Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Puja Sutro Dhar | -- | 2254 | 2023-03-23 12:34:12 | | | |
| 2 | Sirius Huang | Meta information modification | 2254 | 2023-03-24 01:49:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Islam, M.R.; Rahman, M.M.; Dhar, P.S.; Nowrin, F.T.; Sultana, N.; Akter, M.; Rauf, A.; Khalil, A.A.; Gianoncelli, A.; Ribaudo, G. Role of Natural Compounds in Ovarian Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/42476 (accessed on 16 January 2026).
Islam MR, Rahman MM, Dhar PS, Nowrin FT, Sultana N, Akter M, et al. Role of Natural Compounds in Ovarian Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/42476. Accessed January 16, 2026.
Islam, Md. Rezaul, Md. Mominur Rahman, Puja Sutro Dhar, Feana Tasmim Nowrin, Nasrin Sultana, Muniya Akter, Abdur Rauf, Anees Ahmed Khalil, Alessandra Gianoncelli, Giovanni Ribaudo. "Role of Natural Compounds in Ovarian Cancer" Encyclopedia, https://encyclopedia.pub/entry/42476 (accessed January 16, 2026).
Islam, M.R., Rahman, M.M., Dhar, P.S., Nowrin, F.T., Sultana, N., Akter, M., Rauf, A., Khalil, A.A., Gianoncelli, A., & Ribaudo, G. (2023, March 23). Role of Natural Compounds in Ovarian Cancer. In Encyclopedia. https://encyclopedia.pub/entry/42476
Islam, Md. Rezaul, et al. "Role of Natural Compounds in Ovarian Cancer." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Ovarian cancer represents a major health concern for the female population. Combining innovative therapeutic techniques with established approaches can aid in improving treatment outcomes. Because of their multi-target actions, long application history, and widespread availability, natural compounds have particular advantages in this connection.
ovarian cancer
natural compounds
semi-synthetic compounds
medicinal chemistry
anti-metastasis
apoptosis
1. Introduction
Mounting evidence demonstrates that plant-derived natural components like phytochemicals can have a role as adjuvants to conventional chemotherapy and may represent promising options for the future development of treatments against ovarian cancer [1][2].
The interest of researchers in the identification of small molecules acting as anticancer agents towards ovarian cancer is constantly growing, and this is testified by the increasing number of contributions in the field. In the 2019–2021 timeframe, some relevant reviews on this topic were published. Shafabakhsh and Asemi as well as Vafadar et al. reviewed the antiproliferative potential of one of the most widely studied natural compounds, quercetin, in the context of ovarian cancer [3][4]. On the other hand, Kubczak et al. reviewed the molecular targets for anticancer natural compounds identified so far, and organized their contribution into sections according to chemical classes [5]. Eventually, Wu et al. focused their attention on ovarian cancer, and classified the studied compounds according to the mechanisms by which natural molecules may act [6].
The following sections briefly report the role of natural constituents against ovarian cancer, and their proposed mode of action is also discussed herein. In general, natural compounds potentially modulate chemotherapeutic resistance, autophagy, inflammation, propagation, and apoptosis [7]. A brief overview of the several cellular events is reported below. The studied molecules have been grouped according to the proposed mechanism of action.
2. Compounds Inducing Apoptosis and Cytotoxicity and Inhibiting Proliferation
Apoptosis is a kind of organized cell death, and it represents a crucial process for maintenance of homeostasis [8]. The induction of apoptosis and inhibition of cell proliferation are the main general mechanisms through which several natural compounds exert their anticancer role [9][10], and the main examples in the field of ovarian cancer are reported below.
Pro-apoptotic activity in ovarian cancer cell lines has been reported for procyanidins from cocoa [11], zeylenone from Uvaria grandiflora Roxb [12], and sanguiin H-6, a natural constituent present in red raspberry [13].
Similarly, methyl lucidone from L. erythrocarpa has cytotoxic effects and induced apoptosis in SKOV-3 and OVCAR-8 cell lines [14], while tanshinones from Salvia miltiorrhiza (Danshen), such as cryptotanshinone, tanshinone-I (Tan-I) and tanshinone-IIA (TII-A), were reported to induce apoptosis by interaction with TNF receptors. In particular, TII-A showed the highest activity [15].
Sulforafane (SFN) is a biologically relevant component found in cruciferous vegetables, including broccoli, and it suppressed cell growth by downregulating the cell cycle regulators cyclin D1 and cyclin-dependent kinases 4 and 6 [16].
Additionally, dihydroartemisinin (DHA), traditionally used to treat fever symptoms and recently investigated as a potential tool against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) [17][18], can be found in Artemisia annua [19] and induced apoptosis in ovarian cancer cells [20].
