Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kenan Aloss | -- | 4008 | 2023-03-24 09:31:08 | | | |
| 2 | Jessie Wu | + 5 word(s) | 4013 | 2023-03-24 09:46:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aloss, K.; Hamar, P. Thermosensitive Liposomal Doxorubicin. Encyclopedia. Available online: https://encyclopedia.pub/entry/42502 (accessed on 02 March 2026).
Aloss K, Hamar P. Thermosensitive Liposomal Doxorubicin. Encyclopedia. Available at: https://encyclopedia.pub/entry/42502. Accessed March 02, 2026.
Aloss, Kenan, Peter Hamar. "Thermosensitive Liposomal Doxorubicin" Encyclopedia, https://encyclopedia.pub/entry/42502 (accessed March 02, 2026).
Aloss, K., & Hamar, P. (2023, March 24). Thermosensitive Liposomal Doxorubicin. In Encyclopedia. https://encyclopedia.pub/entry/42502
Aloss, Kenan and Peter Hamar. "Thermosensitive Liposomal Doxorubicin." Encyclopedia. Web. 24 March, 2023.
Copy Citation
Thermo-sensitive liposomes (TSLs) offer the most promising approach for more efficient doxorubicin (DOX) delivery to the tumor at mild hyperthermic temperatures (39-42 ̊C)
doxorubicin
liposome
grain
TSL
lysolipids
pTSL
DPPG-TSL
1. Traditional Thermosensitive Liposome (TTSL)
Thermo-sensitive liposomes (TSLs) are spherical vesicles composed of phospholipids that undergo a phase transition from a solid gel to a liquid-crystalline phase at a temperature above physiological temperature called melting phase transition temperature (Tm).In the gel phase, the phospholipids are well-arranged and immobile with fully extended hydrocarbon tails, preserving the liposome membrane impermeability. When the temperature approaches the Tm, the phospholipid heads become mobile, and the trans to a gauche shift in the configuration form of the C-C single bonds in the hydrocarbon chains takes place [1]. In other words, at the gel phase, the rotation around carbon bonds is restricted, and the hydrocarbon chains are in trans conformation (i.e., the dihedral angle = 180°). The trans conformers allow the hydrocarbon chains to pack tightly together. When the temperature approaches the Tm, the carbon atoms in the hydrocarbon chains become less restricted to move and rotate 120° relative to trans conformation resulting in the formation of gauche conformers (Figure 1). As the number of gauche conformers increases in the lipid hydrocarbon chains, the lipid pack becomes loose [2]. At this point, leaky and highly disordered microscopic regions start to form at the interface between the membrane domains that have become liquid and the ones that are still in the gel phase. Those permeable regions are called grain boundaries (Figure 2A). At a temperature higher than the Tm, the liposome membrane becomes fully fluid and permeable resulting in drug release [1][3].
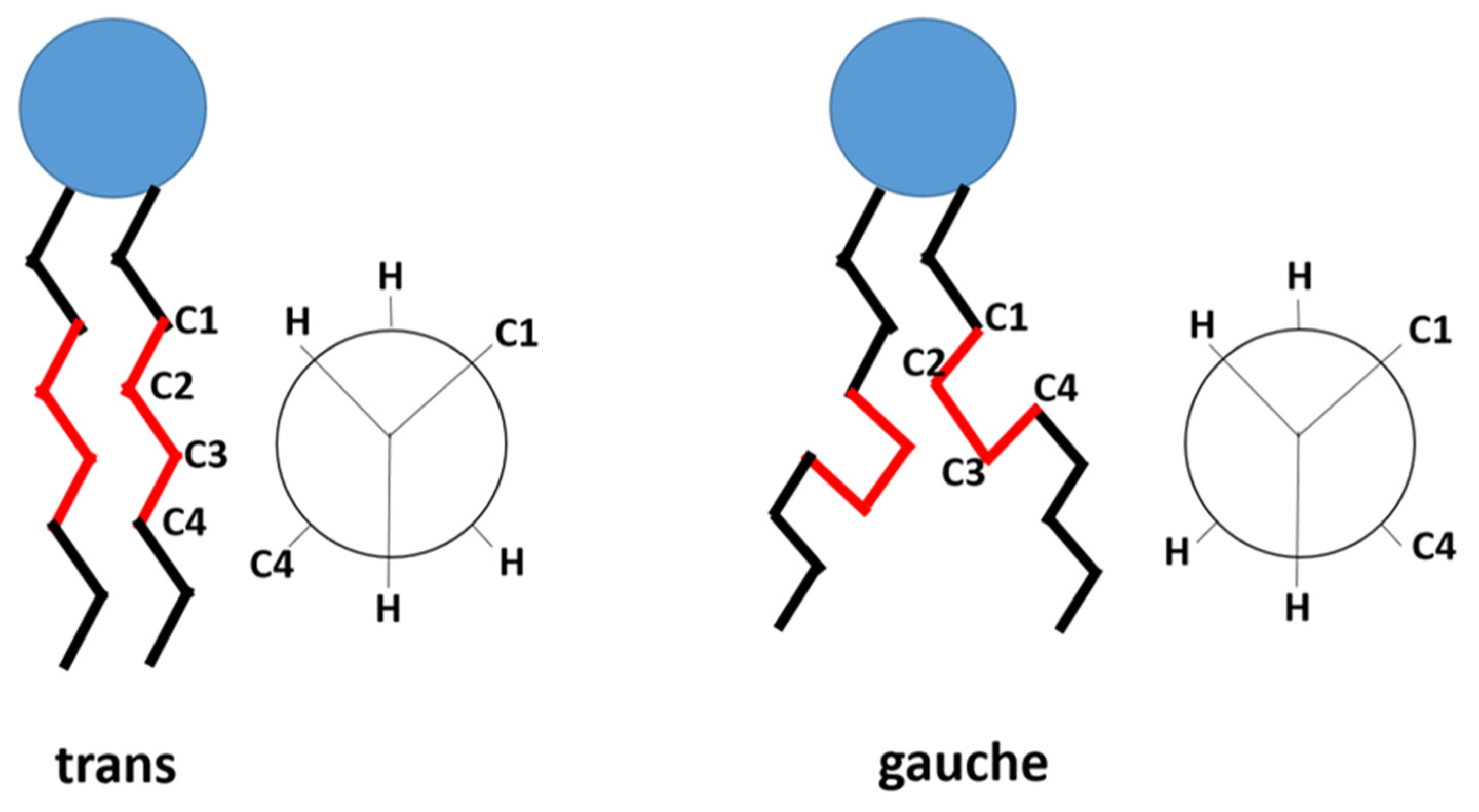
Figure 1. The trans and gauche conformers in the hydrocarbon chain of the phospholipid. In gel phase, trans conformers are predominant. In liquid-crystalline phase, both gauche and trans are present. (based on Kuc et al. [4]).
