Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pedro Miguel Dias Fernandes | -- | 3167 | 2023-03-25 22:34:51 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fernandes, P.D.; Magalhães, F.D.; Pereira, R.F.; Pinto, A.M. Metal-Organic Frameworks in Phototherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/42540 (accessed on 07 March 2026).
Fernandes PD, Magalhães FD, Pereira RF, Pinto AM. Metal-Organic Frameworks in Phototherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/42540. Accessed March 07, 2026.
Fernandes, Pedro D., Fernão D. Magalhães, Rúben F. Pereira, Artur M. Pinto. "Metal-Organic Frameworks in Phototherapy" Encyclopedia, https://encyclopedia.pub/entry/42540 (accessed March 07, 2026).
Fernandes, P.D., Magalhães, F.D., Pereira, R.F., & Pinto, A.M. (2023, March 25). Metal-Organic Frameworks in Phototherapy. In Encyclopedia. https://encyclopedia.pub/entry/42540
Fernandes, Pedro D., et al. "Metal-Organic Frameworks in Phototherapy." Encyclopedia. Web. 25 March, 2023.
Copy Citation
The use of metal-organic frameworks (MOFs) in the biomedical field has grown significantly in recent years. Due to their distinct properties, including their porous structure, large surface area, and inherent photo-responsive properties, MOFs can be particularly useful in the fields of cancer phototherapy and immunotherapy. MOF nanoplatforms have successfully demonstrated their ability to address several drawbacks associated with cancer phototherapy and immunotherapy, enabling an effective and low-side-effect combinatorial synergistical treatment for cancer.
nanomaterials
cancer therapy
photodynamic therapy
photothermal therapy
1. Structure and Properties
MOFs are a class of highly organized porous nanomaterials with a crystalline inorganic-organic hybrid structure, assembled from multiple coordination of organic linkers and inorganic metal ions as cluster nodes (Figure 1) [1][2]. The inorganic components of MOFs, known as secondary building units (SBUs), may contain a variety of alkaline earth metals, alkali metals, transition metals, actinides, lanthanides, and several main groups of metal ions that are primarily in carboxylate form to coordinate with a variety of organic ligands (bipyridyl, imidazolate, and carboxylate-based) and biological macromolecules (amino acids, peptides, nucleobase, and saccharide) [3]. Organic linkers act as bridging ligands between metal nodes, with di-, tri-, and tetra-carboxylate ligands (e.g., Terephthalic acid, 2-aminoterephthalic acid, benzene tricarboxylic acid (BTC) and trimesic acid) being commonly used due to their sterically rigid and highly polarized aromatic structures, allowing for complex morphologies as well as more rigid frameworks [4][5]. In contrast to other porous nanomaterials, such as zeolites and carbons, the MOFs structure can be tailored to the desired application, since SBUs geometry and the size and shape of organic ligands are determinant structural factors that can be selected to achieve the desired pore size, structure, and function [6]. Recently, researchers have been building more complex MOFs, or highly organized meso- and macroscopic superstructures, using nanocrystals as building blocks, exploiting the different metal-ligand geometries (tetrahedral, octahedral, and cubic) [7][8][9]. Such complex superstructures are classified into four dimensions: (i) zero-dimensional (0D) in the form of hollow capsules or microspheres; (ii) one-dimensional (1D) as nanorods and nanofibers; (iii) two-dimensional (2D) nanostructures of platelets, sheets, plates, films, and membranes; and (iv) three dimensional (3D) nanostructures as an extension of 0D, 1D, and 2D superstructures across multiple length scales [8][9][10].
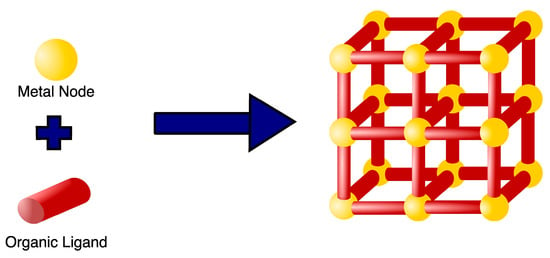
Figure 1. Example of a basic MOF structure. MOFs are highly organized porous nanomaterials composed of metal nodes coordinated with several organic ligands serving as a bridge between nodes.
