Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Islam El-Sharkawy | -- | 1717 | 2023-03-23 16:37:25 | | | |
| 2 | Sirius Huang | Meta information modification | 1717 | 2023-03-24 02:00:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Moniruzzaman, M.; Darwish, A.G.; Ismail, A.; El-Kereamy, A.; Tsolova, V.; El-Sharkawy, I. Genetic Engineering Strategies for Seedlessness Breeding. Encyclopedia. Available online: https://encyclopedia.pub/entry/42487 (accessed on 07 February 2026).
Moniruzzaman M, Darwish AG, Ismail A, El-Kereamy A, Tsolova V, El-Sharkawy I. Genetic Engineering Strategies for Seedlessness Breeding. Encyclopedia. Available at: https://encyclopedia.pub/entry/42487. Accessed February 07, 2026.
Moniruzzaman, Md, Ahmed G. Darwish, Ahmed Ismail, Ashraf El-Kereamy, Violeta Tsolova, Islam El-Sharkawy. "Genetic Engineering Strategies for Seedlessness Breeding" Encyclopedia, https://encyclopedia.pub/entry/42487 (accessed February 07, 2026).
Moniruzzaman, M., Darwish, A.G., Ismail, A., El-Kereamy, A., Tsolova, V., & El-Sharkawy, I. (2023, March 23). Genetic Engineering Strategies for Seedlessness Breeding. In Encyclopedia. https://encyclopedia.pub/entry/42487
Moniruzzaman, Md, et al. "Genetic Engineering Strategies for Seedlessness Breeding." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Seedless fruit occurs naturally and can be produced using hormone application, crossbreeding, or ploidy breeding. However, the two types of breeding are time-consuming and sometimes ineffective due to interspecies hybridization barriers or the absence of appropriate parental genotypes to use in the breeding process. The genetic engineering approach provides a better prospect, which can be explored based on an understanding of the genetic causes underlying the seedlessness trait.
genome editing
molecular breeding
ovule abortion
parthenocarpy
seedlessness
stenospermocarpy
1. Introduction
Seedlessness is one of the most valuable agricultural traits in fruit crops that consumers appreciate for fresh consumption and value-added processed products [1][2]. It enriches the eating quality of the fruits due to their expanded edible pulp and the absence of hard seeds with an awful taste. Further, seedlessness could prevent browning and bitterness caused by seeds [3]. Moreover, it improves many other fruit biometric characteristics regarding acid/sugar levels, dry matter, firmness, and overall shelf-life qualities of climacteric fruit due to reduced ethylene generated by seeds [4]. Seedlessness can also mitigate fruit yield losses caused by environmental stresses affecting pollination and fertilization [5]. Finally, it occurred independently of pollination and fertilization, which increases fruit production, particularly in dioecious species, due to the uselessness of the pollen source staminate trees. Studies on fruit seedlessness suggest that the trait is coordinated by intricate systems involving hormonal, genetic, and environmental factors [6][7]. Therefore, there are many causes underlying the seedless fruit set program [8]. The most classical reasons include male sterility, degradation of mother pollen cells, embryonic abortion, and chromosomal irregularities during meiosis leading to triploidy.
In typical seeded fruit, the ovary proliferates after fertilization through a coordinated program of molecular, biochemical, and structural changes that stimulate fruit size enlargement due to the interplay of cell division, differentiation, and expansion of sporophytic and gametophytic tissues [9]. Research on mechanisms underlying fruit seedlessness has highlighted the potential involvement of two distinct strategies, parthenocarpy and stenospermocarpy [10]. In parthenocarpy, true seedlessness occurs, and the ovary develops into fruit independent of pollination and fertilization [11]. However, two different procedures were identified for the parthenocarpic fruit set program. The obligatory-parthenocarpy, where a plant always produces seedless fruits (i.e., pineapple), and the facultative-parthenocarpy, where seedless fruits only develop if pollination is prevented (i.e., watermelon) [12]. Parthenocarpic fruit development is triggered by the deregulation of the hormone balance in ovary tissues, mainly auxin, gibberellins (GAs), and/or cytokinins (CKs). Applying these hormones to unpollinated ovaries at anthesis can stimulate pollination-independent ovary growth and produce parthenocarpic seedless fruit, strongly supporting their individual and overlapped roles during early fruit development [13]. An earlier study reported that the growth of tomato fruit is coordinated by a delicate balance between auxin and GA, whereby auxin is needed to mediate cell division and GA is required to organize cell expansion [7]. The parthenocarpy trait stability in fruit crops primarily occurs through elective pressure for seedlessness during domestication and breeding [8]. However, parthenocarpic genotypes were also identified in wild species and non-fruit crops [14].