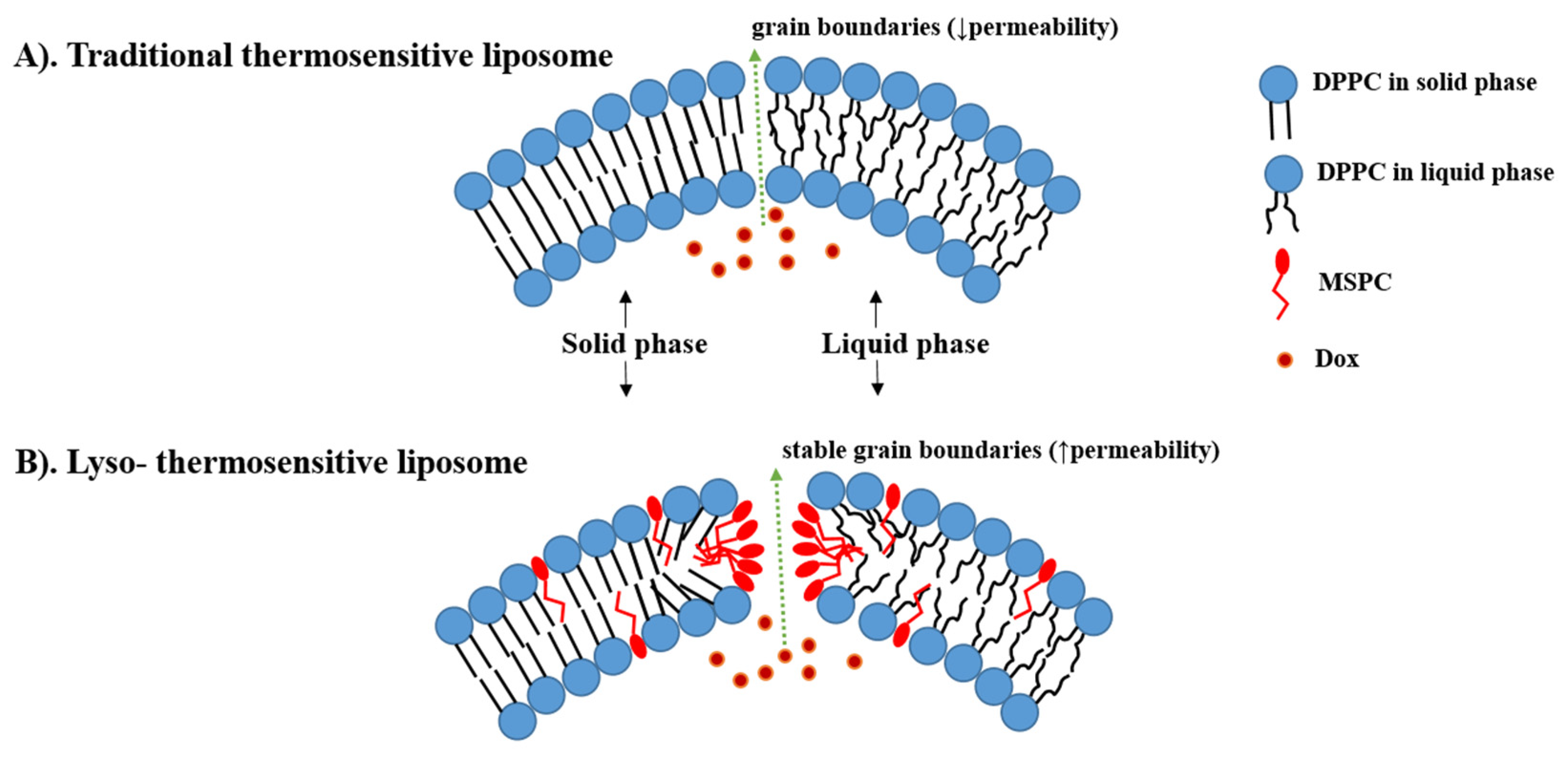
Figure 2. The difference between traditional thermosensitive liposomes (TTSL) (A) and lyso-thermosensitive liposome (LTSL) (B) during the phase transition. The lysolipids stabilize the grain boundaries resulting in rapid doxorubicin (DOX) release. (based on Ta et al. [5]).
The main component in all TSL formulations is a 1,2-DiPalmitoyl-sn-glycero-3-PhosphoCholine (DPPC) (Tm = 41.4 °C) which is usually mixed with small amounts of phospholipids with a higher Tm such as 1,2-DiStearoyl-sn-glycero-3-PhosphoCholine (DSPC) (Tm = 54.9 °C) to increase the membrane stability. The difference between the lower Tm DPPC and the higher Tm DSPC is that in DPPC palmitoyl, whereas in DSPC stearoyl, the residue is included. This explains the higher Tm in DSPC due to the longer hydrocarbon chain in the stearoyl residue [6]. The DPPC + DSPC mixture was the first described TSL formulation, developed by Yatvin et al. in 1978 and known now as traditional thermosensitive liposomes (TTSL) [7]. The main drawback of this formulation was the low rate and amount of release [7]. Therefore, Yatvin’s work was followed by several attempts to increase the membrane permeability by the inclusion of other phospholipids. Gaber et al. tested a combination of DPPC, hydrogenated soy phosphocholine (HSPC), cholesterol, and PEG at a molar ratio of (100:50:30:6). This mixture enhanced DOX release slightly (≈60% within 30 min), but the thermal range of phase transition was 42–45 °C which is not achievable clinically by mild hyperthermia (39–42 °C) [8]. Therefore, the aim in the more advanced TSL strategies is to enhance DOX release by the modification of the lipid bilayer using lysolipids, synthetic polymers and phosphatidylglycerol lipids.
2. Lyso-Thermosensitive Liposome (LTSL)
Lyso is a prefix that refers to the removal of one of the two fatty acid chains in phospholipids by hydrolysis [9]. Thus, lysolipids are small bioactive lipid molecules that contain only one acyl chain instead of two, such as Mono-Stearoyl-2-hydroxy-sn-glycero-3-PhosphoCholine (MSPC) and Mono-Palmitoyl-2-hydroxy-sn-glycero-3-PhosphoCholine (MPPC) [10]. The difference with DPPC/DSPC making up TTSL is that 2-hydroxy-sn-glycero-3-PhosphoCholine part is complexed with 1-stearoyl and 1-palmitoyl residues instead of two in DPPC/ DSPC. Due to the shorter hydrocarbon chain in palmitoyl, the addition of a small amount of MPPC leads to a lower peak phase transition compared to MSPC (40.5 °C vs. 41.3 °C) [11].
Incorporation of these shorter lysolipids into the TSL membrane dramatically increases the DOX released. It is postulated that lysolipids accumulate in the grain boundaries and result in stable pores in the lipid bilayer during the phase transition, leading to high membrane permeability and rapid DOX release from the liposome at the heated tumor (i.e., ≈80% of DOX released at 40–42 °C) (Figure 2B) [10][12]. This idea was first introduced in 1999 by Needham, who added 10% of MPPC into PEGylated DPPC membranes of TTSL (DPPC:MPPC:DSPE-PEG-2000, molar ratio 90:10:4) [13][14]. After that, Needham et al. and Kong et al. evaluated this formulation in mice bearing human squamous cell carcinoma xenograft (FaDu) and demonstrated higher DOX tumor accumulation and tumor growth inhibition after treatment with Lyso-thermosensitive liposome (LTSL) at 42 °C compared to treatment with TTSL and non-TSL liposome (NTSL) [14][15].