In recent decades, MOFs have emerged as intriguing nanotechnology materials due to their potential in a wide array of applications, including gas storage, chemical separation, catalysis, magnetism, sensing and detection, drug delivery, and other biomedical applications such as cancer therapy, osmotic and diffusion-controlled membranes, tissue engineering, gasotransmitter therapies, biosensing, bioimaging, biocatalysts, and antibacterial [11][12][13][14][15][16]. Many MOFs, for example, have previously been developed for biomedical applications in the domains of bone treatment and bone repair, such as Cu-TCPP-TCP for bone tumors, ZIF-8/VAN for osteoarthritis, and Zr-MOFs for bone regeneration [17]. Titanium MOFs (Ti-MOF) are yet another type of MOF that has been developed for biomedical applications, including antibacterial, anti-inflammatory, bone damage, and cancer therapy [18]. MOFs have unique properties that cannot be found in organic or inorganic systems due to their hybrid nature [19]. MOFs comprising different metal ions and organic linker structures feature different morphologies, pore diameters, and unique electrical, magnetic, and optical properties that can be used in specific applications [6][11]. One of the most appealing properties of MOFs as a basis for their functions is their constant highly organized porosity. Until recently, the majority of MOFs developed have been microporous (<2 nm) with a large surface area imparting good adsorption of various gases such as hydrogen and carbon [6][20]. However, this pore size is unsuitable for other applications, such as catalysis and drug delivery, that require mesoporous (2–50 nm) and microporous (>50 nm) MOFs with a larger surface area [6][21]. The linear extension of organic linkers tends to be a solution to provide large storage space and a higher number of adsorption sites within the crystal framework. The increased space between the pores, may stimulate the formation of interpenetrating structures (the intertwined growth of two or more frameworks) [20]. As a result, the synthesis of mesoporous and macroporous MOFs remains a problem for the various applications of MOFs [6].
In addition to the high porosity and large surface area, other properties, such as easy functionalization, inherent biocompatibility, water solubility, biodegradability, and thermal stability, aroused the interest in MOFs, particularly as drug delivery systems (DDS) [22][23]. Since MOFs are tunable, they can accommodate a wide range of molecules with varying physicochemical properties that can be incorporated into the MOF via surface attachment, covalent bonding, pore encapsulation, and in situ encapsulation through multiple interactions with the linkers (e.g., hydrogen bonds, electrostatic interactions, van der Waals forces, stacking, covalent bonds, and coordination bonds) [11][23]. Additionally, due to constant porosity, these flexible network structures are stimuli-responsive under stress, changing their properties and/or structures in different environments [19][24]. MOFs’ inherent ability to undergo a molecular change in response to specific stimuli allows for controlled induction in a desired environment for several applications [24]. MOF transformation can be triggered by the presence of specific endogenous environments (pH, ATP, redox, glutathione (GSH)) or by the reaction to external stimuli (different wavelengths of light, temperature, pressure, magnetic field, ions, and humidity) [24][25]. Stimuli-responsive MOFs can act as delivery systems for several bioactive molecules chemotherapeutic agents, fluorescent agents, and organic dyes for application in chemotherapy, biomedical imaging, PDT, and PTT, enhancing their efficiency and potentially diminishing side effects [24][26][27]. As an example, Qin et al. developed a novel hydrostable 2D Zn-based MOF as drug delivery system for 5-fluorouracil (5-FU), a typical anticancer drug. 5-FU encapsulation in the MOF could potentially inhibit poor biodistribution, as a release assay, reports a slow release of 5-FU with no bursting effects. Moreover, cytotoxicity, evaluated by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H tetrazolium bromide (MTT) assay, displayed >90% cell survival [28].
Despite the promise promoted by their distinct properties, early MOFs, primarily developed using divalent metals such as Cu2+ and Zn2+, proved unsuitable for certain applications due to stability issues under harsh conditions (e.g., moisture or aqueous solutions), limiting their application and commercialization [29][30]. Several factors, including metal ions and organic ligand composition, metal-ligand coordination geometry, pore surface hydrophobicity, and the operating environment (e.g., the presence of water, temperature, pH, and pressure), have been reported to affect MOF stability, resulting in poor water stability, acid/base stability, thermal stability, and mechanical stability [6][30][31]. The rationalization of MOFs stability under certain conditions is critical for employment in the desired application. As a result, the consideration of metal-ligand bond strength and various kinetic parameters is critical for the development of more stable MOFs [6][32]. In recent years, several solutions have been developed to tackle different stability problems, including increasing the strength of coordination bonds and the surface hydrophobicity of MOFs for better water stability, combining high oxidation-state metal ions or hard acids with carboxylate linkers (hard bases) to generate strong bonds and increase acid/base stability, and using high valence metal ions (e.g., Ln3+, Al3+, Zr4+, and Ti4+) to achieve higher thermal stabilities [6]. Although studies on improving mechanical stability are scarce, the functional groups of organic ligands appear to have an impact MOF mechanical stability [6][33]. Recent advances in MOF-hydrogel composites may provide a solution for improving MOF stability, not only for biomedical applications but also in other sectors [34]. Nonetheless, a better understanding of the factors influencing structural stability has resulted in the growing development of more stable MOFs and the expansion of many applications [6].