In stenospermocarpy, pollination and fertilization typically occur; however, the seed growth is prematurely aborted due to the cessation of seed coat and endosperm development, resulting in expanded fruit size with seminal rudiments or seed traces [15]. The fact that different degrees of seedlessness were observed in progeny grapevines resulting from crossing seeded and stenospermocarpy seedless parents adds more complexity to the integrative regulatory network and signaling pathways underlying the stenospermocarpy seedless fruit set machinery [16]. Despite recent advances in grape biology, the molecular basis that triggers stenospermocarpy fruit development is largely unknown [10]. The efforts to unravel the molecular basis for stenospermocarpy in grapes were able to identify and functionally characterize several genes that can be potentially involved in the procedure [17][18][19]. Although the results did not show an ultimate gene network, they at least shed light on potential molecular mechanisms that synchronize stenospermocarpy machinery.
Fruit size and weight are positive commercial attributes, through which the number of developed seeds per fruit is positively correlated with the two characters [20]. Parthenocarpy fruit set results in considerably smaller fruit size than seeded fruit due to the absence of seed initiation, leading to reduced hormone levels necessary to sustain fruit growth [7]. However, stenospermocarpy does not compromise or, in the worst-case scenario, slightly reduce the fruit size because the ovary-growth event occurs after pollination and fertilization, making stenospermocarpy seedlessness a more attractive trait for breeding (Figure 1). CKs are essential to determining ovary size before fertilization. However, the slightly compromised size of the stenospermocarpy fruit is due to the availability of CKs post-fertilization, which negatively regulates cell expansion during fruit development [21].

Figure 1. Close-up cluster views of Thompson seeded mutant (DVIT 1334), Thompson seedless, and Centennial seedless grape genotypes exhibiting seeded, stenospermocarpy, and parthenocarpy fruit set programs, respectively.
Parthenocarpy seedlessness can be induced by applying hormones to unpollinated inflorescences at anthesis, via fostering self-incompatibility, or through generating triploid plants using conventional breeding practices [4][22]. Nevertheless, all the strategies are laborious, time-consuming, and sometimes impossible to use due to the absence of proper parental genetic resources. In the meantime, no treatment or application that can induce stenospermocarpy seedlessness has been identified yet. Accordingly, both seedlessness mechanisms are important, depending on growth conditions and commercial value. This has opened up the opportunity for genetic engineering approaches that have given encouraging results, both in the quality and quantity of seedless fruit production.
2. Genetic Engineering Strategies for Seedlessness Breeding
Parthenocarpy seedless fruits can be accomplished either by exogenous application of plant growth regulators, conventional breeding, interspecies hybridization, or polyploidy breeding. However, none of these strategies is feasible to induce stenospermocarpy seedless. For instance, muscadine seedless grape breeding is not viable due to the absence of the trait within the species. The only available seedless muscadine genotype, “Fry Seedless”, is parthenocarpic with limited commercial value and cannot be used as a crossing parent in the breeding program due to male sterility (Figure 2) [23]. Muscadine and bunch grape are classified under the Euvitis genera; however, the pronounced differences in their phenomic, metabolomic, and genomic characteristics represented by the dissimilarities in stress responses, horticultural and reproductive growth characteristics, and genome structure enabled us to classify them into two different genera, Muscadinia and Vitis [24]. Accordingly, introducing the stenospermocarpy seedless trait to muscadine grapes via generating Vitis x Muscadinia interspecific hybrids is challenging due to the differences in chromosome number and genetic incompatibility [25]. Alternatively, developing triploid seedless muscadine grapes might be an option that avoids the genetic barrier between species. However, the attempt to establish a triploid seedless muscadine grape did not produce satisfactory genotypes that can be promoted into new cultivars due to limited reproductive growth qualities [26]. Hence, genetic engineering could be a promising strategy for introducing a seedless trait.

Figure 2. Close-up cluster view of muscadine cultivars Majesty (female flower, seeded), Floriana (perfect flower, seeded), and Fry seedless (perfect flower, parthenocarpy seedless).