In contrast to NTSL, LTSL releases DOX into the bloodstream in the heated tumor, followed by DOX diffusion from the blood vessels into the tumor interstitium. This intravascular release approach increases DOX accumulation in the tumor and bypasses the dependence on the EPR effect [16]. A histological analysis conducted by Manzoor et al. demonstrated that administration of LTSL+ HT doubled DOX penetration compared to Doxil + HT [16]. Moreover, Li et al. introduced a novel two-steps hyperthermia to maximize the benefits of the TSLs by firstly enhancing the tumor vasculature permeability and, as a result, the accumulation of the TSL in the tumor and secondly activating DOX release from the extravasated TSL to achieve higher interstitial levels. However, the two-step HT combined with the extravasation-based TSL was less effective on tumor growth inhibition in mice bearing BLM melanoma as compared to a combination of a one-step HT and TSL that provided fast intravascular release [17]. This conclusion was confirmed recently by Al-jamal et al., who investigated the additive effect of 2-HT on the efficacy of LTSL, TTSL, and NTSL in the human breast MDA-MB-435 xenograft model. After a single HT, LTSL was the most effective in suppressing tumor growth, with the longest median survival. Interestingly, applying the second HT 24 h after the first one did not improve either DOX tumor levels or the therapeutic effect of any of the administrated liposomal formulations as compared to the single HT approach. The lack of improvement in tumoral DOX and tumor growth inhibition was attributed to the enhanced blood perfusion in the heated tumor after the second HT. This enhanced perfusion can lead to DOX washing out from the tumor [18].
The main drawback of the LTSL is the possible dissociation of the lysolipids from the liposome membrane due to their desorption into biological components such as serum proteins. Lysolipid dissociation can lead to undesirable leakage at body temperature, followed by systematic toxicity and alteration in the thermosensitivity. Banno et al. demonstrated that LTSL lost ≈70% of lysolipids within one hour after injection into the circulation at 37 °C [19]. Moreover, LTSL released ≈ 35% of the encapsulated cargo within 1 h of incubation in fetal bovine serum (FBS) at 37 °C [20]. Needham attributed this DOX leakage from LTSL to H+ ions leakage through the membrane grain boundary defects. H+ ions leakage disrupts the protonated–unprotonated DOX balance inside the liposome and results in leakage of unprotonated soluble DOX [21].
The inclusion of 5–10 mol% cholesterol into the LTSL membrane has been proposed as a possible solution to stabilize the LTSL and reduce premature leakage. Sadeghi et al. exhibited that LTSL containing 5 and 10 mol% cholesterol was more stable than conventional LTSL, with approximately 13% leakage after incubation in FBS at 37 °C. Importantly, the incorporation of low amounts of cholesterol kept the fast-release kinetics feature of LTSL with complete DOX release within 2 min incubation in FBS at 42 °C [20]. Thus, the cholesterol-containing LTSL can provide some advantages over the conventional LTSL; however, more in vivo studies are required to investigate the effect of cholesterol inclusion on circulation kinetics and tumor accumulation.
Due to the premature leakage and short circulation time, the timing of LTSL administration (i.e., before or during HT) is crucial to achieving the possible clinical benefits of LTSL. Most of the previous preclinical studies applied HT immediately after [18][22] or shortly before LTSL administration [23][24]. Ponce et al. demonstrated that tumoral DOX was double when LTSL was injected during HT versus 15 min before HT [23]. This improvement in tumoral DOX was associated with better anti-tumor activity in rats bearing fibrosarcomas [23]. In addition, LTLD infusion during HT achieved higher DOX accumulation in the bladder wall of pigs bearing bladder cancer compared to DOX + HT [25].
Thermodox (LTLD) is an LTSL that was developed by Celsion corporation using the same formulation of Needham [14] with slight modification (DPPC:MSPC:DSPE-PEG-2000, molar ratio 86:10:4). LTLD is the first TSL to enter human clinical trials where it was used in combination with Radiofrequency ablation (RFA), a monotherapy for the treatment of small tumors (˂3 cm) in Hepatocellular carcinoma (HCC). The phase I clinical trial using this combination was conducted on 24 patients with HCC. This study exhibited a dose-response relationship of LTLD regarding the time until the failure of the treatment. Interestingly, most of the patients had tumors ˃ 3 cm. Following the promising results of the phase I study, FDA permitted Celsion to progress directly to the phase III study called HEAT [26].
The aim of the HEAT study (NCT00617981) was to evaluate the additive effect of a single dose (50 mg/m2) of LTLD administrated 15 min prior to RFA as compared to RFA alone in HCC patients with tumors 3–7 cm. Unfortunately, the study failed to reach its primary endpoint (i.e., PFS) as both PFS and OS rates were similar in both groups [27]. However, a subgroup analysis exhibited a significant enhancement in OS in patients who received LTLD + RFA ≥ 45 min in comparison to RFA alone. In contrast, OS was similar in both groups when RFA was applied ˂45 min [28]. Based on that, a second phase III study, the OPTIMA trial, was launched, where HCC patients were treated for a minimum of 45 min with RFA, and OS was chosen as the primary endpoint (NCT02112656). In 2021, Celsion corporation declared the cessation of the study because futility criteria were met at a planned interim analysis [29].
The similarities in the design of phase III trials might be responsible for the insufficient efficacy of LTLD. These similarities include using the same cancer type (i.e., HCC), the single administration of LTLD, and the application of RFA to trigger DOX release. Hence, the necessity for some modifications in future trials with LTLD is justified. These modifications can be (i) the utilization of a cancer type that is more sensitive to DOX (i.e., breast cancer), (ii) testing the multiple-dosage regimen of LTLD, (iii) the selection of other HT approaches to activate LTLD [30], such as magnetic resonance-guided high intensity focused ultrasound (MR-HIFU), an HT modality that can be used to perform tissues ablation (˃60 °C) [31] or mild HT (≈42 °C) [32], and modulated electro-hyperthermia (mEHT) that heats the tumor selectively (42 °C) [33][34]. Most of these aspects have been considered in the ongoing clinical trials with LTLD in different solid tumors (NCT04791228, NCT02536183, NCT03749850) (Table 1).
