Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marica Falzarano | -- | 3178 | 2023-03-24 12:25:22 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Falzarano, M.; Polettini, A.; Pomi, R.; Rossi, A.; Zonfa, T. Bioplastics Biodegradation. Encyclopedia. Available online: https://encyclopedia.pub/entry/42516 (accessed on 07 February 2026).
Falzarano M, Polettini A, Pomi R, Rossi A, Zonfa T. Bioplastics Biodegradation. Encyclopedia. Available at: https://encyclopedia.pub/entry/42516. Accessed February 07, 2026.
Falzarano, Marica, Alessandra Polettini, Raffaella Pomi, Andreina Rossi, Tatiana Zonfa. "Bioplastics Biodegradation" Encyclopedia, https://encyclopedia.pub/entry/42516 (accessed February 07, 2026).
Falzarano, M., Polettini, A., Pomi, R., Rossi, A., & Zonfa, T. (2023, March 24). Bioplastics Biodegradation. In Encyclopedia. https://encyclopedia.pub/entry/42516
Falzarano, Marica, et al. "Bioplastics Biodegradation." Encyclopedia. Web. 24 March, 2023.
Copy Citation
Bioplastics have entered everyday life as a potential sustainable substitute for commodity plastics. The wide array of biopolymers and commercial blends available make predicting the biodegradation degree and kinetics quite a complex issue that requires specific knowledge of the multiple factors affecting the degradation process. Understanding the material-related and environment-related aspects that determine the actual biodegradation of bioplastics is necessary to harmonize their treatment with biowaste using the typical processing conditions of waste treatment plants.
anaerobic digestion
biopolymers
PHA
PHB
PLA
starch-based
bioplastics
Mater-Bi
PCL
PBAT
1. General Concepts and Influencing Factors
Biodegradation of organic matter involves microbially mediated conversion of the original compounds into water, biomass cells, CO2 (under aerobic conditions) or CO2, CH4, and minor amounts of other gaseous products (under anaerobic conditions).
The process can occur in natural environments under uncontrolled conditions or in dedicated systems where the operating parameters, the process factors, and the metabolic products can be monitored more easily.
Based on the current state of the art, most biodegradable plastics are engineered to be degraded in aerobic environments, which has fostered a large quantity of scientific studies on the assessment of the aerobic degradability of such materials. On the other hand, the research about the biodegradation features of commercial bioplastic products under anaerobic conditions has only very recently developed systematically. As a result, definitive conclusions on the degree of anaerobic biodegradability, the governing mechanisms, and the influence of key factors are still far from having been achieved.
The anaerobic degradation of organic matter has been intensively explored over the past three decades to elucidate the underlying biochemical pathways, the microbial species involved, the reaction products, as well as the main influencing factors of the process. Anaerobic digestion is a complex biochemical process resulting from the synthrophic activity of an array of microbial species having different functions and physiology, metabolic capabilities, and operating conditions requirements. Such microorganisms, therefore, play a specific role in one of the sequential process phases (hydrolysis, acidogenesis, acetogenesis, and methanogenesis). In general, and particularly for complex substrates such as the polymeric structures of bioplastics, hydrolysis—which involves the breakdown of the original substrate molecules into simpler species that can be further metabolized by the microorganisms—is recognized to be the rate-limiting step of the whole process and is therefore crucial for the subsequent biochemical pathways. Acidogenic microorganisms convert the hydrolyzed compounds into short-chain fatty acids, lactate, alcohols, and chetons. These are in turn further transformed by acetogenic microorganisms into H2, CO2, and acetate; this can also be synthesized by autotrophic homoacetogens directly from the H2 and CO2 generated in the previous stage. The final methanogenic stage mainly involves the formation of CH4 and CO2 through either the acetoclastic or hydrogenotrophic pathways [1][2]. The main microbial species taking part in the process include hydrolytic bacteria, primary/secondary fermentative bacteria, and methanogenic archaea, which are synthrophically connected through the exchange of H2, formate (as electron carriers), and other metabolites such as acetate [3] to sustain the related microbial reactions.