2. MOFs in Phototherapy
Phototherapy uses near-infrared region (NIR) light to kill cancer cells by generating ROS in PDT and inducing thermal ablation in PTT [35]. The selection of the best PSs or PTAs in both therapies has a significant impact on the therapy’s efficacy [36]. Ideal photosensitizers are non-toxic or have minimal toxicity, display high absorbance in the NIR wavelength, and exhibit high photostability [37]. Despite the development of several inorganic materials and nanoparticles (e.g., noble metals, semiconductors, carbon nanomaterials, magnetic nanoparticles, and manganese dioxide) and organic compounds (e.g., indocyanine green and porphyrin) as photosensitizers, the in vivo non-biodegradability, high toxicity, possible long-term toxicity of inorganic nanoparticles, and easy photobleaching of organic compounds limits their phototherapeutic applications [37][38]. Furthermore, other drawbacks, such as hydrophobicity-induced aggregation, limited diffusion of ROS, oxygen dependence, undesirable penetration depth in PSs, and limited penetration depth and lack of selectivity in PTAs, highlight the need for improvement and the development of more elaborate phototherapeutic strategies [38].
Over the last decade, there has been an increasing interest in the intrinsic photodynamic and photothermal capabilities of certain MOFs [38]. Another appealing feature is that the porous structure of MOFs enables the loading of phototherapeutic agents for photo-responsive release, preventing self-aggregation and self-quenching of PSs and improving photothermal responses and thermal stability of PTAs [37][38]. Furthermore, the nanomaterials’ superior biocompatibility, ease of modification, passive targeting of enhanced permeability and retention (EPR), and TME-responsive degradation make them attractive candidates for enhanced phototherapy treatments [37]. On the other hand, MOFs demonstrate difficulties in adapting to the TME due to poor water stability. However, by using core-shell structures, where MOFs may act as the core or shell that binds to other materials, it is possible to solve stability issues while keeping the original functional capabilities. Examples of those other materials includes metal oxides, organic polymers, and carbon nanoparticles [38].
PDT is a novel and non-invasive therapy that specifically destroys tumor cells; it is dependent on the efficiency of the photosensitizer, light, and oxygen available in the TME [37]. Synthesis of intrinsic photodynamic MOFs often involves the use of porphyrins and their derivatives (dihydroporphyphenol, chlorophyllin) [38][39]. Porphyrins are organic heterocyclic macrocycles composed of four pyrrole groups connected by methylene bridges [39][40]. Their application in phototherapy is attractive due to their prevalence in natural systems; this makes them ideal for use in biological singlet oxygen production with the absence of significant cytotoxicity without light [41]. Furthermore, porphyrin has 22 π-electrons of which 18 are conjugated, facilitating π–π* transitions to yield a Soret, or B band at ~400 nm (electronic transition from the ground state to a second excited singlet state (S0 → S2)) and four lower energy and low-intensity Q-bands between ~450 and 650 nm (S0 → S1) [40]. Porphyrin derivatives with fewer π-electrons show increased red-shift absorptivity Q-bands at wavelengths ranging from 650 to 800 nm [38][40]. The overlap of absorption in the red with the highest tissue penetration region raises interest in PDT applications [41]. High hydrophobicity and aggregation, on the other hand, restrict bioavailability and accumulation at target sites, limiting their therapeutic applicability [40]. The use of porphyrin-based MOFs improves PS efficiency by preventing aggregation and enhancing ROS diffusion due to the porous structures of the MOFs. Therefore, several porphyrin-based MOFs have been developed for application in PDT [38].
Lu et al. created the first porphyrin-based MOF in a rational design of a hafnium (Hf)-porphyrin nanoscale MOF. DBP-UiO MOF (DBP referring to dibenzoporphyrin and UiO referring to University of Oslo) was created via a solvothermal reaction involving HfCl4 and the porphyrin derivative, 5,15-di(p-benzoato) porphyrin (H2DBP), originating a UiO-type MOF crystal structure composed of hexanuclear clusters of SBU twelve-fold bonded to bridging ligands. Through the isolation of the photosensitizer, which prevents aggregation and self-quenching, and the enhancement of intersystem crossing by the heavy Hf center, DBP-UiO efficiently boosted ROS formation and, as a result, PDT efficacy. In vivo studies revealed that half of the treated mice exhibited tumor volume reduction while the other half experienced tumor eradication, emphasizing the significant promise of nanoscale MOFs (nMOFs) as strong PDT agents [42].