Advanced genome-editing tools, such as CRISPR-TSKO, precise base editing, or prime editing approaches, were efficiently applied in different crops for precise genome editing that prevented or minimized the pleiotropic effects [27][28]. However, this approach is considered GMO and may affect consumer acceptance. Interestingly, the Cas9-free lines can be selected by crossing out the transgenes from the segregating populations or through RNP-mediated protoplast transformation and regeneration [29][30]. Moreover, the CRISPR reagent (RNP) can be delivered to the germline cells using viral vectors like the Tobacco rattle virus (TRV). Thus, an inherited mutation could be achieved using the seeds from the germline-edited plant [31].
The model presented in Figure 3 illustrates the suggested strategy for introducing a seedless trait using genome editing technology.
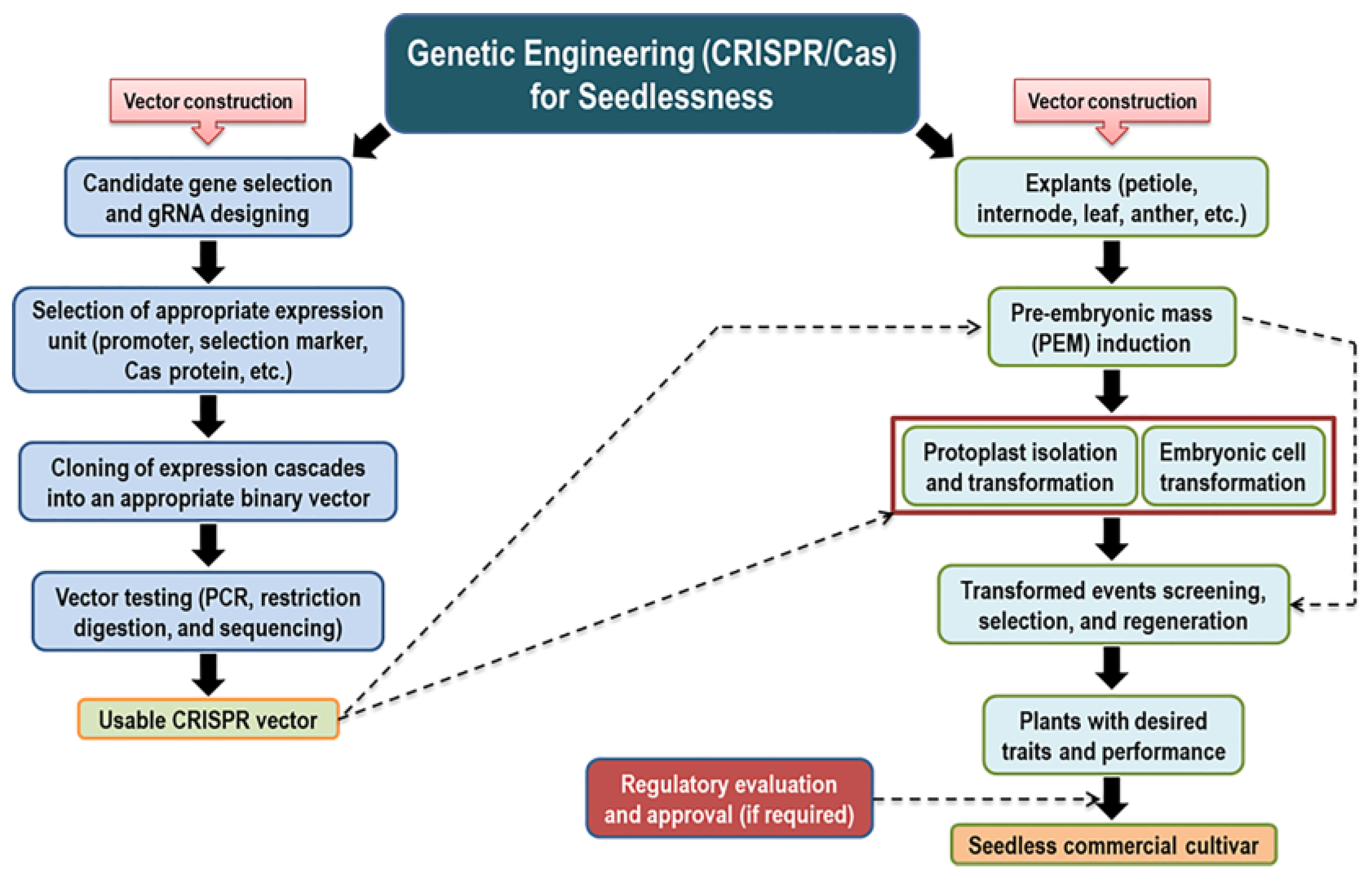
Figure 3. Visual scheme of gaining the seedlessness trait using genetic engineering (CRISPR/Cas) strategy.