Anaerobic digestion is commonly regarded as a valuable and sustainable strategy to recover materials (compost, digestate, nutrients) and energy from wastes [4][5], while at the same time contributing to reducing the net emissions of greenhouse gases from waste treatment. With regard to such aspects, anaerobic digestion can represent a valuable technological option for the management of end-of-life bioplastics, assuming that they are collected and managed together with the organic fraction of municipal solid waste. Optimized anaerobic degradation conditions—as for other biological processes—require well-balanced amounts of carbon and nutrients. Since it is well recognized that typical substrates for anaerobic digesters, such as food/kitchen waste, the organic fraction of municipal solid waste, and sewage sludge, have a typically low C/N ratio while most bioplastics are poor in nitrogen, the co-digestion of such materials may be an operating strategy to adjust the C/N ratio to optimize the digestion condition and enhance the degree of substrate conversion into biogas [6].
The estimation of biodegradability is commonly made on the basis of the volume of biogas evolved. Under aerobic conditions, the CO2 volume is used as an index of assimilation and mineralization of the substrate and biodegradability is expressed as the ratio between the evolved CO2 and the theoretical amount of CO2 expected (Equation (1)). Under anaerobic conditions, biodegradability is usually quantified from the ratio between the total biogas (CH4 + CO2) produced and the corresponding theoretical amount of biogas expected (Equation (2)), or as the equivalent ratio for methane instead of total biogas (Equation (3)). Equation (3) is sometimes preferred over Equation (2) since CO2 is relatively water-soluble (especially under elevated CO2 partial pressures as in digesters’ headspace); therefore, the quantification of the total biogas volume evolved requires direct determination of the dissolved inorganic carbon that should be made without altering the thermodynamic and chemical conditions of the system.
The theoretical volumes of CO2 and biogas produced are calculated from the polymer’s carbon content under the hypothesis that this is totally converted into the final products, e.g., neglecting the amount of carbon incorporated in the microbial cells due to biomass growth. For instance, under anaerobic conditions, the Buswell equation is commonly adopted (Equation (4)) [7]:
It should be considered that the Buswell equation does not take into account the substrate conversion into biomass; therefore, the actual biogas production has an upper limit that is obviously lower than that expected from Equation (4) [8].
Biodegradation is a process governed by the combination of different factors, depending on the polymer characteristics and on the environmental conditions it is subjected to.
The configuration of the monomeric units constituting the polymer, the bonds among the elements, and their orientation dictate the material properties, which, in turn, influence its biodegradation profile. In general, the presence of hydrolyzable groups in biopolymers (ether, ester, amide, and carbonate) is the factor that determines their susceptibility to microbial attack [9]. The solubility of polymers typically decreases as the polymeric chain length and molecular weight increase. Crystallinity improves water resistance, therefore limiting both hydrolysis and the microbial activity that are instead favored in amorphous regions. On the other hand, hydrophilicity determines higher vulnerability to water.
Flexibility is another characteristic that lowers the degradation enthalpy since it improves the possibility to fit better into the active sites of enzymes. Aliphatic polyesters have, in general, a larger flexibility compared to the aromatic and aliphatic-aromatic counterparts and are therefore particularly suited for degradation [10].
Polymers with lower molecular weights, a higher amorphous character, and higher flexibility are in principle more prone to biological attack [11].
Furthermore, exposure conditions to potential degradation agents/factors can complement polymers characteristics and improve degradability. The main external factors affecting biodegradation can be both biotic and abiotic. Each environment typically has a specific microbial community and the main abiotic factors, such as temperature, pH, and moisture, can promote their growth and activity [12].
Biodegradation is an enzymatic reaction and proceeds very specifically depending on the chemical bonds/linkages of the polymer and the structure of particular functional groups. In general, microorganisms are only capable of attacking specific functional groups at specific sites.
Temperature has an effect on enhancing the hydrolysis and the overall process rate [13] by increasing polymer chains mobility and enzymatic activity. When temperature is in the range of the polymer’s Tg, the material becomes more flexible. Acidic or basic environments have been found to accelerate hydrolysis as well. Of course, moisture is involved in the hydrolysis of polymeric materials as well as in sustaining microbial activity. Another mechanism of biopolymer alteration involves photodegradation, which depends on the interaction between the polymer and UV radiation.
