Anabaena sphaerica biomass. J. Adv. Res. 2013, 4, 367–374.
- Zhang, S.; Rensing, C.; Zhu, Y.-G. Cyanobacteria-Mediated Arsenic Redox Dynamics Is Regulated by Phosphate in Aquatic Environments. Environ. Sci. Technol. 2014, 48, 994–1000.
- Badisa, V.L.D.; Latinwo, L.M.; Odewumi, C.O.; Ikediobi, C.O.; Badisa, R.B.; Ayuk-Takem, L.T.; Nwoga, J.; West, J. Mechanism of DNA damage by cadmium and interplay of antioxidant enzymes and agents. Environ. Toxicol. 2007, 22, 144–151.
- Al-Hasawi, Z.M.; Abdel-Hamid, M.I.; Almutairi, A.W.; Touliabah, H.E. Response of Pseudokirchneriella subcapitata in Free and Alginate Immobilized Cells to Heavy Metals Toxicity. Molecules 2020, 25, 2847.
- Eroglu, E.; Smith, S.M.; Raston, C.L. Application of Various Immobilization Techniques for Algal Bioprocesses. In Biomass and Biofuels from Microalgae: Advances in Engineering and Biology; Moheimani, N.R., McHenry, M.P., de Boer, K., Bahri, P.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 19–44.
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14.
- Sánchez-Saavedra, M.d.P.; Molina-Cárdenas, C.A.; Castro-Ochoa, F.Y.; Castro-Ceseña, A.B. Protective effect of glycerol and PEG-methyl ether methacrylate coatings on viability of alginate-immobilized Synechococcus elongatus after cold storage. J. Appl. Phycol. 2019, 31, 2289–2297.
- Fu, M.; Liang, J.; Wang, S.; Geng, C.; Zhang, W.; Wu, T. The Response of Microalgae Chlorella sp. to Free and Immobilized ZrO2 and Mg(OH)2 Nanoparticles: Perspective from the Growth Characteristics. Environ. Eng. Sci. 2020, 37, 429–438.
- Xie, B.; Gong, W.; Yu, H.; Tang, X.; Yan, Z.; Luo, X.; Gan, Z.; Wang, T.; Li, G.; Liang, H. Immobilized microalgae for anaerobic digestion effluent treatment in a photobioreactor-ultrafiltration system: Algal harvest and membrane fouling control. Bioresour. Technol. 2018, 268, 139–148.
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.G.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J. Clean. Prod. 2017, 150, 58–64.
- Zeng, X.; Guo, X.; Su, G.; Danquah, M.K.; Zhang, S.; Lu, Y.; Sun, Y.; Lin, L. Bioprocess considerations for microalgal-based wastewater treatment and biomass production. Renew. Sustain. Energy Rev. 2015, 42, 1385–1392.
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184.
- Lee, H.; Jeong, D.; Im, S.; Jang, A. Optimization of alginate bead size immobilized with Chlorella vulgaris and Chlamydomonas reinhardtii for nutrient removal. Bioresour. Technol. 2020, 302, 122891.
- Kumar, R.; Ghosh, A.K.; Pal, P. Fermentative ethanol production from Madhuca indica flowers using immobilized yeast cells coupled with solar driven direct contact membrane distillation with commercial hydrophobic membranes. Energy Convers. Manag. 2019, 181, 593–607.
- Murujew, O.; Whitton, R.; Kube, M.; Fan, L.; Roddick, F.; Jefferson, B.; Pidou, M. Recovery and reuse of alginate in an immobilized algae reactor. Environ. Technol. 2021, 42, 1521–1530.
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell immobilization strategies for biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565.
- Han, M.; Zhang, C.; Ho, S.-H. Immobilized microalgal system: An achievable idea for upgrading current microalgal wastewater treatment. Environ. Sci. Ecotechnol. 2023, 14, 100227.
- Shen, L.; Wang, J.; Li, Z.; Fan, L.; Chen, R.; Wu, X.; Li, J.; Zeng, W. A high-efficiency Fe2O3@Microalgae composite for heavy metal removal from aqueous solution. J. Water Process Eng. 2020, 33, 101026.
- Zhang, Q.; Liu, C.; Li, Y.; Yu, Z.; Chen, Z.; Ye, T.; Wang, X.; Hu, Z.; Liu, S.; Xiao, B.; et al. Cultivation of algal biofilm using different lignocellulosic materials as carriers. Biotechnol. Biofuels 2017, 10, 115.