Table 1. Summary of the clinical trials on lyso-thermosensitive liposomal doxorubicin (LTLD).
| Trial ID/Name | Status | Phase | Disease | Intervention |
|---|---|---|---|---|
| NCT03749850/i-GO | recruiting | I | breast cancer | LTLD + Cyclophosphamide + MR-HIFU |
| NCT02536183 | recruiting | I | relapsed/refractory solid tumors | LTLD + MR-HIFU |
| NCT02112656/OPTIMA | completed | III | HCC | LTLD + RFA vs. RFA ( ≥ 45 min) |
| NCT00617981/HEAT | completed | III | HCC | LTLD + RFA vs. RFA (12–60 min based on tumor size) |
| NCT02181075/TARDOX | completed | I | Liver tumors | LTLD + Focused Ultrasound |
| NCT00826085/DIGNITY | completed | I/II | breast cancer | LTLD + Microwave HT (60 min) |
| NCT00441376 | completed | I | HCC | LTLD + RFA |
The ongoing i-Go trial (NCT03749850) is the first study to combine LTLD with MR-HIFU in breast cancer and the second study in breast cancer after the DIGNITY study (NCT00826085), which combined LTLD with microwave HT. The aim of the i-Go study is to replace DOX in the AC chemotherapy regimen (i.e., DOX+ cyclophosphamide) with a combination of LTLD and MR-HIFU (60 min, 40–42 °C). Thus, 12 patients with de novo stage IV her2-negative breast cancer will receive six cycles of LTLD combined with MR-HIFU and followed by cyclophosphamide. While the primary endpoints are the tolerability and feasibility in terms of the number of cycles and HT duration that can be completed, treatment efficacy represented by the radiological response is the secondary endpoint.
LTLD has the same toxicity profile as DOX in terms of hematological and gastrointestinal adverse effects. Alopecia, leukopenia, and neutropenia (grades 3 and 4) are the most frequently observed [28][35]. No dose-limiting cardiotoxicity was reported after LTLD treatment except few cases of asymptomatic declines in left ventricular ejection fraction (LVEF) after six cycles of LTLD [35]. However, most of the previous preclinical and clinical studies have administrated LTLD in a single dose. Thus, more evaluation of the cardiac function after repeated dosing of LTLD is required.
Similar to Doxil and Myocet, LTLD-induced hypersensitivity reaction was observed in dogs and pigs. Therefore, a prophylactic regimen consisting of corticosteroids (i.e., Dexamethasone) and antihistamines (H1 and H2) was applied before LTLD infusion [24][25][36]. This premedication, accompanied by LTLD slow infusion over 30 min, successfully eliminated the hypersensitivity in these animals and thus applied in the clinical trials [28][35]. On the other hand, the hypersensitivity reaction was avoided in many mice studies by using nude mice [14][22][37][38].
3. Polymer-Modified Thermosensitive Liposome (pTSL)
Another strategy to achieve thermosensitivity in the liposome membrane is the inclusion of thermosensitive polymers. Such polymers are characterized by their lower critical solution temperature (LCST), at which a coil−globule transition and phase separation occurs [39]. At temperatures below the LCST, the polymers are in a water-soluble coil state, preserving the liposome membrane stability and preventing drug release. As the temperature exceeds the LCST, the polymers start to lose their hydrogen bonds with water molecules and precipitate into a dehydrated globular form, resulting in the disruption of the liposome membrane and the release of its cargo (Figure 3) [40]. The desired LCST of the polymer (e.g., 38–42 °C) can be obtained by copolymerization with hydrophilic or hydrophobic co-monomers that can elevate or reduce the LCST, respectively [39][41].
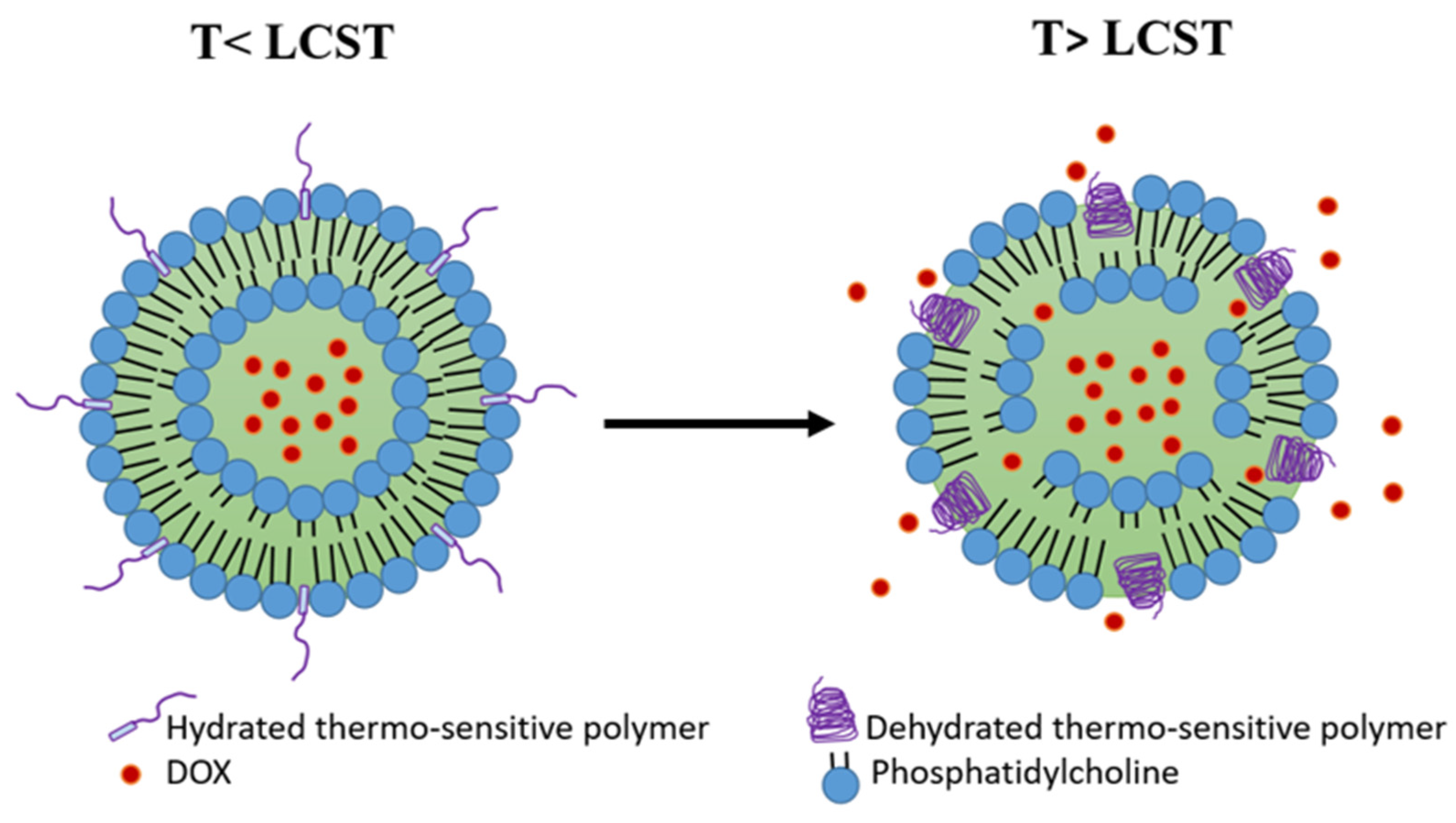
Figure 3. Polymer-modified thermosensitive liposome (pTSL). At temperatures below the lower critical solution temperature (LCST), the polymers are in a hydrated coil form and the membrane is stable. At temperatures higher than LCST, polymers become dehydrated and globular, destabilizing the TSL membrane and releasing DOX. (based on Mo et al. [42]).