2. Biodegradation Mechanisms
Polymers biodegradation is the result of the competition and combination of multiple mechanisms. As illustrated in Figure 1, both abiotic and biotic (enzymatic) actions can lead to the cleavage of the polymer’s chemical bonds, and later to matrix erosion [14]. The process can be carried out at different levels: surface level, bulk level, or through autocatalysis [15]. Surface degradation is a heterogeneous process which may also be detected visually, while bulk erosion affects the whole matrix at the same time, so that the material remains apparently the same for a long time until it disaggregates abruptly [16]. Bulk erosion is more related to the influence of abiotic factors, which may include mechanical stresses (resulting from compression, tension, or shear forces), thermal alteration, water absorption, chemical hydrolysis, oxidation, or photolysis [17][18]. The resulting fractures can favor the microbial degradation pathways. Autocatalysis is a phenomenon that happens internally, where the oligomers and monomers released remain trapped into the matrix and are able to continue cleaving the polymeric backbone from the inside. Regardless of the mechanisms involved, the degradation of the polymeric matrix can be tracked with the monitoring of molecular weight and monomers release [16].
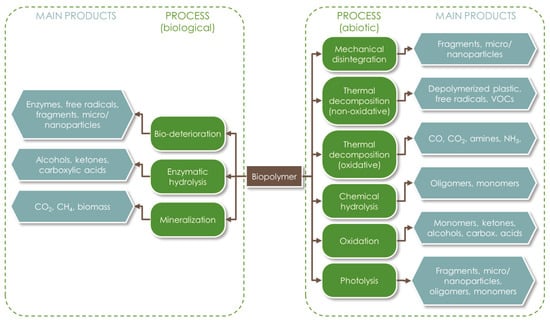
Figure 1. Main abiotic and biotic degradation mechanisms of biopolymers and related products.
In general terms, the main steps in the degradation of polymers include: (i) biodeterioration; (ii) depolymerization; (iii) assimilation; and (iv) mineralization [19]. Biodeterioration causes changes in the physical, mechanical, and chemical characteristics of the material. It begins with the adhesion of microorganisms on the material surface and the formation of a biofilm. Extracellular depolymerase enzymes and free radicals are generated and their action leads to the formation of cavities, microfractures, and the cleavage of the polymer backbone. A physical surface embrittlement and bulk erosion may also complement the enzymatic degradation, increasing the material’s surface area exposed to microbial attack, thus promoting the subsequent biodegradation reactions. In this phase, hydrolysis occurs thanks to the diffusion of water into the amorphous regions of the polymeric matrix. For instance, the butylene adipate and butylene terephthalate components of PBAT degrade at different rates, with the former being less crystalline [20]. Moreover, the kinetics of this process depend on the polymer hydrophilicity; thus, it is generally very slow for PCL [21].
Depolymerization and assimilation are carried out by two categories of enzymes that are extracellular and intracellular. Extracellular enzymes are secreted by microorganisms and can act randomly on the disruption of specific bonds or linkages in the polymeric structure, releasing intermediate metabolic products with simpler molecular structures, with an associated reduction in the molecular weight of the material [22]. Some authors observed that the efficacy of enzymatic hydrolysis is dependent on the degree of adsorption of the enzyme onto the polymer surface, which is the pre-condition required for surface erosion of the polymer [23].
Extracellular enzymes exert their action according to two different polymer cleaving modes: endo-type hydrolysis involves random scission of ester bonds along the main chain of the polymer, releasing either monomers or short-chain soluble oligomers; on the other hand, in exo-type hydrolysis, the material is degraded stepwise from the chain ends of the polymeric structure (for instance, either the hydroxyl or the carbonyl end of the molecule in the case of polyesters), with oligomers being mainly generated at first by the cleavage action [24].
In particular, the ester bond in the polyesters’ backbone is susceptible to non-enzymatic scission that occurs through the following reaction [25]:
The formation of carboxylic groups, in particular, determines the further autocatalysis of the breakage of ester linkages, since polymer oligomers have a lower pKa compared to most carboxylic groups [25][26]. In PBAT, the cleavage of ester linkages is coupled with the reaction between water and the carbonyl groups located in the proximity of the benzene rings [20].