In addition to Hf, other metal centers, such as manganese (Mn) and, most commonly, zirconium (Zr), can be used for the construction of MOFs with intrinsic photodynamic properties [43]. Among several MOFs developed, the porous coordination network (PCN) family, consisting of Zr6-based porphyrinic MOFs with high surface areas, is particularly important for structurally guided strategies in photodynamic therapies [43][44][45]. Park et al. developed size-controllable PCN-224 through a solvothermal reaction of 6 Zr6 clusters (primarily octahedral) coupled to a tetrakis(4-carboxyphenyl)-porphyrin (TCPP) ligand into a spheric morphology. A size-controlled PCN-224 might increase cellular uptake and, as a result, PDT efficiency, highlighting the relevance of size parameters of the nanoplatforms in cellular response [46]. Furthermore, post-synthetic modifications of MOFs can be an efficient method of increasing PDT efficacy. Porphyrin MOF surface modification approaches have included cell-penetrating peptide, folic acid (FA), hyaluronic acid (HA), erythrocyte membrane, cancer cell membrane, exosome, metal nanoparticles, and nano enzymes, to name a few [43][47][48][49][50][51][52][53][54]. According to Park et al., further functionalization with FA in PCN-224 enabled active targeting of tumor cells and further improved PDT performance [46].
Non-intrinsic photodynamic MOFs can also be employed in PDT by incorporating PSs into the MOF structure through encapsulation, surface attachment, or the construction of a core-shell structure. MOF alternatives are more diversified without the constraint of porphyrins and their derivatives, including ZIF-8 (zeolitic imidazolate framework), MIL-101 (Materials Institute Lavoisier), and UiO-66, often used in biomedical applications [38][55]. ZIF-8 consists of a robust 3D network composed of tetrahedral zinc (Zn) ions connected by 2-methyl imidazolate ligands, normally with a sodalite topology [56]. Zheng et al. reported a pH-responsive ZIF-8-based nanoplatform by incorporating gold nanoclusters (AuNCs) as photosensitizer and doxorubicin (DOX) as a chemotherapeutic agent for a PDT/chemotherapy synergistic therapy. Under the acidic conditions of the TME, the ZIF-8 structure is destroyed promoting the delivery of both AuNCs and DOX for an enhanced PDT/Chemotherapy therapeutic effect [57]. MIL-101, on the other hand, is composed of a metal-(III) trimer consisting of three octahedra that laterally bind to two carboxylic groups of two terephthalic acids [1,4-benzene dicarboxylate (H2BDC)] molecules, culminating in a super tetrahedron topology. MIL-101 has an extraordinarily large surface area and pore volume, as well as good air, water, and acid stability [58][59]. In a strategy for PDT target switching, Liu et al. modified MIL-101(Fe) with amino groups (NH2) for surface attachment of the photosensitizer chlorine e6 (Ce6)-labeled cathepsin B (CaB) substrate peptide. The MOF composite was then loaded with a camptothecin anticancer agent for a combined PDT and chemotherapy treatment. The transfer of the excited electron to the MOF hindered the fluorescence of Ce6. When Ce6 came into contact with CaB in TME, it was cleaved off of the MOF surface, regaining its fluorescence and the ability to activate PDT for effective combined cancer therapy [60]. UiO-66 is a conventional MOF composed of Zr4+ ions as metal nodes coupled by terephthalic acid molecules that exhibits desirable drug carrier properties, including a large surface area, physicochemical stability, and low toxicity [55][61]. Ding et al. designed a novel multifunctional MOF for a PDT/chemotherapy synergistic antitumor treatment through the functionalization of UiO-66-NH2 with the encapsulation of 5-aminolevulanic acid (ALA-5), a protoporphyrin precursor, as a photosensitizer and the formation of a core-shell structure promoted by the affinity of pemetrexed (MTA) (a chemotherapeutic agent that possesses high antitumor activity and targeting ability as a folate antagonist) to the unsaturated Zr active site of UiO-66-NH2,, reaching high loading rates. MTA's greater affinity for folate receptors improved tumor cell targeting and uptake. Moreover, an effective PDT/chemotherapy combination therapy remarkably suppressed tumor growth [61].