Reduced cell proliferation was also achieved with use of berbamine, an alkaloid obtained from Berberis amurensis, through the involvement of the Wnt/catenin signaling pathway [21].
Epigallocatechin gallate (EGCG), one of the main catechins found in green tea, inhibited the development and proliferation of OVCAR3 [22], as well as Pulchrin A from Enicosanthellum pulchrum [23].
Kadsuphilactone B, a nortriterpenoid from Schisandra chinensis (Turcz) B. [24], resveratrol [25] and curcumin [26], which will be discussed in a separate section, represent other examples of compounds promoting ovarian cancer cell death.
Other naturally occurring mixtures, such as those containing silybin analogs, demonstrated a potential inhibitory effect on cancer development, including inhibition of elongation, pro-apoptotic effects, and cytotoxicity [27]. For a detailed list of extracts, the reader should refer to the review by Wu et al. [6].
Overall, the main involved mechanisms targeted by the abovementioned compounds include induction of DNA damage, caspase-3, reduction of Janus family tyrosine kinase (p-JAK), Akt phosporilation and SERCA, increased apoptosis-inducing factor (AIF), PARP and Bcl-2 family proteins.
3. Interference with Reactive Oxygen Species (ROS) Damage and with Nucleic Acid Repair
Excessive oxidative stress is generally believed to play a role in a wide range of diseases, from inflammation to cancer. Carcinogenesis has been connected to enhanced ROS formation and damage [28], and several studies have highlighted the involvement of antioxidant and radical scavenger properties of natural and synthetic compounds [29][30].
In various experiments, the abovementioned antioxidant SFN induced apoptosis in the OVCAR3, OVCAR4, OVCAR5, and SKOV3 cell lines and diminished cancer development in vivo [31].
Several flavones, including quercetin [32], and isoflavones have been previously reported to show antiproliferative activity [33]. Quercetin is a relevant natural compound that has been widely studied, and the properties of this molecule will be overviewed in another section. It has been demonstrated that the isoflavone formononetin (FMN), which is found in red clovers and soy, has anticancer and cancer-preventive actions in a variety of cell types. FMN combats ROS and cell division [6][34].
DNA can be harmed directly or indirectly by events such as oxidative stress, radiations, alkylating agents, and a range of other chemotherapeutic techniques, but the capability of ovarian cancer cells to repair DNA damage is believed to be a crucial element in determining the resistance to chemotherapy [35].
In this context, sideroxylin from Callistemon lanceolatus induced apoptosis and reduced proliferation in ovarian cancer cells by influencing lipid peroxidation and ROS activity [36].
Additionally, berberine, another example of a common alkaloid that can be retrieved from several natural sources [37], which has been proven to stop cell division by interfering with DNA repair processes, inhibited the effects of PARP1, which is involved in oxidative states of damaged DNA [38].
Besides, alone or in combination with cisplatin, the abovementioned compound WFA induced the formation of reactive oxygen species (ROS) in A2780 ovarian cancer cells, which caused DNA harm. The compound acted in a synergistic cytotoxic manner with cisplatin, which formed DNA adducts [39].
On the other hand, in the context of the role played by nucleic acid sequences as targets for anticancer agents, aberrant RNAs have been discovered to play critical oncogenic roles in several human cancers. For example, astragalus polysaccharide (APS), a bioactive substance from Astragalus membranaceus, increased apoptosis while decreasing cell invasion targeting such sequences [40].
4. Modulation of Inflammation
It is now widely accepted that inflammation has a direct association with carcinogenesis as it contributes in initiation, proliferation, invasion, and metastasis [41]. Pro-inflammatory cytokines like TNF-α and IL-6 are blocked by anti-inflammatory compounds including baicalein, apigenin, curcumin, EGCG, genistein, luteolin, and wogonin [6]. Alongside, signal transducer and activator of transcription 3 (STAT-3) prevention, cyclooxygenase-2 (COX-2) inhibition, and nitric oxide synthase (iNOS) downregulation are considered as the main anti-inflammatory mechanisms of phytochemicals [42].
5. Suppression of Events Related to Disease Progression: Cell Migration and Angiogenesis
Cell migration and invasion are among the hallmarks of disease progression, and some natural compounds have been reported to target such events.
Among these, tetramethylpyrazine (TMP) from Ligusticum wallichil decreased cell viability and motility in SKOV-3 cells [43], and emodin, which is contained in several preparations of Chinese herbs, was found to suppress cell division, invasion, and migration by hindering the ILK/GSK-3β pathway [44].
Another event that contributes to disease progression is angiogenesis. BLP, the abovementioned mixture containing proanthocyanidins from Chinese bayberry leaves, is probably the most promising in this context, as it demonstrated an anti-angiogenic effect in the IOSE-364 ovarian cell line due to an inhibition of vascular endothelial growth factor (VEGF) [45].