The type of intermediate metabolites produced in the depolymerization phase depends on both the specific polymer of concern and the type of enzymes involved [27].
It was observed that PLA degradation into lactic acid oligomers begins when a molecular weight drop to below 10,000 Da [25] and the main enzymes involved are proteases and lipases [28][29]. The same enzymes were found to be responsible for PCL ester bond cleavage [30]; as a result of such bond breaking, the polymer is broken down to carboxyl terminal groups and 6-hydroxylcaproic acid [31].
During degradation of PBS, degrading enzymes including esterases, lipases, and cutinases were identified[32][24][33]. Exo-type cleavage was observed in the presence of lipase, with 4-hydroxybutyl succinate dimer as the main hydrolysis product by some investigators [23][24]. In another study [34], an enzyme extracted from Aspergillus sp. was found to be capable of degrading PBS, again through exo-type hydrolysis at the carboxylic chain end; in this case, the degradation products were found to include succinic acid, butylene succinate, succinic acid-butylene succinate, and their salts. PBS degradation using cutinase was tested in a number of studies [35][36] that revealed endo-type hydrolysis of the polymer, although different chain scission modes (either at the hydroxyl or at the carbonyl end of the polymer) were found to occur based on the observed degradation products.
A series of enzymes (hydrolase, lipase, esterase, and cutinase) were identified in both composting and anaerobic digestion environments in PBAT degradation [37], with the subsequent production of terephthalic acid, adipic acid, and 1,4-butanediol [38].
PHB and PHBV were found to be broken down by depolymerases and hydrolases to 3-hydroxybutyric acid and both 3-hydroxybutyric acid and 3-hydroxyvaleric acid, respectively [39].
During starch degradation, the amylose and amylopectin acetal links are hydrolyzed by amylase and glucosidase, respectively, which generate glucose, maltose, and maltotriose [40][41].
After depolymerization, long- and short-chain oligomers and soluble monomers released are able to cross the cell membranes and can then be directly exposed to the assimilation reactions, which are catalyzed by intracellular enzymes [42]. They are used by the microorganisms in both catabolic and anabolic reactions to generate energy and other metabolic products and synthesize new microbial cells. The last stage of the biodegradation process, i.e., mineralization, involves the final substrate conversion into water, biomass cells, CO2 (under aerobic conditions) or CO2, CH4, and minor amounts of other gaseous products (under anaerobic conditions).
3. Microbiology of Bioplastics Biodegradation
The specific type of microbial pathways occurring and the related microbial species involved are crucial for the degradation of the polymeric matrix of bioplastic products. More than 90 types of microbes were found to be involved in bioplastics degradation [43], mainly deriving from compost or soil environments. Currently, little is known on the specific role of each microbial species in the biodegradation process, particularly regarding anaerobic conditions [44][45]. In general terms, the microorganisms found in anaerobic digesters are mainly bacteria; archaea are present as well and take part in the methanogenic phase [46].
The operating temperature has a large influence on the microbial community development. During mesophilic treatment of bioplastics, a prevalence of Bacteroidota, Chloroflexi, Desulfobacterota, Firmicutes, and Euryarchaeota was observed, while at thermophilic temperatures, Firmicutes, Proteobacteria, and Coprothermobacter were found to be predominant [46][47]. Increased temperatures were also observed to favor the growth of hydrogenotrophic methanogens [48]. Some attempts have been made at isolating bacterial strains, which were also found to become more efficient as the degradation time was reduced [49][50].
A number of authors attempted to identify the microbial strains participating in the degradation of specific bioplastic matrices. For starch-based products, a prevalence of Firmicutes and Synergistetes operational taxonomic units (OTUs) was observed under thermophilic conditions, while a dominance of Bacteroidetes, Firmicutes, Chloroflexi, and Proteobacteria was detected under mesophilic conditions [48].[51]
PHB was found to be degraded by the genus Clostridium botulinum [49] and by consortia of Ilyobacter delafieldii, Enterobacterm and Cupriavidus [52]. Moreover, Yagi and colleagues tested PHB and detected Arcobacter thereius and Clostridium sp. when operating under mesophilic temperatures [53], and Peptococcaceae bacterium Ri50, Bacteroides plebeius, and Catenibacterium mitsuokai at thermophilic temperatures [54].