PTT, like PDT, is a new and non-invasive therapy that uses the conversion of light energy into heat energy to increase the temperature and achieve therapeutic effects at the lesion site [62]. PTT can be directly mediated by MOFs with inherent photothermal properties without the introduction of an exogenous PTA by using several PTAs as ligands (e.g., IR825 and ferrocene (Fc)) [38]. In this regard, Yang et al. designed a self-assembling MOF with Mn2+ as the metal node and PTA, IR825, as the ligand to achieve excellent NIR absorbance and photothermal stability. To improve biocompatibility, the nanoparticles were further modified with polydopamine (PDA) and polyethylene glycol (PEG), generating Mn-IR825@PDAPEG nanoscale metalorganic particles (NMOPs). Under 808 nm light irradiation, the NMOPs demonstrated strong photothermal performance and effective tumor ablation [63]. In another example, Deng et al. built a Zr-Fc MOF nanosheet for a PTT/CDT synergetic method. Zr clusters were bridged by 1,1-ferrocenedicarboxylic acid [Fc(COOH)2] ligands in the Zr-Fc MOF, resulting in a nanosheet with excellent light absorbance and good photo-thermal conversion efficiency (PCE). Additionally, Zr-Fc MOF has endowed a Fenton catalytic activity from the Fc ligand that converts H2O2 into the hydroxyl radical (•OH), for an additional chemotherapeutic effect. The combined action of PDT and chemotherapy led to the death of >80% of 4T1 tumor cells in vitro under 808 nm irradiation for 3 min and nearly 100% after 5 min. Furthermore, in vivo tumor growth was effectively suppressed, suggesting a viable MOF-based nanoplatform with the potential for PTT cancer therapy without the use of exogenous PTAs [64].
Prussian blue (PB) is a MOF archetype that has been authorized as a clinical antidote for internal radioactive contamination by the United States Food and Drug Administration (FDA) [65][66]. PB is a coordination polymer with a cubic porous network structure composed of ferric ions (FeIII) and ferrous ions (FeII) coupled to a nitrogen atom and carbon atom of a cyanide molecule that bridges both iron ions, assuming an ideal formula of FeIII4[FeII(CN)6]3nH2O [66]. PB has been widely employed in PTT as a MOF with inherent photothermal capabilities due to its strong light absorption and photo-thermal conversion efficiency in the NIR. NIR light is converted into heat by electron migration between Fe III and Fe II via the cyanide ligand, promoting therapeutic hyperthermia [66][67][68]. Furthermore, PB has minimal biotoxicity and good biodegradability for biomedical applications [67]. Peng et al. established a simple, low-cost, and environmentally friendly approach to synthesize carbon dot (CD)-decorated Prussian blue nanoparticles (CDs/PBNP) nanocomposites, combining CD photoluminescent capabilities with PBNPs photothermal conversion ability. CD/PNBP presented high photothermal conversion efficiency (30%) and photothermal stability, evoking an efficient photothermal cytotoxic effect on C6-tumor-bearing mice subjected to light irradiation at 808 nm for 10 min [69].
In a similar fashion to PDT, exogenous PTAs can also be incorporated into several MOFs (e.g., ZIF-8 and MIL-100) by encapsulation in the porous structure or as either the core or shell of a core-shell MOF structure [26]. As an example, Tian et al. reported the encapsulation of graphene quantum dots (GQD) and of the chemotherapeutic agent DOX into ZIF-8 to generate DOX-ZIF-8/CQD nanoparticles for a controlled drug delivery system in a synergistic therapeutic approach. GQD provided the nanoparticles significant NIR absorbance, PCE, and outstanding thermal conductivity for a good photothermal effect while also endowing the capability to adjust the therapeutic temperature through NIR intensity, time of irradiation, and DOX-ZIF-8/GQD nano-particle concentration. Furthermore, CQD dissociation increased ZIF-8 pH-sensitive DOX release. As a result, the synergistic impact of chemo- and photothermal treatment, as well as the improved delivery mechanism, demonstrated the development of a multifunctional nanoplatform with the potential for effective cancer cell ablation [70]. In another study, Fan et al. developed PPy@MIL-100 core-shell nanoparticles in a synergistic PTT/chemotherapeutic strategy, making use of polypyrrole’s (PPy) high photothermal conversion efficiency and excellent biocompatibility. The nanoparticles were synthesized with PPy as the core coated by an iron (III) carboxylate MOF (MIL-100) outer shell, which was then loaded with DOX anticancer drug. Under 808 nm light irradiation, the nanoparticles displayed an improved pH and NIR-responsive drug release, as well as a photothermal effect for enhanced tumor cell cellular death [71].
References
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-Organic Frameworks with Different Dimensionalities: An Ideal Host Platform for Composites. Coord. Chem. Rev. 2022, 454, 214327.