(I) Establishing a plant regeneration system based on embryonic suspension cell culture. Genetic transformation using embryogenic cell suspension cultures is a good opportunity because of their higher organogenetic potential [32][33][34][35]. Regeneration of putatively transformed cells and subsequent grafting of transgenic micro-shoots on rootstocks may shorten the juvenile period for flowering and fruiting [36].
(II) Selecting appropriate candidate gene(s) and regulatory elements for targeted genome editing. The AGL11 is considered the only identified upstream regulatory gene that controls ovule/seed development, and its Arabidopsis mutant phenotype (stk) displayed compromised seed characteristics. [37]. In the case of V. vinifera, stenospermocarpy seedlessness is associated with an SNP mutation in VviAGL11 (R197L) [16]. The availability of whole genome sequence (i.e., muscadine whole genome sequence) [38] facilitates target gene selection. Researchers highlighted many other genes associated with seed development/abortion. These genes could be target candidates for genome editing. Organ-specific promoter-driven Cas protein expression has been reported on the CRISPR platform [39][40][41][42]. Using a seed-specific promoter could achieve the goal more effectively and efficiently because the desired expression will occur only in seeds. Seed development-specific promoters have been characterized using various genes and different plant species [43][44][45][46].
(III) Cloning and assembly of a binary vector. Guide RNA (gRNA) design for the particular gene and cloning the gRNAs into appropriate vector backbones are the primary tasks for genome editing vector construction. Different online tools for gRNA design (i.e., CRISPOR [47], CRISPR-P [48], CCTop [49], CHOPCHOP [50], and GuideMaker [51]) with customized features are openly accessible. High-efficiency cloning technology (MoClo) [52] and readymade cloning materials of different expression modules are readily available from addgene (https://www.addgene.org/) and other sources. Adopting the MoClo cloning technology would accelerate vector construction efficiency. Recently, it has been shown that gRNA possessing the same restriction site as the type II restriction enzyme used for the GG reaction does not affect MoClo cloning and subsequent genome editing efficiency, which expanded the gRNA selection options [53].
(IV) Transformation of embryogenic cells or protoplasts and regeneration of putatively transformed cells into a complete plant. Using the appropriate transformation system increases the chances of getting transformed events. Among the different types of transformation processes, Agrobacterium-mediated or direct protoplast transformation could be adopted. Interestingly, RNP (ribonucleoprotein) mediated genome editing of protoplasts could avoid current GMO regulations, as the USDA does not consider the plant a GMO if the engineering involves a plant self-repair mechanism.
(V) Screening for potential transgenic events and securing approval from regulatory agencies. The procedure is associated with both molecular and phenotypic evaluations. Molecular screening means genomic and proteomic studies of the desired genome engineering plant(s) to confirm that desired change(s) in the genome, and phenotypic screening means the study of visual changes (either positive or negative) in the plants. If satisfactory performance is achieved, the new plant genotype needs approval from regulatory authorities before releasing for commercialization.
References
- Wijesinghe, S.A.E.C.; Evans, L.J.; Kirkland, L.; Rader, R. A global review of watermelon pollination biology and ecology: The increasing importance of seedless cultivars. Sci. Hortic. 2020, 271, 109493.
- Vignati, E.; Lipska, M.; Dunwell, J.M.; Caccamo, M.; Simkin, A.J. Options for the generation of seedless cherry, the ultimate snacking product. Planta 2022, 256, 90.
- Maestrelli, A.; Scalzo, R.L.; Rotino, G.L.; Acciarri, N.; Spena, A.R.; Vitelli, G.; Bertolo, G. Freezing effect on some quality parameters of transgenic parthenocarpic eggplants. J. Food Eng. 2003, 56, 285–287.
- Pandolfini, T. Seedless fruit production by hormonal regulation of fruit set. Nutrients 2009, 1, 168–177.