Several studies on PLA anaerobic degradation also reported the main microbial strains detected during the process. In many cases, lactic acid bacteria were observed, such as Moorella, Tepidimicrobium, Thermogutta [47][52][55]. When treating the polymer under mesophilic conditions, Xanthomonadaceae bacterium and Mesorhizobium sp. were detected [53], while Ureibacillus sp. was identified under thermophilic conditions [54]. Methanosaeta, Methanoculleus, and Methanobacterium were the methanogenic archaea mainly found during the anaerobic degradation of PLA [53][51].
PCL was found to be degraded by strains of the Clostridium genus [49] and A thereius [53], although there were also other reported cases in which PCL displayed a remarkable resistance to microbial attack under anaerobic conditions compared to compost or soil environments [56][49].
The understanding and control of the microbial consortia operating during the anaerobic degradation process may be used to maximize substrate conversion and the related biogas production. Molecular biology techniques could be used as a tool to this aim. In the past years, many attempts have been made to improve bioplastic production processes through the use of modified enzymes by protein engineering [57], while investigation on applications to enhance bioplastic degradation is still in its infancy. However, enzymatic degradation of bioplastics could represent a viable option if correctly assessed and standardized [58]. Bioaugmentation may also be a useful tool; however, so far, it has been explored mainly for composting conditions. For instance, Mistry and colleagues tested high molecular weight PLA films with an ad hoc degrading bacterial consortium with Nocardioides zeae EA12, Stenotrophomonas pavanii EA33, Gordonia desulfuricans EA63, and Chitinophaga jiangningensis EA02 and observed a 50% increase in mineralization compared to the test with indigenous microorganisms [59]. Expanding the research in the way of engineered enzymes or introducing the assessment of bioaugmentation strategies could improve the current understanding of the anaerobic degradation of bioplastics.
4. Biodegradation Monitoring Techniques
Since the degradation of biopolymers and biopolymer-based materials is a complex process, it can be monitored and assessed using different approaches and viewpoints. The assessment of biogas and methane production can be complemented with further analyses, which can provide additional information on the physical, mechanical, chemical, and microstructural characteristics of the material at different stages of degradation. The data retrieved using different approaches can then be used to derive correlations and draw more detailed conclusions on the biodegradation process.
The additional methodologies that can be used belong to five main categories, including disintegration measures, morphologic/visual inspection, microbiological characterization, thermal behavior, and spectroscopic analyses.
Disintegration can be assessed through mass loss measurements at different times to monitor the evolution of polymer disruption.
Visual inspection can be carried out at a macroscopic level by observing the plastic fragments at the end of the experiment, provided that they are still visible at the naked eye. More advanced particle observation techniques, such as optical microscopy or scanning electron microscopy (SEM), can be used to monitor the physical changes at the microscopic level.
The analysis of the microbial community involved during the degradation process can provide further information on the adaptability of microorganisms to the polymeric substrate and the compatibility of the material with the environmental conditions it was subjected to.
The analysis of the thermal behavior of the material can give an insight into the changes occurring in its physical and chemical properties. To this aim, the most used techniques are thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) that can identify key temperatures in polymer phase transitions.
Spectroscopic analysis can also be carried out using Fourier-transform infrared (FT-IR) or X-ray diffraction (XRD) techniques, which can assist the identification of major chemical bonds in the matrix and their rearrangement as a result of biodegradation.
References
- Appels, L.; Lauwers, J.; Degrve, J.; Helsen, L.; Lievens, B.; Willems, K.; Van Impe, J.; Dewil, R. Anaerobic Digestion in Global Bio-Energy Production: Potential and Research Challenges. Renewable and Sustainable Energy Reviews 2011, 15, 4295–4301.
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013.. Renewable and Sustainable Energy Reviews 2014, 36, 412–427, 10.1016/j.rser.2014.04.039.
- Batstone, D.J.; Hülsen, T.; Oehmen, A. Metabolic Modelling of Mixed Culture Anaerobic Microbial Processes.. Current Opinion in Biotechnology 2019, 57, 137–144.