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry: Occurrence and Taxonomy of Nets and Grammar for the Design of Frameworks. Acc. Chem. Res. 2005, 38, 176–182.
- Moumen, E.; Bazzi, L.; el Hankari, S. Metal-Organic Frameworks and Their Composites for the Adsorption and Sensing of Phosphate. Coord. Chem. Rev. 2022, 455, 214376.
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.v.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.v.; Khozina, E.v.; Grachev, V.A. Metal-Organic Framework Structures: Adsorbents for Natural Gas Storage. Russ. Chem. Rev. 2019, 88, 925–978.
- Jeyaseelan, C.; Jain, P.; Soin, D.; Gupta, D. Metal Organic Frameworks: An Effective Application in Drug Delivery Systems. Inorg. Nano-Met. Chem. 2022, 52, 1463–1475.
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68.
- Natarajan, S.; Mahata, P. Metal–Organic Framework Structures—How Closely Are They Related to Classical Inorganic Structures? Chem. Soc. Rev. 2009, 38, 2304–2318.
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of Metal-Organic Frameworks at the Mesoscopic/Macroscopic Scale. Chem. Soc. Rev. 2014, 43, 5700–5734.
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of Various Dimensional Metal Organic Frameworks (MOFs) and Their Hybrid Composites for Emerging Applications—A Review. Chemosphere 2022, 298, 134184.
- Bai, Y.; Liu, C.; Shan, Y.; Chen, T.; Zhao, Y.; Yu, C.; Pang, H. Metal-Organic Frameworks Nanocomposites with Different Dimensionalities for Energy Conversion and Storage. Adv. Energy Mater 2022, 12, 2100346.
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846.
- Gu, C.; Guo, C.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-Based Metal-Organic Framework Embedded with Carbon Dots: Ultra-Sensitive Platform for Early Diagnosis of HER2 and HER2-Overexpressed Living Cancer Cells. Biosens. Bioelectron. 2019, 134, 8–15.
- Liao, L.G.; Ke, D.; Li, G.C.; Zhang, S.; Li, B.J. Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters. Molecules 2023, 28, 852.
- Valizadeh Harzand, F.; Mousavi Nejad, S.N.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Chiang, W.-H.; Buonomenna, M.G.; Lai, C.W. Recent Advances in Metal-Organic Framework (MOF) Asymmetric Membranes/Composites for Biomedical Applications. Symmetry 2023, 15, 403.
- Moribe, S.; Takeda, Y.; Umehara, M.; Kikuta, H.; Ito, J.; Ma, J.; Yamada, Y.; Hirano, M. Spike Current Induction by Photogenerated Charge Accumulation at the Surface Sites of Porous Porphyrinic Zirconium Metal-Organic Framework Electrodes in Photoelectrochemical Cells. Bull. Chem. Soc. Jpn. 2023.
- Alabyadh, T.; Albadri, R.; Es-haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. J. Funct. Biomater. 2022, 13, 139.
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current Status and Prospects of Metal-Organic Frameworks for Bone Therapy and Bone Repair. J. Mater. Chem. B 2022, 10, 5105–5128.
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent Advances in Ti-Based MOFs in Biomedical Applications. Dalton Trans. 2022, 51, 14817–14832.
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal–Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2018, 30, 1704124.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444.
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous Metal–Organic Framework Materials. Chem. Soc. Rev. 2012, 41, 1677–1695.
- Nezhad-Mokhtari, P.; Arsalani, N.; Javanbakht, S.; Shaabani, A. Development of Gelatin Microsphere Encapsulated Cu-Based Metal-Organic Framework Nanohybrid for the Methotrexate Delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 174–180.
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal-Organic Frameworks for Stimuli-Responsive Drug Delivery. Biomaterials 2020, 230, 119619.
- Oroojalian, F.; Karimzadeh, S.; Javanbakht, S.; Hejazi, M.; Baradaran, B.; Webster, T.J.; Mokhtarzadeh, A.; Varma, R.S.; Kesharwani, P.; Sahebkar, A. Current Trends in Stimuli-Responsive Nanotheranostics Based on Metal–Organic Frameworks for Cancer Therapy. Mater. Today 2022, 57, 192–224.
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526.
- Lan, G.; Ni, K.; Lin, W. Nanoscale Metal–Organic Frameworks for Phototherapy of Cancer. Coord. Chem. Rev. 2019, 379, 65–81.
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Mostafavi, E. Metal-Organic Frameworks-Based Nanomaterials for Drug Delivery. Materials 2021, 14, 3652.
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202.