Similarly, Tan-IIA, already mentioned above, interfered with disease progression in an A2780 xenograft model. Concerning the underlying mechanism of action, Tan-IIA promoted antiangiogenetic effects, mediated by the interference with VEGF, and induced apoptosis in the ID-8 and A2780 cell lines [46].
Several natural flavonoids were also reported to act on the EGF/VEGF pathway, including apigenin, taxifolin, luteolin, quercetin, genistein, kaempferol [47], harmine [48], and cranberry proanthocyanidin-1 [49].
6. Regulation of Tumor Micro Environment
The tumor microenvironment is a complex and dynamic combination of elements in which cancer cells are embedded. It comprises nonmalignant cells, the extracellular matrix and several cytokines, chemokines, and growth factors. Considering their multi-target action, natural compounds can modulate several aspects of the microenvironment. In particular, Dias et al. highlighted how natural derivatives can influence metabolic crosstalk to “re-educate” tumor microenvironment cells towards potential anticancer activity. In particular, curcumin, resveratrol, EGCG, shikonin, and phloretin were reported to alter the metabolism of stromal cells [50].
The abovementioned effect is achieved through the modulation of the expression of cancer-associated genes by the natural products, and this mechanism has also been reported to explain the anticancer activity of quercetin, berberine, and tanshinones [51].
In addition, β-escin was recently reported to combat ovarian cancer metastasis by targeting both cancer and stromal cells in the tumor microenvironment [52].
7. Other Mechanisms Related to Dysregulation of Cell Cycle
Dysregulation of the cell cycle is a relevant contributing factor in the carcinogenesis of ovarian cancer, and interference with the G0/G1 stages is the most commonly reported mechanism of natural compounds with an anticancer role targeting this process [53]. This mechanism was reported for asiatic acid from Centella asiatica [54], mentoflavone from Selaginella tamariscina [55], proanthocyanidins from Chinese bayberry leaves (BLPs) [56], and pulchrin A [57], which were found to combat cell proliferation and cancer progression, in particular by targeting such phases of the cell cycle.
Moreover, co-treatment with herbal extracts from Fritillaria cirrhosa (FC) and Scutellaria baicalensis (SB) resulted in G0/G1 stage cell cycle arrest also in OVCA 420 and OVCA 429 ovarian cancer cells [58].
Additionally, cucurbitacin-A, isolated from Momordica charantia L., was found to show anticancer potential, causing cell cycle arrest in the G2/M phase [59].
In this context, licorice plants contain large amounts of the flavonoid isoliquiritigenin (ISL). In OVCAR-5 and ES-2 cell lines, ISL also decreased cell proliferation in a dose- and time-dependent manner, targeting the G2/M phase of the cell cycle [60].
Autophagy is another physiological cell process that contributes to the maintenance of a normal cell cycle. According to increasing evidence, autophagy and ovarian cancer also appear to be connected [61]. Thus, natural compounds that help in modulating autophagy may find an application in ovarian cancer treatment. Among the natural constituents reported to act against ovarian cancer through this mechanism, Emblica officinalis (Amla) extracts [62], resveratrol [63], withaferin A (WFA) [64], and grifolin [65] were reported.
Moreover, Tan-I, a compound from the class of tanshinones, cited above, increased levels of the autophagy-related proteins beclin1, ATG7, and p62 as well as LC3II/LC3I and caspase-3 in A2780 and ID8, boosting apoptosis and inducing autophagy [66][67].
Finally, genistein promoted autophagy of caspase-independent cells [68] and induced apoptosis in cisplatin-sensitive and resistant ovarian cancer cells (A2780/CaOV3, ES-2).
8. Natural Constituents Modulating Resistance to Chemotherapeutic Agents
In ovarian cancer cells, plant-derived constituents were found to enhance sensitivity to chemotherapeutics, an aspect which, as anticipated, is crucial in this pathology.
For example, pre-treatment with either ellagic acid or resveratrol 48-h before cisplatin administration was reported to increase cytotoxicity of cisplatin itself in A2780CisR cisplatin-resistant cells, while synergistic treatment with either cisplatin–ellagic acid or cisplatin–resveratrol for 26 weekly cycles completely prevented cisplatin resistance in A2780 cells [69].
Moreover, in A2780 cells, SFN diminished the xenobiotic-reaction component (XRE). SFN also interferes with cell pH regulation and migration, and in this context it has been proposed as an agent to combat chemoresistance [70].
In doxorubicin-resistant human ovarian cancer cell lines (NCI/ADR-RES), treatment with RCM, also known as Korean dark raspberry, led to apoptosis through phosphorylation of c-Jun N-terminal kinase (JNK) [71]. In the same model, ellagic acid and quercetin, two phytochemicals also found in RCM as well as in many other natural sources, were shown to influence JNK and Akt phosphorylation, thus inducing apoptosis [72].