- Vasilieva, S.; Lobakova, E.; Grigoriev, T.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Gotovtsev, P.; Antipova, C.; Zagoskin, Y.; Scherbakov, P.; et al. Bio-inspired materials for nutrient biocapture from wastewater: Microalgal cells immobilized on chitosan-based carriers. J. Water Process Eng. 2021, 40, 101774.
- Mahmoud, M.E.; Abdou, A.E.H.; Mohamed, S.M.S.; Osman, M.M. Engineered staphylococcus aureus via immobilization on magnetic Fe3O4-phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824.
- Whitton, R.; Ometto, F.; Villa, R.; Pidou, M.; Jefferson, B. Influence of light regime on the performance of an immobilised microalgae reactor for wastewater nutrient removal. Algal Res. 2019, 44, 101648.
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption Properties toward Trivalent Rare Earths by Alginate Beads Doping with Silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461.
- Qin, L.; Gao, M.; Zhang, M.; Feng, L.; Liu, Q.; Zhang, G. Application of encapsulated algae into MBR for high-ammonia nitrogen wastewater treatment and biofouling control. Water Res. 2020, 187, 116430.
- Han, M.; Zhang, C.; Li, F.; Ho, S.-H. Data-driven analysis on immobilized microalgae system: New upgrading trends for microalgal wastewater treatment. Sci. Total Environ. 2022, 852, 158514.
- Maswanna, T.; Phunpruch, S.; Lindblad, P.; Maneeruttanarungroj, C. Enhanced hydrogen production by optimization of immobilized cells of the green alga Tetraspora sp. CU2551 grown under anaerobic condition. Biomass Bioenergy 2018, 111, 88–95.
- Papageorgiou, S.K.; Katsaros, F.K.; Favvas, E.P.; Romanos, G.E.; Athanasekou, C.P.; Beltsios, K.G.; Tzialla, O.I.; Falaras, P. Alginate fibers as photocatalyst immobilizing agents applied in hybrid photocatalytic/ultrafiltration water treatment processes. Water Res. 2012, 46, 1858–1872.
- Kube, M.; Spedding, B.; Gao, L.; Fan, L.; Roddick, F. Nutrient removal by alginate-immobilized Chlorella vulgaris: Response to different wastewater matrices. J. Chem. Technol. Biotechnol. 2020, 95, 1790–1799.
- Saxena, A.; Mishra, B.; Tiwari, A. Development of diatom entrapped alginate beads and application of immobilized cells in aquaculture. Environ. Technol. Innov. 2021, 23, 101736.
- Giese, E.C.; Silva, D.D.V.; Costa, A.F.M.; Almeida, S.G.C.; Dussán, K.J. Immobilized microbial nanoparticles for biosorption. Crit. Rev. Biotechnol. 2020, 40, 653–666.
- Zheng, Z.; Ali, A.; Su, J.; Zhang, S.; Fan, Y.; Sun, Y. Self-immobilized biochar fungal pellet combined with bacterial strain H29 enhanced the removal performance of cadmium and nitrate. Bioresour. Technol. 2021, 341, 125803.
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600.
- Vasilieva, S.; Shibzukhova, K.; Morozov, A.; Solovchenko, A.; Bessonov, I.; Kopitsyna, M.; Lukyanov, A.; Chekanov, K.; Lobakova, E. Immobilization of microalgae on the surface of new cross-linked polyethylenimine-based sorbents. J. Biotechnol. 2018, 281, 31–38.
- Tran, N.-A.T.; Seymour, J.R.; Siboni, N.; Evenhuis, C.R.; Tamburic, B. Photosynthetic carbon uptake induces autoflocculation of the marine microalga Nannochloropsis oculata. Algal Res. 2017, 26, 302–311.
- Alam, M.A.; Wan, C.; Guo, S.-L.; Zhao, X.-Q.; Huang, Z.-Y.; Yang, Y.-L.; Chang, J.-S.; Bai, F.-W. Characterization of the flocculating agent from the spontaneously flocculating microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014, 118, 29–33.
- Vasilieva, S.; Lobakova, E.; Solovchenko, A. Biotechnological Applications of Immobilized Microalgae. In Environmental Biotechnology; Gothandam, K.M., Ranjan, S., Dasgupta, N., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 3, pp. 193–220.
- Martins, S.C.S.; Martins, C.M.; Fiúza, L.M.C.G.; Santaella, S.T. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. Afr. J. Biotechnol. 2013, 12, 4412–4418.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Entry Collection:
Wastewater Treatment
Revisions:
2 times
(View History)
Update Date:
27 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