- Acciarri, N.; Restaino, F.; Vitelli, G.; Perrone, D.; Zottini, M.; Pandolfini, T.; Spena, A.; Rotino, G. Genetically modified parthenocarpic eggplants: Improved fruit productivity under both greenhouse and open field cultivation. BMC Biotechnol. 2002, 2, 4.
- Bouquet, A.; Danglot, Y. Inheritance of seedlessness in grapevine (Vitis vinifera L.). Vitis 1996, 35, 35–42.
- de Jong, M.; Mariani, C.; Vriezen, W.H. The role of auxin and gibberellin in tomato fruit set. J. Exp. Bot. 2009, 60, 1523–1532.
- Varoquaux, F.; Blanvillain, R.; Delseny, M.; Gallois, P. Less is better: New approaches for seedless fruit production. Trends Biotechnol. 2000, 18, 233–242.
- El-Sharkawy, I.; Sherif, S.; El Kayal, W.; Mahboob, A.; Abubaker, K.; Ravindran, P.; Jyothi-Prakash, P.A.; Kumar, P.P.; Jayasankar, S. Characterization of gibberellin-signalling elements during plum fruit ontogeny defines the essentiality of gibberellin in fruit development. Plant Mol. Biol. 2014, 84, 399–413.
- Ledbetter, C.A.; Ramming, D.W. Seedlessness in grapes. Hortic. Rev. 1989, 11, 159–184.
- Ingrosso, I.; Bonsegna, S.; De Domenico, S.; Laddomada, B.; Blando, F.; Santino, A.; Giovinazzo, G. Over-expression of a grape stilbene synthase gene in tomato induces parthenocarpy and causes abnormal pollen development. Plant Physiol. Biochem. 2011, 49, 1092–1099.
- Gorguet, B.; van Heusden, A.W.; Lindhout, P. Parthenocarpic fruit development in tomato. Plant Biol. 2005, 7, 131–139.
- Mapelli, S.; Frova, C.; Torti, G.; Soressi, G.P. Relationship between set, development, and activities of growth regulators in tomato fruits. Plant Cell Physiol. 1978, 19, 1281–1288.
- Picarella, M.E.; Mazzucato, A. The occurrence of seedlessness in higher plants; Insights on roles and mechanisms of parthenocarpy. Front. Plant Sci. 2019, 9, 2018.
- Doligez, A.; Bouquet, A.; Danglot, Y.; Lahogue, F.; Riaz, S.; Meredith, P.; Edwards, J.; This, P. Genetic mapping of grapevine (Vitis vinifera L.) applied to the detection of QTLs for seedlessness and berry weight. Theor. Appl. Genet. 2002, 105, 780–795.
- Royo, C.; Torres-Pérez, R.; Mauri, N.; Diestro, N.; Cabezas, J.A.; Marchal, C.; Lacombe, T.; Ibáñez, J.; Tornel, M.; Carreño, J.; et al. The major origin of seedless grapes is associated with a missense mutation in the MADS-box gene VviAGL11. Plant Physiol. 2018, 177, 1234–1253.
- Hanania, U.; Velcheva, M.; Or, E.; Flaishman, M.; Sahar, N.; Perl, A. Silencing of chaperonin 21 that was differentially expressed in inflorescence of seedless and seeded grapes, promoted seed abortion in tobacco and tomato fruits. Transgenic Res. 2007, 16, 515–525.
- Malabarba, J.; Buffon, V.; Mariath, J.E.A.; Gaeta, M.L.; Dornelas, M.C.; Margis-Pinheiro, M.; Pasquali, G.; Revers, L.F. The MADS-box gene Agamous-like 11 is essential for seed morphogenesis in grapevine. J. Exp. Bot. 2017, 68, 1493–1506.
- Tang, Y.; Liu, B.; Li, Y.; Van Nocker, S.; Wang, Y.; Zhang, C. Differential expression of the seed-specific gene ABCG20 between seedless and seeded grapes and its roles in tomato seed development. S. Afr. J. Bot. 2020, 131, 428–436.
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014, 65, 4561–4575.
- Di Marzo, M.; Herrera-Ubaldo, H.; Caporali, E.; Novák, O.; Strnad, M.; Balanzà, V.; Ezquer, I.; Mendes, M.A.; de Folter, S.; Colombo, L. SEEDSTICK controls Arabidopsis fruit size by regulating cytokinin levels and FRUITFULL. Cell Rep. 2020, 30, 2846–2857.