While TTSLs release their cargo slowly and insufficiently and LTLD shows premature leakage at physiological temperature, the polymer-modified thermosensitive liposomes (pTSLs) could find the balance between TTSL and LTLD and overcome their limitations. Particularly, pTSLs display a more preferred balance between stability and DOX release profile. It has been found that at physiological temperature, pTSL was more stable than LTLD, releasing ˂7% of DOX within 90 min in serum [43]. Moreover, pTSL exhibited a rapid and higher release rate at 42 °C compared to TTSL (≈70% within 3 min) [44].
Different pTSL formulations have been investigated to encapsulate DOX. Poly (N-IsoPropyl-acryl-AMide) (pNIPAM) is the most used thermosensitive polymer for this purpose. PNIPAM is usually copolymerized with co-monomers to increase its LCST [45]. Terence et al. synthesized a DOX-pTSL by attachment of a copolymer composed of pNIPAM and pH-responsive PropylAcrylic Acid (PAA) to TTSL. The TTSL was composed of DPPC:HSPC:CHOL:DSPE-PEG-2000 at molar ratios: 100:50:30:6, respectively. The prepared pTSL exhibited a dramatic reduction in the thermal dose following 5 min incubation in HEPES buffer at 43 °C compared to TTSL. Additionally, the thermal dose was further decreased under slightly acidic conditions. This finding can be exploited clinically due to the acidic environment in the tumor [43]. Moreover, pTSL combined with FUS (43 °C, 5 min) was more effective on tumor growth inhibition in rats bearing tumors with greater penetration to the tumor center compared to free DOX and TTSL [44].
Mo et al. copolymerized pNIPAM with N-(2-HydroxyPropyl) MethacrylAmide (HPMA). The strong hydrophilicity of HPMA can increase the LCST of pNIPAM. Thus, the poly (NIPAM-r-HPMA) was incorporated into TTSL to prepare DOX-pTSL, which exhibited phase transition at 42 °C. The resulting pTSL had high stability at 37 °C while releasing around 70% DOX within 1 min at 42 °C. Moreover, the pTSL enhanced the cellular uptake in vitro and the tumor penetration in vivo as compared to TTSL. Interestingly, applying 5 min HT 24 h post-injection of pTSL was enough to exhibit superior anti-tumor activity in 4T1-bearing mice as compared to TTSL+HT with no cardiotoxicity. The shortly applied HT is highly important in the clinic as it results in less damage to normal tissues [42].
Another polymer that has been used for the modification of TSL is poly(2-(2-EthOxy) EthOxyethyl Vinyl Ether) (EOEOVE), which displays an LCST of around 40 °C. Poly (EOEOVE) has a greater ability to sensitize the liposomes to temperature than (pNIPAM) [46]. Kono et al. copolymerized poly(EOEOVE) with OctaDecyl Vinyl Ether (ODVE) that acts as a moiety anchor for stronger attachment of poly(EOEOVE) onto the liposome membrane. The resulting poly (EOEOVE)-OD4 copolymer was used to prepare pTSL for DOX. The poly(EOEOVE)-OD4-TSL was stable at 37 °C with less than 5% leakage. However, this pTSL exhibited significant DOX release at temperatures above 40 °C (50% at 43 °C, 85% within 1 min at 45 °C). In addition, the poly(EOEOVE)-OD4-TSL combined with HT (10 min, 45 °C) had a greater effect on tumor growth suppression in mice bearing C26 colon carcinoma than non-modified TSL [47].
Elk et al. encapsulated DOX into liposome grafted with copolymer composed of cholesterol and poly(N-(2-Hydroxypropyl) MethacrylAmide mono/dilactate) (chol-pHPMAlac). Chol-pHPMAlac exhibits tuneable critical solution temperature behavior. The ratio between pHPMmonolactate and pHPMdilactate was 43:57 [48]. The higher content of pHPM dilactate can reduce the LCST of the polymer due to the higher hydrophobicity of pHPM dilactate over pHPM monolactate. Moreover, cholesterol serves as an anchor to fix the polymer onto the liposome membrane [49]. The in vitro study revealed that (chol-pHPMAlac) TSL did not induce platelet activation in whole blood, which makes this TSL safe for intravenous administration. However, the complete release of DOX was obtained at 47 °C or higher, making (chol-pHPMAlac) TSL unsuitable for application with mild HT modalities [48].
The use of a variety of temperature-responsive copolymers in previous studies has resulted in pTSLs with different DOX release behavior and variance in the HT time required for the complete release. Therefore, these factors need to be optimized before reaching the clinical investigation.
4. Phosphatidylglycerol-Based Thermosensitive Liposome (DPPG-TSL)
DPPG-based TSLs are a new generation of long-circulating TSLs that combines both stability and rapid release. DPPG-TSL was first introduced by Lindner et al., using 30% of 1,2-dipalmitoyl-sn-glycero-3-phosphodiglycerol (DPPG2) in combination with DPPC and DSPC, omitting PEG and lysolipids [50]. Despite the absence of PEG, the long circulation of DPPG-based TSL was achieved by the inclusion of the synthetic lipid DPPG containing free hydroxyl groups, which presents strong hydrophilicity and inhibits interactions with serum proteins [50]. In contrast to lysolipids, DPPG does not affect the stability of TSL at physiological temperatures. It was shown that DOX leakage from DPPG-TSL did not exceed 5% after 1 h of incubation in serum at 37 °C compared to approximately 30% leakage from LTLD. Furthermore, DPPG-TSL exhibited a DOX release profile similar to LTLD at 42 °C in vitro, indicating that the fast and complete release of DOX can be achieved without the inclusion of lysolipids [51][52].
Due to the favorable pharmacokinetics, DPPG-TSL showed promising results in preclinical studies in different animal models. Hossann et al. revealed that DOX accumulation was higher in tumors but lower in the heart of sarcoma-bearing rats after treatment with DPPG-TSLDOX+ HT compared to LTLD+ HT. Consequently, rats treated with DPPG-TSL had the highest survival rate [52]. Similarly, DPPG-TSLDOX was evaluated in cats suffering from feline sarcoma [52][53]. Importantly, DPPG-TSLDOX administration was safe without serious toxicity [53]. In addition, a combination of DPPG-TSLDOX and HT exhibited a better response than conventional DOX, as demonstrated by the metabolic response determined with 18F-FDG-PET/MRI and histopathological analysis after tumor resection [52].
DPPG-TSLDOX was investigated as a treatment for muscle-invasive bladder cancer in rats and pigs [54][55]. Valenberg et al. evaluated DOX accumulation in the bladder wall of pigs treated with 20 mg/kg and 60 mg/kg of DPPG-TSLDOX and free DOX. The highest DOX accumulation in the bladder wall was observed after a combination of DPPG-TSLDOX and HT. On the other hand, lower DOX accumulation was detected in the heart and kidney of (DPPG-TSLDOX + HT)-treated pigs [54]. Consistent with this finding, rats treated with DPPG-TSLDOX + HT demonstrated higher complete tumor response than free DOX-treated rats (70% vs. 18%) [55].