- Cecchi, F.; Bolzonella, D.; Pavan, P.; Macé, S.; Mata-Alvarez, J. Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste for Methane Production: Research and Industrial Application.. Comprehensive Biotechnology 2011, 6, 411–420.
- Batstone, D.J.; Hülsen, T.; Mehta, C.M.; Keller, J. Platforms for Energy and Nutrient Recovery from Domestic Wastewater: A Review.. Chemosphere 2015, 140, 2–11.
- Benn, N.; Zitomer, D. Pretreatment and Anaerobic Co-Digestion of Selected PHB and PLA Bioplastics.. Frontiers in Environmental Science 2018, 5, 93.
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation.. Industrial & Engineering Chemistry 1952, 44, 550–552.
- Ryan, C.A.; Billington, S.L.; Criddle, C.S. Methodology to Assess End-of-Life Anaerobic Biodegradation Kinetics and Methane Production Potential for Composite Materials.. Composites Part A: Applied Science and Manufacturing 2017, 95, 388–399.
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview.. Macromolecular Bioscience 2007, 7, 255–277.
- Lim, B.K.H.; Thian, E.S. Biodegradation of Polymers in Managing Plastic Waste—A Review.. Sci. Total Environ. 2022, 813, 151880.
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526–536.
- Massardier-Nageotte, V.; Pestre, C.; Cruard-Pradet, T.; Bayard, R. Aerobic and Anaerobic Biodegradability of Polymer Films and Physico-Chemical Characterization. Polym. Degrad. Stab. 2006, 91, 620-627.
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of Important Abiotic and Biotic Factors in the Biodegradation of Poly(l-Lactic Acid). Int. J. Biol. Macromol. 2014, 71, 155–162.
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1-20.
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256.
- Göpferich, A. Mechanisms of Polymer Degradation and Erosion1. The Biomaterials: Silver Jubilee Compendium 1996, 17, 117–128.
- Mergaert, J.; Glorieux, G.; Hauben, L.; Storms, V.; Mau, M.; Swings, J. Biodegradation of Poly(3-Hydroxyalkanoates) in Anaerobic Sludge and Characterization of a Poly(3-Hydroxyalkanoates) Degrading Anaerobic Bacterium. Syst. Appl. Microbiol. Syst. Appl. Microbiol., 19, 407–413.
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. Biodegradation of Biopolymers. Current Developments in Biotechnology and Bioengineering 2017, ., 739–755.
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442.
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y.; Polymeric, F. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater Sediment. Molecules 2020, 25, 3946.
- Heimowska, A.; Morawska, M.; Bocho-Janiszewska, A. Biodegradation of Poly(ϵ-Caprolactone) in Natural Water Environments. Pol. J. Chem. Technol. 2017, 19, 120-126.
- Husárová, L.; Pekařová, S.; Stloukal, P.; Kucharzcyk, P.; Verney, V.; Commereuc, S.; Ramone, A.; Koutny, M. Identification of Important Abiotic and Biotic Factors in the Biodegradation of Poly(l-Lactic Acid). Int. J. Biol. Macromol. 2014, 71, 155-162.
- Taniguchi, I.; Nakano, S.; Nakamura, T.; El-Salmawy, A.; Miyamoto, M.; Kimura, Y. Mechanism of Enzymatic Hydrolysis of Poly(Butylene Succinate) and Poly(Butylene Succinate-Co-L-Lactate) with a Lipase from Pseudomonas Cepacia. Macromol. Biosci. 2002, 2, 447-455.
- Lee, C.W.; Kimura, Y.; Chung, J.-D. Mechanism of Enzymatic Degradation of Poly(Butylene Succinate). Macromol. Res. 2008, 16, 651-658.
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(Lactic Acid): A Review. Polym. Degrad. Stab. 2017, 137, 122-130.
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-Term Hydrolytic Degradation Study of Polycaprolactone Films and Fibers Grafted with Poly(Sodium Styrene Sulfonate): Mechanism Study and Cell Response. Biointerphases 2020, 15, 061006.
- Mat Yasin, N.; Akkermans, S.; Van Impe, J.F.M. Enhancing the Biodegradation of (Bio)Plastic through Pretreatments: A Critical Review. Waste Manag. 2022, 150, 1-12.