- Baisouny, F.M.; Himelrick, D.G. Muscadine grapes. J. Am. Pomol. Soc. 2002, 56, 207.
- Park, M.; Sarkhosh, A.; Tsolova, V.; El-Sharkawy, I. Horizontal transfer of LTR retrotransposons contributes to the genome diversity of Vitis. Int. J. Mol. Sci. 2021, 22, 10446.
- Lu, J.; Schell, L.; Ramming, D. Interspecific hybridization between Vitis rotundifolia and Vitis vinifera and evaluation of the hybrids. Acta Hortic. 1998, 528, 481–486.
- Xu, X.; Lu, J.; Bradley, F. Applications of polyploids in muscadine grape (Vitis rotundifolia Michx.) breeding. Acta Hortic. 2014, 1046, 411–417.
- Decaestecker, W.; Buono, R.A.; Pfeiffer, M.L.; Vangheluwe, N.; Jourquin, J.; Karimi, M.; Van Isterdael, G.; Beeckman, T.; Nowack, M.K.; Jacobs, T.B. CRISPR-TSKO: A technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell 2019, 31, 2868–2887.
- Moniruzzaman, M.; Zhong, Y.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Exploration of susceptible genes with clustered regularly interspaced short palindromic repeats–tissue-specific knockout (CRISPR-TSKO) to enhance host resistance. CRC Crit. Rev. Plant Sci. 2020, 39, 387–417.
- Pyott, D.E.; Sheehan, E.; Molnar, A. Engineering of CRISPR/Cas9-mediated potyvirus resistance in transgene-free Arabidopsis plants. Mol. Plant Pathol. 2016, 17, 1276–1288.
- Gao, X.; Chen, J.; Dai, X.; Zhang, D.; Zhao, Y. An effective strategy for reliably isolating heritable and Cas9-free Arabidopsis mutants generated by CRISPR/Cas9-mediated genome editing. Plant Physiol. 2016, 171, 1794–1800.
- Zaidi, S.S.; Mansoor, S. Viral vectors for plant genome engineering. Front. Plant Sci. 2017, 8, 539.
- Omar, A.A.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. Somatic embryogenesis: Still a relevant technique in citrus improvement. Methods Mol. Biol. 2016, 1359, 289–327.
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Yan, H.; Yuanda, L.; Jiang, B.; Zhong, G. Citrus cell suspension culture establishment, maintenance, efficient transformation and regeneration to complete transgenic plant. Plants 2021, 10, 664.
- Moniruzzaman, M.; Yaakob, Z.; Anuar, N. Factors affecting in vitro regeneration of Ficus carica L. and genetic fidelity studies using molecular marker. J. Plant Biochem. Biotechnol. 2021, 30, 304–316.
- Li, Z.T.; Kim, K.-H.; Dhekney, S.A.; Jasinski, J.R.; Creech, M.R.; Gray, D.J. An optimized procedure for plant recovery from somatic embryos significantly facilitates the genetic improvement of Vitis. Hortic. Res. 2014, 1, 14027.
- Dutt, M.; Grosser, J.W. An embryogenic suspension cell culture system for Agrobacterium-mediated transformation of citrus. Plant Cell Rep. 2010, 29, 1251–1260.
- Mizzotti, C.; Ezquer, I.; Paolo, D.; Rueda-Romero, P.; Guerra, R.F.; Battaglia, R.; Rogachev, I.; Aharoni, A.; Kater, M.M.; Caporali, E.; et al. SEEDSTICK is a master regulator of development and metabolism in the Arabidopsis seed coat. PLoS Genet. 2014, 10, e1004856.
- Park, M.; Vera, D.; Kambiranda, D.; Gajjar, P.; Cadle-Davidson, L.; Tsolova, V.; El-Sharkawy, I. Chromosome-level genome sequence assembly and genome wide association study of Muscadinia rotundifolia reveal the genetics of 12 berry-related traits. Hortic. Res. 2021, 9, uhab011.
- Mao, Y.; Zhang, Z.; Feng, Z.; Wei, P.; Zhang, H.; Botella, J.R.; Zhu, J.K. Development of germ-line-specific CRISPR-Cas9 systems to improve the production of heritable gene modifications in Arabidopsis. Plant Biotechnol. J. 2016, 14, 519–532.