Recently, a clinically feasible protocol has been developed to trigger DOX release from DPPG-TSL using MR-HIFU modality in healthy landrace pigs. Specifically, 50 mg DOX per m2 of DPPG2-TSL-DOX was infused for 30 min, followed by two local HT treatments initiated at 10 min and 60 min after the beginning of DPPG2-TSL-DOX infusion. The temperature of the heated muscle (i.e., thigh muscle) was kept at 42 °C for 30 min. Importantly, DOX quantification revealed much higher DOX in the heated muscle compared to unheated muscles. Moreover, low DOX concentrations were detected in the heart of the animals; however, cardiotoxicity was not evaluated in this study [56]. This study represents a step toward the clinical translation of DPPG-TSL-DOX. According to Regenold et al., DPPG-TSL-DOX is currently in clinical development by Thermosome GmbH in Germany [29].
5. Multifunctional-Thermosensitive Liposome
Applying one strategy to deliver DOX to the target tumor might not be enough. The difficulties with demonstrating significant clinical benefits from the LTLD in phase III clinical trials support this presumption. DOX can be delivered more efficiently to tumors by loading it in liposomes that combine the thermosensitivity with other strategies. Similar to the functionalized PLDs, TSLs can be surface modified with some molecules, such as targeting ligands. For instance, an LTSL modified with iRGD tumor-homing peptide was prepared and combined with HIFU for 10 min at 42 °C. Interestingly, iRGD-LTSL-DOX+ HIFU was more effective on tumor growth suppression in mice bearing the 4T1 model than LTSL-DOX+ HIFU. This finding was attributed to the ability of iRGD to selectively bind the αν integrins on the tumor angiogenic endothelial cells [57]. Similar results were obtained after DOX encapsulation in LTSL modified with a tumor-homing peptide, namely Cys-Arg-Glu-Lys-Ala (CREKA), a peptide that targets the clotted plasma proteins on the tumor vessel walls and stroma [38].
Lin et al. conjugated DOX with a CPE and encapsulated the conjugate along with magnetic fluid Fe3O4 in a TSL. The TSL was composed of DPPC: MSPC: DSPE-PEG2000 in a molar ratio of 87:3:10. The aim of the Fe3O4 was to generate heating (42 °C) under the application of an AC magnetic field. The magnetic TSL (DOX-CPE TSML) was able to release more than 80% of the cargo within 30 min at 42 °C with 7% leakage at 37 °C. Under the magnetic field, DOX-CPE TSML exhibited superior tumor growth inhibition to a non-magnetic DOX-CPE TSL activated by HT in MCF-7 tumor-bearing mice. Significantly, both formulations did not exhibit systematic toxicity demonstrated by the body weight [58]. In another study, Dorjsuren et al. synthesized a TSL consisting of DPPC, DSPE-PEG2000, and MPPC. The prepared TSL was loaded with DOX and Fe3O4 magnetic nanoparticles (MNP). After that, the TSL was conjugated with Cetuximab to target EGFR-expressing breast cancer cells. The resulting TSL revealed an increased DOX release under near-infrared (NIR) laser irradiation and acidic pH. Moreover, when exposed to NIR irradiation in vivo, the TSL elevated the temperature of the tumor surface to 48.7 °C due to the presence of the MNP. However, the in vivo anti-tumor activity of the prepared TSL has not been investigated [59].
A multifunctional pTSL was developed by Kono et al. by the incorporation of trastuzumab (HER) to target the HER2-positive cells and a fluorescence dye for NIR imaging to follow the biodistribution of the liposome. The HER-TSL accumulated more in the tumor in SK-OV3-bearing mice than the non-modified TSL. When combined with HT (44 °C) for 10 min, HER-TSL exhibited better anti-tumor activity than the non-modified TSL [60].
Alawak et al. designed a novel multifunctional magnetic DOX-TSL conjugated with MAB 1031 antibody to target the transmembrane metalloprotease-disintegrin (ADAM8) protein which exhibits high expression in triple-negative breast cancer (TNBC) cells and contributes negatively in tumor progression. In addition, gadolinium (Gd), a paramagnetic agent, was incorporated into the liposome to increase the contrast and cell detection by ultra-high field MR imaging (UHF-MRI). The incorporation of MAB antibody enhanced the in vitro cell-binding efficiency of MDA-MB-231 cells compared to non-modified TSL. In addition, MDA-MB-231 cells treated with MAB-TSL revealed higher viability reduction after 1 h of exposure to UHF-MRI. Moreover, the liposome was hemocompatible and safe for intravenous administration [61].
References
- Kim, K.Y. Nanotechnology platforms and physiological challenges for cancer therapeutics. Nanomedicine 2007, 3, 103–110.
- Leekumjorn, S. and A.K. Sum, Molecular studies of the gel to liquid-crystalline phase transition for fully hydrated DPPC and DPPE bilayers. Biochim. Biophys. Acta Biomembr. 2007, 1768, 354–365.
- Papahadjopoulos, D.; Jacobson, K.; Nir, S.; Isac, T. Phase transitions in phospholipid vesicles Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta Biomembr. 1973, 311, 330–348.
- Kuć, M.; Cieślik-Boczula, K.; Rospenk, M. Anesthetic-dependent changes in the chain-melting phase transition of DPPG liposomes studied using near-infrared spectroscopy supported by PCA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 37–43.
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125.
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98.
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia. Science 1978, 202, 1290–1293.
- Gaber, M.H.; Hong, K.; Huang, S.K.; Papahadjopoulos, D. Thermosensitive Sterically Stabilized Liposomes: Formulation and in Vitro Studies on Mechanism of Doxorubicin Release by Bovine Serum and Human Plasma. Pharm. Res. 1995, 12, 1407–1416.
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140.
- Landon, C.D.; Park, J.Y.; Needham, D.; Dewhirst, M.W. Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. Open Nanomed. J. 2011, 3, 38–64.
- Mills, J.K.; Needham, D. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim. Biophys. Acta Biomembr. 2005, 1716, 77–96.
- De Matos, M.B.C.; Beztsinna, N.; Heyder, C.; Fens, M.H.A.M.; Mastrobattista, E.; Schiffelers, R.M.; Leneweit, G.; Kok, R.J. Thermosensitive liposomes for triggered release of cytotoxic proteins. Eur. J. Pharm. Biopharm. 2018, 132, 211–221.
- Anyarambhatla, G.R.; Needham, D. Enhancement of the Phase Transition Permeability of DPPC Liposomes by Incorporation of MPPC: A New Temperature-Sensitive Liposome for use with Mild Hyperthermia. J. Liposome Res. 1999, 9, 491–506.
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A New Temperature-sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model1. Cancer Res. 2000, 60, 1197–1201.
- Kong, G.; Anyarambhatla, G.; Petros, W.P.; Braun, R.D.; Colvin, O.M.; Needham, D.; Dewhirst, M.W. Efficacy of Liposomes and Hyperthermia in a Human Tumor Xenograft Model: Importance of Triggered Drug Release1. Cancer Res. 2000, 60, 6950–6957.