- Feng, L.; Wang, K.Y.; Day, G.S.; Ryder, M.R.; Zhou, H.C. Destruction of Metal-Organic Frameworks: Positive and Negative Aspects of Stability and Lability. Chem. Rev. 2020, 120, 13087–13133.
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303.
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal-Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018.
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the Stability of Metal-Organic Frameworks. Adv. Chem. 2014, 2014, 1155.
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal-Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839.
- Lim, J.Y.C.; Goh, L.; Otake, K.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-Relevant Metal Organic Framework-Hydrogel Composites. Biomater. Sci. 2023.
- Zhou, S.; Li, D.; Lee, C.; Xie, J. Nanoparticle Phototherapy in the Era of Cancer Immunotherapy. Trends Chem. 2020, 2, 1082–1095.
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal-Organic Frameworks: A Future Toolbox for Biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153.
- Bao, Z.; Li, K.; Hou, P.; Xiao, R.; Yuan, Y.; Sun, Z. Nanoscale Metal-Organic Framework Composites for Phototherapy and Synergistic Therapy of Cancer. Mater. Chem. Front. 2021, 5, 1632–1654.
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The Recent Progress on Metal-Organic Frameworks for Phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125.
- Yang, M.; Zhang, J.; Shi, W.; Zhang, J.; Tao, C. Recent Advances in Metal-Organic Frameworks and Their Composites for the Phototherapy of Skin Wounds. J. Mater. Chem. B 2022, 10, 4695–4713.
- Rajora, M.A.; Lou, J.W.H.; Zheng, G. Advancing Porphyrin’s Biomedical Utility: Via Supramolecular Chemistry. Chem. Soc. Rev. 2017, 46, 6433–6469.
- DeRosa, M.; Crutchley, R. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371.
- Lu, K.; He, C.; Lin, W. Nanoscale Metal-Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715.
- Ye, Y.; Zhao, Y.; Sun, Y.; Cao, J. Recent Progress of Metal-Organic Framework-Based Photodynamic Therapy for Cancer Treatment. Int. J. Nanomed. 2022, 17, 2367–2395.
- Liu, T.F.; Feng, D.; Chen, Y.P.; Zou, L.; Bosch, M.; Yuan, S.; Wei, Z.; Fordham, S.; Wang, K.; Zhou, H.C. Topology-Guided Design and Syntheses of Highly Stable Mesoporous Porphyrinic Zirconium Metal-Organic Frameworks with High Surface Area. J. Am. Chem. Soc. 2015, 137, 413–419.
- Matlou, G.G.; Abrahamse, H. Nanoscale Metal–Organic Frameworks as Photosensitizers and Nanocarriers in Photodynamic Therapy. Front. Chem. 2022, 10, 971747.
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.C. Size-Controlled Synthesis of Porphyrinic Metal-Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525.
- Jia, J.; Zhang, Y.; Zheng, M.; Shan, C.; Yan, H.; Wu, W.; Gao, X.; Cheng, B.; Liu, W.; Tang, Y. Functionalized Eu(III)-Based Nanoscale Metal-Organic Framework to Achieve Near-IR-Triggered and -Targeted Two-Photon Absorption Photodynamic Therapy. Inorg. Chem. 2018, 57, 300–310.
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering Phototheranostic Nanoscale Metal-Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051.
- Liu, C.; Xing, J.; Akakuru, O.U.; Luo, L.; Sun, S.; Zou, R.; Yu, Z.; Fang, Q.; Wu, A. Nanozymes-Engineered Metal-Organic Frameworks for Catalytic Cascades-Enhanced Synergistic Cancer Therapy. Nano Lett. 2019, 19, 5674–5682.
- Gao, S.; Zheng, P.; Li, Z.; Feng, X.; Yan, W.; Chen, S.; Guo, W.; Liu, D.; Yang, X.; Wang, S.; et al. Biomimetic O2-Evolving Metal-Organic Framework Nanoplatform for Highly Efficient Photodynamic Therapy against Hypoxic Tumor. Biomaterials 2018, 178, 83–94.
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal–Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, 1808200.
- Illes, B.; Hirschle, P.; Barnert, S.; Cauda, V.; Wuttke, S.; Engelke, H. Exosome-Coated Metal-Organic Framework Nanoparticles: An Efficient Drug Delivery Platform. Chem. Mater. 2017, 29, 8042–8046.
- Zhang, L.; Cheng, Q.; Li, C.; Zeng, X.; Zhang, X.Z. Near Infrared Light-Triggered Metal Ion and Photodynamic Therapy Based on AgNPs/Porphyrinic MOFs for Tumors and Pathogens Elimination. Biomaterials 2020, 248, 120029.