- Zaborowska, M.; Bernat, K.; Pszczółkowski, B.; Cydzik-Kwiatkowska, A.; Kulikowska, D.; Wojnowska-Baryła, I. Multi-Faceted Analysis of Thermophilic Anaerobic Biodegradation of Poly(Lactic Acid)-Based Material. Waste Manag. 2023, 155, 40-52.
- Oda, Y.; Yonetsu, A.; Urakami, T.; Tonomura, K. Degradation of Polylactide by Commercial Proteases. J. Polym. Environ. 2000, 8, 29-32.
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1-20.
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217-1256.
- Sisti, L.; Totaro, G.; Marchese, P. PBS Makes Its Entrance into the Family of Biobased Plastics. Biodegrad. Biobased Polym. Environ. Biomed. Appl. 2016, 7, 225-285.
- Rosato, A.; Romano, A.; Totaro, G.; Celli, A.; Fava, F.; Zanaroli, G.; Sisti, L. Enzymatic Degradation of the Most Common Aliphatic Bio-Polyesters and Evaluation of the Mechanisms Involved: An Extended Study. Polymers 2022, 14, 1850.
- Li, F.; Hu, X.; Guo, Z.; Wang, Z.; Wang, Y.; Liu, D.; Xia, H.; Chen, S. Purification and Characterization of a Novel Poly(Butylene Succinate)-Degrading Enzyme from Aspergillus Sp. XH0501-A. World J. Microbiol. Biotechnol. 2011, 27, 2591–2596.
- Rosato, A.; Romano, A.; Totaro, G.; Celli, A.; Fava, F.; Zanaroli, G.; Sisti, L. Enzymatic Degradation of the Most Common Aliphatic Bio-Polyesters and Evaluation of the Mechanisms Involved: An Extended Study. Polymers 2022, 27, 2591-2596.
- Shi, K.; Su, T.; Wang, Z. Comparison of Poly(Butylene Succinate) Biodegradation by Fusarium Solani Cutinase and Candida Antarctica Lipase. Polym. Degrad. Stab. 2019, 164, 55-60.
- Wallace, P.W.; Haernvall, K.; Ribitsch, D.; Zitzenbacher, S.; Schittmayer, M.; Steinkellner, G.; Gruber, K.; Guebitz, G.M.; Birner-Gruenberger, R. PpEst Is a Novel PBAT Degrading Polyesterase Identified by Proteomic Screening of Pseudomonas Pseudoalcaligenes. Appl. Microbiol. Biotechnol. 2017, 101, 2291-2303.
- Jia, H.; Zhang, M.; Weng, Y.; Zhao, Y.; Li, C.; Kanwal, A. Degradation of Poly(Butylene Adipate-Co-Terephthalate) by Stenotrophomonas Sp. YCJ1 Isolated from Farmland Soil. J. Environ. Sci. 2021, 103, 50-58.
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates (PHAs)—Production, Properties, and Biodegradation. Biodegradable Polymers in the Circular Plastics Economy 2022, 6, 145-204.
- Araújo, M.A.; Cunha, A.M.; Mota, M. Enzymatic Degradation of Starch-Based Thermoplastic Compounds Used in Protheses: Identification of the Degradation Products in Solution.. Biomaterials 2004, 25, 2687-2693.
- Rosa, D.S.; Carvalho, C.L.; Gaboardi, F.; Rezende, M.L.; Tavares, M.I.B.; Petro, M.S.M.; Calil, M.R. Evaluation of Enzymatic Degradation Based on the Quantification of Glucose in Thermoplastic Starch and Its Characterization by Mechanical and Morphological Properties and NMR Measurements. Polym. Test. 2008, 27, 827-834.
- Gu, J.D. Microbiological Deterioration and Degradation of Synthetic Polymeric Materials: Recent Research Advances. Int. Biodeterior. Biodegrad. 2003, 52, 69-91.
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of Bioplastics in Natural Environments. Waste Manag. 2017, 59, 526-536.
- Bandini, F.; Vaccari, F.; Soldano, M.; Piccinini, S.; Misci, C.; Bellotti, G.; Taskin, E.; Cocconcelli, P.S.; Puglisi, E. Rigid Bioplastics Shape the Microbial Communities Involved in the Treatment of the Organic Fraction of Municipal Solid Waste. Front. Microbiol. 2022, 13, 1035561.