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575.
- Li, L.; ten Hagen, T.L.; Haeri, A.; Soullié, T.; Scholten, C.; Seynhaeve, A.L.; Eggermont, A.M.; Koning, G.A. A novel two-step mild hyperthermia for advanced liposomal chemotherapy. J. Control. Release 2014, 174, 202–208.
- Al-Jamal, W.T.; Kostarelos, K. Mild hyperthermia accelerates doxorubicin clearance from tumour-extravasated temperature-sensitive liposomes. Nanotheranostics 2022, 6, 230–242.
- Banno, B.; Ickenstein, L.M.; Chiu, G.N.; Bally, M.B.; Thewalt, J.; Brief, E.; Wasan, E.K. The functional roles of poly(ethylene glycol)-lipid and lysolipid in the drug retention and release from lysolipid-containing thermosensitive liposomes in vitro and in vivo. J. Pharm. Sci. 2010, 99, 2295–2308.
- Sadeghi, N.; Deckers, R.; Ozbakir, B.; Akthar, S.; Kok, R.J.; Lammers, T.; Storm, G. Influence of cholesterol inclusion on the doxorubicin release characteristics of lysolipid-based thermosensitive liposomes. Int. J. Pharm. 2018, 548, 778–782.
- Needham, D.; Park, J.Y.; Wright, A.M.; Tong, J. Materials characterization of the low temperature sensitive liposome (LTSL): Effects of the lipid composition (lysolipid and DSPE-PEG2000) on the thermal transition and release of doxorubicin. Faraday Discuss. 2013, 161, 515–534; discussion 563–589.
- Besse, H.C.; Barten-van Rijbroek, A.D.; van der Wurff-Jacobs, K.M.G.; Bos, C.; Moonen, C.T.W.; Deckers, R. Tumor Drug Distribution after Local Drug Delivery by Hyperthermia, In Vivo. Cancers 2019, 11, 1512.
- Ponce, A.M.; Viglianti, B.L.; Daohai, Y.u.; Yarmolenko, P.S.; Michelich, C.R.; Woo, J.; Bally, M.B.; Dewhirst, M.W. Magnetic Resonance Imaging of Temperature-Sensitive Liposome Release: Drug Dose Painting and Antitumor Effects. J. Natl. Cancer Inst. 2007, 99, 53–63.
- Hauck, M.L.; LaRue, S.M.; Petros, W.P.; Poulson, J.M.; Yu, D.; Spasojevic, I.; Pruitt, A.F.; Klein, A.; Case, B.; Thrall, D.E.; et al. Phase I trial of doxorubicin-containing low temperature sensitive liposomes in spontaneous canine tumors. Clin. Cancer Res. 2006, 12, 4004–4010.
- Mikhail, A.S.; Negussie, A.H.; Pritchard, W.F.; Haemmerich, D.; Woods, D.; Bakhutashvili, I.; Esparza-Trujillo, J.; Brancato, S.J.; Karanian, J.; Agarwal, P.K.; et al. Lyso-thermosensitive liposomal doxorubicin for treatment of bladder cancer. Int. J. Hyperth. 2017, 33, 733–740.
- Poon, R.T.P.; Borys, N. Lyso-thermosensitive liposomal doxorubicin: A novel approach to enhance efficacy of thermal ablation of liver cancer. Expert Opin. Pharmacother. 2009, 10, 333–343.
- Lencioni, R.; Cioni, D. RFA plus lyso-thermosensitive liposomal doxorubicin: In search of the optimal approach to cure intermediate-size hepatocellular carcinoma. Hepatic Oncol. 2016, 3, 193–200.
- Tak, W.Y.; Lin, S.M.; Wang, Y.; Zheng, J.; Vecchione, A.; Park, S.Y.; Chen, M.H.; Wong, S.; Xu, R.; Peng, C.Y.; et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin. Cancer Res. 2018, 24, 73–83.
- Regenold, M.; Bannigan, P.; Evans, J.C.; Waspe, A.; Temple, M.J.; Allen, C. Turning down the heat: The case for mild hyperthermia and thermosensitive liposomes. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102484.
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release 2017, 249, 63–73.
- Hsiao, Y.H.; Kuo, S.J.; Tsai, H.D.; Chou, M.C.; Yeh, G.P. Clinical Application of High-intensity Focused Ultrasound in Cancer Therapy. J. Cancer 2016, 7, 225–231.
- Zhu, L.; Partanen, A.; Talcott, M.R.; Gach, H.M.; Greco, S.C.; Henke, L.E.; Contreras, J.A.; Zoberi, I.; Hallahan, D.E.; Chen, H.; et al. Feasibility and safety assessment of magnetic resonance-guided high-intensity focused ultrasound (MRgHIFU)-mediated mild hyperthermia in pelvic targets evaluated using an in vivo porcine model. Int. J. Hyperth. 2019, 36, 1147–1159.
- Schvarcz, C.A.; Danics, L.; Krenács, T.; Viana, P.; Béres, R.; Vancsik, T.; Nagy, Á.; Gyenesei, A.; Kun, J.; Fonović, M.; et al. Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts. Cancers 2021, 13, 1744.
- Danics, L.; Schvarcz, C.A.; Viana, P.; Vancsik, T.; Krenács, T.; Benyó, Z.; Kaucsár, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581.
- Zagar, T.M.; Vujaskovic, Z.; Formenti, S.; Rugo, H.; Muggia, F.; O’Connor, B.; Myerson, R.; Stauffer, P.; Hsu, I.C.; Diederich, C.; et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int. J. Hyperth. 2014, 30, 285–294.
- Swenson, C.E.; Haemmerich, D.; Maul, D.H.; Knox, B.; Ehrhart, N.; Reed, R.A. Increased Duration of Heating Boosts Local Drug Deposition during Radiofrequency Ablation in Combination with Thermally Sensitive Liposomes (ThermoDox) in a Porcine Model. PLoS ONE 2015, 10, e0139752.
- Yarmolenko, P.S.; Zhao, Y.; Landon, C.; Spasojevic, I.; Yuan, F.; Needham, D.; Viglianti, B.L.; Dewhirst, M.W. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperth. 2010, 26, 485–498.
- Wang, C.; Wang, X.; Zhong, T.; Zhao, Y.; Zhang, W.Q.; Ren, W.; Huang, D.; Zhang, S.; Guo, Y.; Yao, X.; et al. The antitumor activity of tumor-homing peptide-modified thermosensitive liposomes containing doxorubicin on MCF-7/ADR: In vitro and in vivo. Int. J. Nanomed. 2015, 10, 2229–2248.
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) Copolymers That Respond Sharply to Temperature and pH. Biomacromolecules 2006, 7, 1381–1385.
- Kono, K.; Nakai, R.; Morimoto, K.; Takagishi, T. Thermosensitive polymer-modified liposomes that release contents around physiological temperature. Biochim. Biophys. Acta Biomembr. 1999, 1416, 239–250.