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.; Ma, L.; Du, Y.; Peng, N.; Ma, L.; Zhang, Q.; et al. Intercalation-Activated Layered MoO3 Nanobelts as Biodegradable Nanozymes for Tumor-Specific Photo-Enhanced Catalytic Therapy. Angew. Chem.–Int. Ed. 2022, 61, e202115939.
- Pourmadadi, M.; Eshaghi, M.M.; Ostovar, S.; Shamsabadipour, A.; Safakhah, S.; Mousavi, M.S.; Rahdar, A.; Pandey, S. UiO-66 Metal-Organic Framework Nanoparticles as Gifted MOFs to the Biomedical Application: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2022, 76, 103758.
- Liang, W.; Ricco, R.; Maddigan, N.K.; Dickinson, R.P.; Xu, H.; Li, Q.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Control of Structure Topology and Spatial Distribution of Biomacromolecules in Biocomposites. Chem. Mater. 2018, 30, 1069–1077.
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. PH-Responsive Metal-Organic Framework Encapsulated Gold Nanoclusters with Modulated Release to Enhance Photodynamic Therapy/Chemotherapy in Breast Cancer. J. Mater. Chem. B 2020, 8, 1739–1747.
- Zorainy, M.Y.; Gar Alalm, M.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 Metal-Organic Framework: Design, Synthesis, Modifications, Advances, and Recent Applications. J. Mater. Chem. A Mater 2021, 9, 22159–22217.
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface area. Science 2005, 309, 2040–2042.
- Liu, J.; Zhang, L.; Lei, J.; Shen, H.; Ju, H. Multifunctional Metal-Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158.
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A Multimodal Metal-Organic Framework Based on Unsaturated Metal Site for Enhancing Antitumor Cytotoxicity through Chemo-Photodynamic Therapy. J. Colloid Interface Sci. 2022, 621, 180–194.
- Wang, P.; Chen, B.; Zhan, Y.; Wang, L.; Luo, J.; Xu, J.; Zhan, L.; Li, Z.; Liu, Y.; Wei, J. Enhancing the Efficiency of Mild-Temperature Photothermal Therapy for Cancer Assisting with Various Strategies. Pharmaceutics 2022, 14, 2279.
- Yang, Y.; Liu, J.; Liang, C.; Feng, L.; Fu, T.; Dong, Z.; Chao, Y.; Li, Y.; Lu, G.; Chen, M.; et al. Nanoscale Metal-Organic Particles with Rapid Clearance for Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Nano 2016, 10, 2774–2781.
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.J.; Peng, X. One Stone Two Birds: Zr-Fc Metal-Organic Framework Nanosheet for Synergistic Photothermal and Chemodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20321–20330.
- Mei, X.; Han, Y.; Xi, J.; Liu, J.; Xu, L.; Yuan, J.; Wang, S.; Li, X.; Si, W.; Li, J. Preparation of Hollow Mesoporous Prussian Blue Coated with Mesoporous Silica Shell Nanocubes for Photothermal Therapy and Drug Carrier. Mater. Lett. 2022, 312, 131697.
- Qin, Z.; Li, Y.; Gu, N. Progress in Applications of Prussian Blue Nanoparticles in Biomedicine. Adv. Healthc. Mater. 2018, 7, 1800347.
- Luo, Y.; Li, J.; Liu, X.; Tan, L.; Cui, Z.; Feng, X.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. Dual Metal-Organic Framework Heterointerface. ACS Cent. Sci. 2019, 5, 1591–1601.
- Chen, W.; Zeng, K.; Liu, H.; Ouyang, J.; Wang, L.; Liu, Y.; Wang, H.; Deng, L.; Liu, Y.N. Cell Membrane Camouflaged Hollow Prussian Blue Nanoparticles for Synergistic Photothermal-/Chemotherapy of Cancer. Adv. Funct. Mater. 2017, 27, 1605795.
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon Dots/Prussian Blue Satellite/Core Nanocomposites for Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092.
- Tian, Z.; Yao, X.; Ma, K.; Niu, X.; Grothe, J.; Xu, Q.; Liu, L.; Kaskel, S.; Zhu, Y. Metal-Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy. ACS Omega 2017, 2, 1249–1258.
- Zhu, Y.d.; Chen, S.P.; Zhao, H.; Yang, Y.; Chen, X.Q.; Sun, J.; Fan, H.S.; Zhang, X.D. Nanoparticles as a PH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
991
Revision:
1 time
(View History)
Update Date:
25 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