- Abraham, A.; Park, H.; Choi, O.; Sang, B.-I. Anaerobic Co-Digestion of Bioplastics as a Sustainable Mode of Waste Management with Improved Energy Production—A Review. Bioresour. Technol. 2021, 322, 124537.
- Sundberg, C.; Al-Soud, W.A.; Larsson, M.; Alm, E.; Yekta, S.S.; Svensson, B.H.; Sørensen, S.J.; Karlsson, A. 454 Pyrosequencing Analyses of Bacterial and Archaeal Richness in 21 Full-Scale Biogas Digesters. FEMS Microbiol. Ecol. 2013, 85, 612-626.
- Cazaudehore, G.; Monlau, F.; Gassie, C.; Lallement, A.; Guyoneaud, R. Active Microbial Communities during Biodegradation of Biodegradable Plastics by Mesophilic and Thermophilic Anaerobic Digestion. J. Hazard. Mater. 2023, 443, 130208.
- Cazaudehore, G.; Monlau, F.; Gassie, C.; Lallement, A.; Guyoneaud, R. Methane Production and Active Microbial Communities during Anaerobic Digestion of Three Commercial Biodegradable Coffee Capsules under Mesophilic and Thermophilic Conditions. Sci. Total Environ. 2021, 784, 146972.
- Abou-Zeid, D.-M.; Müller, R.-J.; Deckwer, W.-D. Degradation of Natural and Synthetic Polyesters under Anaerobic Conditions. J. Biotechnol. 2001, 86, 113-126.
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of Bioplastics: Opportunities and Challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68-75.
- Peng, W.; Wang, Z.; Shu, Y.; Lü, F.; Zhang, H.; Shao, L.; He, P. Fate of a Biobased Polymer via High-Solid Anaerobic Co-Digestion with Food Waste and Following Aerobic Treatment: Insights on Changes of Polymer Physicochemical Properties and the Role of Microbial and Fungal Communities. Bioresour. Technol. 2022, 343, 126079.
- Cazaudehore, G.; Guyoneaud, R.; Lallement, A.; Gassie, C.; Monlau, F. Biochemical Methane Potential and Active Microbial Communities during Anaerobic Digestion of Biodegradable Plastics at Different Inoculum-Substrate Ratios. J. Environ. Manag. 2022, 324, 116369.
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Mesophilic Anaerobic Biodegradation Test and Analysis of Eubacteria and Archaea Involved in Anaerobic Biodegradation of Four Specified Biodegradable Polyesters. Polym. Degrad. Stab. 2014, 110, 278-283.
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Thermophilic Anaerobic Biodegradation Test and Analysis of Eubacteria Involved in Anaerobic Biodegradation of Four Specified Biodegradable Polyesters. Polym. Degrad. Stab. 2013, 98, 1182-1187.
- Tseng, H.-C.; Fujimoto, N.; Ohnishi, A. Biodegradability and Methane Fermentability of Polylactic Acid by Thermophilic Methane Fermentation. Bioresour. Technol. Rep. 2019, 8, 100327.
- Lim, B.K.H.; Thian, E.S. Biodegradation of Polymers in Managing Plastic Waste—A Review. Sci. Total Environ. 2022, 813, 151880.
- Hiraishi, Tomohiro; Taguchi, Seiichi. Protein Engineering of Enzymes Involved in Bioplastic Metabolism; IntechOpen: Rijeka, 2013; pp. 6.
- Kaushal, J.; Khatri, M.; Arya, S.K. Recent Insight into Enzymatic Degradation of Plastics Prevalent in the Environment: A Mini—Review. Clean. Eng. Technol. 2021, 2, 100083.
- Mistry, A.N.; Kachenchart, B.; Pinyakong, O.; Assavalapsakul, W.; Jitpraphai, S.M.; Somwangthanaroj, A.; Luepromchai, E. Bioaugmentation with a Defined Bacterial Consortium: A Key to Degrade High Molecular Weight Polylactic Acid during Traditional Composting. Bioresour. Technol. 2023, 367, 128237.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
772
Revision:
1 time
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