- Pennadam, S.S.; Firman, K.; Alexander, C.; Górecki, D.C. Protein-polymer nano-machines. Towards synthetic control of biological processes. J. Nanobiotechnology 2004, 2, 8.
- Mo, Y.; Du, H.; Chen, B.; Liu, D.; Yin, Q.; Yan, Y.; Wang, Z.; Wan, F.; Qi, T.; Wang, Y.; et al. Quick-Responsive Polymer-Based Thermosensitive Liposomes for Controlled Doxorubicin Release and Chemotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2316–2329.
- Ta, T.; Convertine, A.J.; Reyes, C.R.; Stayton, P.S.; Porter, T.M. Thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules 2010, 11, 1915–1920.
- Ta, T.; Bartolak-Suki, E.; Park, E.J.; Karrobi, K.; McDannold, N.J.; Porter, T.M. Localized delivery of doxorubicin in vivo from polymer-modified thermosensitive liposomes with MR-guided focused ultrasound-mediated heating. J. Control. Release 2014, 194, 71–81.
- Hayashi, H.; Kono, K.; Takagishi, T. Temperature-controlled release property of phospholipid vesicles bearing a thermo-sensitive polymer. Biochim. Biophys. Acta Biomembr. 1996, 1280, 127–134.
- Kono, K.; Murakami, T.; Yoshida, T.; Haba, Y.; Kanaoka, S.; Takagishi, T.; Aoshima, S. Temperature sensitization of liposomes by use of thermosensitive block copolymers synthesized by living cationic polymerization: Effect of copolymer chain length. Bioconjugate Chem. 2005, 16, 1367–1374.
- Kono, K.; Ozawa, T.; Yoshida, T.; Ozaki, F.; Ishizaka, Y.; Maruyama, K.; Kojima, C.; Harada, A.; Aoshima, S. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials 2010, 31, 7096–7105.
- van Elk, M.; van den Dikkenberg, J.B.; Storm, G.; Hennink, W.E.; Vermonden, T.; Heger, M. Preclinical evaluation of thermosensitive poly(N-(2-hydroxypropyl) methacrylamide mono/dilactate)-grafted liposomes for cancer thermochemotherapy. Int. J. Pharm. 2018, 550, 190–199.
- Van Elk, M.; Deckers, R.; Oerlemans, C.; Shi, Y.; Storm, G.; Vermonden, T.; Hennink, W.E. Triggered release of doxorubicin from temperature-sensitive poly(N-(2-hydroxypropyl)-methacrylamide mono/dilactate) grafted liposomes. Biomacromolecules 2014, 15, 1002–1009.
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel Temperature-Sensitive Liposomes with Prolonged Circulation Time. Clin. Cancer Res. 2004, 10, 2168–2178.
- Hossann, M.; Wiggenhorn, M.; Schwerdt, A.; Wachholz, K.; Teichert, N.; Eibl, H.; Issels, R.D.; Lindner, L.H. In vitro stability and content release properties of phosphatidylglyceroglycerol containing thermosensitive liposomes. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2491–2499.
- Hossann, M.; Hirschberger, J.; Schmidt, R.; Baumgartner, C.; Zimmermann, K.; Baer, S.; Ratzlaff, C.; Peller, M.; Troedson, K.; Limmer, S.; et al. A Heat-Activated Drug-Delivery Platform Based on Phosphatidyl-(oligo)-glycerol Nanocarrier for Effective Cancer Treatment. Adv. NanoBiomed Res. 2021, 1, 2000089.
- Zimmermann, K.; Hossann, M.; Hirschberger, J.; Troedson, K.; Peller, M.; Schneider, M.; Brühschwein, A.; Meyer-Lindenberg, A.; Wess, G.; Wergin, M.; et al. A pilot trial of doxorubicin containing phosphatidyldiglycerol based thermosensitive liposomes in spontaneous feline soft tissue sarcoma. Int. J. Hyperth. 2017, 33, 178–190.
- Van Valenberg, F.J.P.; Brummelhuis, I.S.G.; Lindner, L.H.; Kuhnle, F.; Wedmann, B.; Schweizer, P.; Hossann, M.; Witjes, J.A.; Oosterwijk, E. DPPG(2)-Based Thermosensitive Liposomes with Encapsulated Doxorubicin Combined with Hyperthermia Lead to Higher Doxorubicin Concentrations in the Bladder Compared to Conventional Application in Pigs: A Rationale for the Treatment of Muscle-Invasive Bladder Cancer. Int. J. Nanomed. 2021, 16, 75–88.
- Brummelhuis, I.S.G.; Simons, M.; Lindner, L.H.; Kort, S.; de Jong, S.; Hossann, M.; Witjes, J.A.; Oosterwijk, E. DPPG(2)-based thermosensitive liposomes as drug delivery system for effective muscle-invasive bladder cancer treatment in vivo. Int. J. Hyperth. 2021, 38, 1415–1424.
- Sebeke, L.C.; Castillo Gómez, J.D.; Heijman, E.; Rademann, P.; Simon, A.C.; Ekdawi, S.; Vlachakis, S.; Toker, D.; Mink, B.L.; Schubert-Quecke, C.; et al. Hyperthermia-induced doxorubicin delivery from thermosensitive liposomes via MR-HIFU in a pig model. J. Control. Release 2022, 343, 798–812.
- Deng, Z.; Xiao, Y.; Pan, M.; Li, F.; Duan, W.; Meng, L.; Liu, X.; Yan, F.; Zheng, H. Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J. Control. Release 2016, 243, 333–341.
- Lin, W.; Xie, X.; Yang, Y.; Fu, X.; Liu, H.; Yang, Y.; Deng, J. Thermosensitive magnetic liposomes with doxorubicin cell-penetrating peptides conjugate for enhanced and targeted cancer therapy. Drug Deliv. 2016, 23, 3436–3443.
- Dorjsuren, B.; Chaurasiya, B.; Ye, Z.; Liu, Y.; Li, W.; Wang, C.; Shi, D.; Evans, C.E.; Webster, T.J.; Shen, Y. Cetuximab-Coated Thermo-Sensitive Liposomes Loaded with Magnetic Nanoparticles and Doxorubicin for Targeted EGFR-Expressing Breast Cancer Combined Therapy. Int. J. Nanomed. 2020, 15, 8201–8215.
- Kono, K.; Takashima, M.; Yuba, E.; Harada, A.; Hiramatsu, Y.; Kitagawa, H.; Otani, T.; Maruyama, K.; Aoshima, S. Multifunctional liposomes having target specificity, temperature-triggered release, and near-infrared fluorescence imaging for tumor-specific chemotherapy. J. Control. Release 2015, 216, 69–77.
- Alawak, M.; Abu Dayyih, A.; Mahmoud, G.; Tariq, I.; Duse, L.; Goergen, N.; Engelhardt, K.; Reddy Pinnapireddy, S.; Jedelská, J.; Awak, M.; et al. ADAM 8 as a novel target for doxorubicin delivery to TNBC cells using magnetic thermosensitive liposomes. Eur. J. Pharm. Biopharm. 2021, 158, 390–400.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