- Ordon, J.; Bressan, M.; Kretschmer, C.; Dall’Osto, L.; Marillonnet, S.; Bassi, R.; Stuttmann, J. Optimized Cas9 expression systems for highly efficient Arabidopsis genome editing facilitate isolation of complex alleles in a single generation. Funct. Integr. Genom. 2020, 20, 151–162.
- Ren, Q.; Zhong, Z.; Wang, Y.; You, Q.; Li, Q.; Yuan, M.; He, Y.; Qi, C.; Tang, X.; Zheng, X.; et al. Bidirectional promoter-based CRISPR-Cas9 systems for plant genome editing. Front. Plant Sci. 2019, 10, 1173.
- Zheng, N.; Li, T.; Dittman, J.D.; Su, J.; Li, R.; Gassmann, W.; Peng, D.; Whitham, S.A.; Liu, S.; Yang, B. CRISPR/Cas9-based gene editing using egg cell-specific promoters in Arabidopsis and Soybean. Front. Plant Sci. 2020, 11, 800.
- Wang, L.; Dai, W.; Shi, Y.; Wang, Y.; Zhang, C. Cloning and activity analysis of the highly expressed gene VviABCG20 promoter in seed and its activity is negatively regulated by the transcription factor VviDof14. Plant Sci. 2022, 315, 111152.
- Gong, P.; Wei, R.; Li, Y.; Wang, R.; Tang, Y.; Wang, L.; Zhu, H.; Wang, Y.; Zhang, C. Molecular cloning and functional characterization of a seed-specific VvβVPE gene promoter from Vitis vinifera. Planta 2019, 250, 657–665.
- Jeong, H.J.; Choi, J.Y.; Shin, H.Y.; Bae, J.M.; Shin, J.S. Seed-specific expression of seven Arabidopsis promoters. Gene 2014, 553, 17–23.
- Tang, G.; Xu, P.; Li, P.; Zhu, J.; Chen, G.; Shan, L.; Wan, S. Cloning and functional characterization of seed-specific LEC1A promoter from peanut (Arachis hypogaea L.). PLoS ONE 2021, 16, e0242949.
- Haeussler, M.; Schönig, K.; Eckert, H.; Eschstruth, A.; Mianné, J.; Renaud, J.-B.; Schneider-Maunoury, S.; Shkumatava, A.; Teboul, L.; Kent, J.; et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016, 17, 148.
- Lei, Y.; Lu, L.; Liu, H.Y.; Li, S.; Xing, F.; Chen, L.L. CRISPR-P: A web tool for synthetic single-guide RNA design of CRISPR-system in plants. Mol. Plant 2014, 7, 1494–1496.
- Stemmer, M.; Thumberger, T.; Del Sol Keyer, M.; Wittbrodt, J.; Mateo, J.L. CCTop: An Intuitive, flexible and reliable CRISPR/Cas9 target prediction tool. PLoS ONE 2015, 10, e0124633.
- Montague, T.G.; Cruz, J.M.; Gagnon, J.A.; Church, G.M.; Valen, E. CHOPCHOP: A CRISPR/Cas9 and TALEN web tool for genome editing. Nucleic Acids Res. 2014, 42, W401–W407.
- Poudel, R.; Rodriguez, L.T.; Reisch, C.R.; Rivers, A.R. GuideMaker: Software to design CRISPR-Cas guide RNA pools in non-model genomes. GigaScience 2022, 11, giac007.
- Weber, E.; Engler, C.; Gruetzner, R.; Werner, S.; Marillonnet, S. A modular cloning system for standardized assembly of multigene constructs. PLoS ONE 2011, 6, e16765.
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Zhong, G. Having a same type IIS enzyme’s restriction site on guide RNA sequence does not affect golden gate (GG) cloning and subsequent CRISPR/Cas mutagenesis. Int. J. Mol. Sci. 2022, 23, 4889.
- Moniruzzaman, M.; Zhong, Y.; Huang, Z.; Zhong, G. Having a same type IIS enzyme’s restriction site on guide RNA sequence does not affect golden gate (GG) cloning and subsequent CRISPR/Cas mutagenesis. Int. J. Mol. Sci. 2022, 23, 4889. https://doi.org/10.3390/ijms23094889.
More
Information
Subjects:
Horticulture
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