Finally, since therapy with WFA and doxorubicin reduced cell proliferation in xenograft mice models of ovarian cancer more effectively than WFA or doxorubicin alone, it has been postulated that WFA may be thought of as an adjuvant to standard doxorubicin therapy to minimize adverse effects [64].
A schematic representation and summary of the pathways targeted by natural compounds are reported in Figure 1. Moreover, Table 1 summarizes the most recently reported updates on the molecular mechanisms underlying the activity of natural compounds and their derivatives in the context of ovarian cancer.
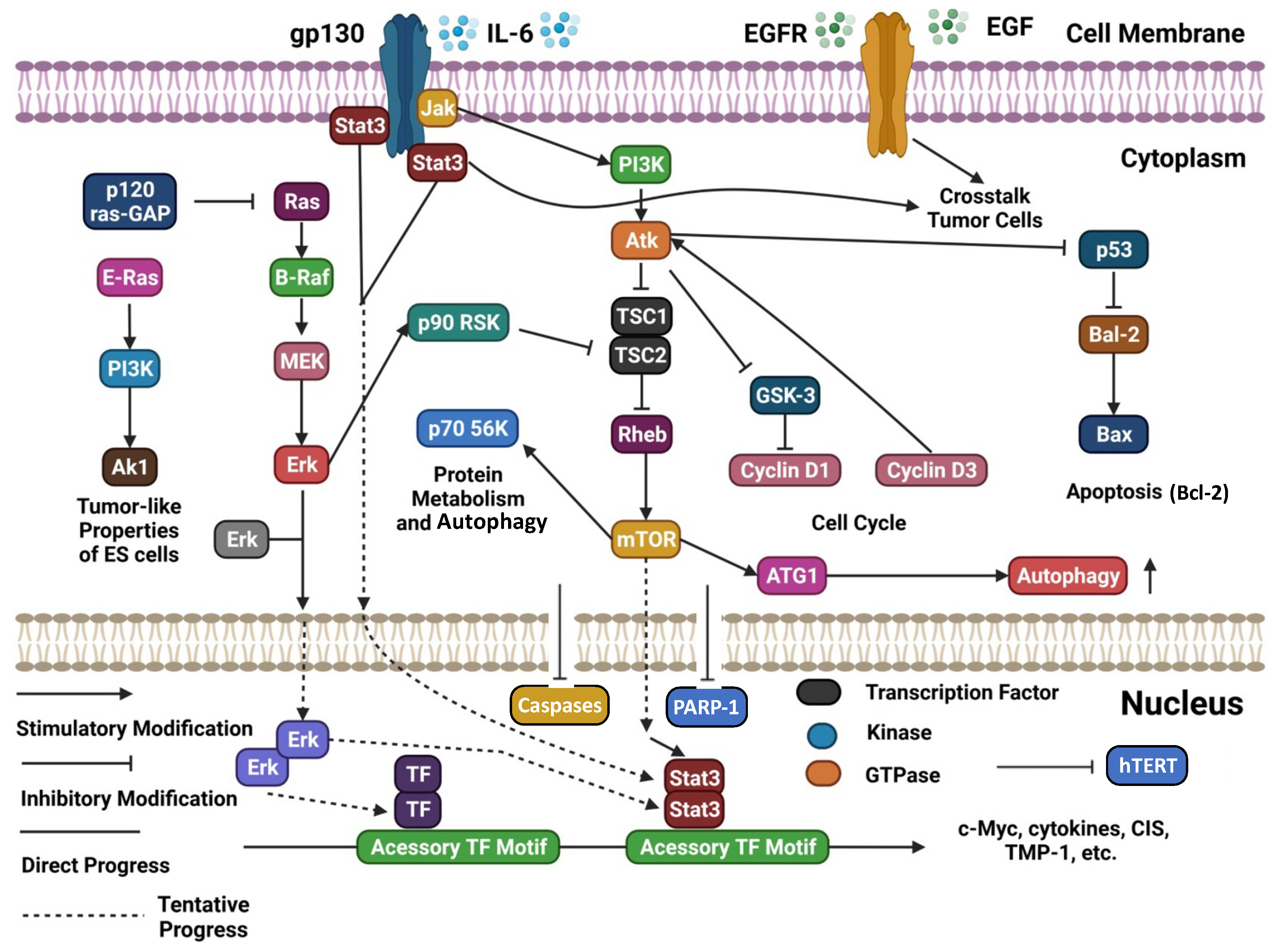
Figure 1. Schematic representation of several cellular signaling pathways potentially targeted by natural compounds (adapted and updated from [6]).
Table 1. Overview of natural substances showing anticancer properties against ovarian cancer models. This table is intended as an addendum to the one reported by Wu et al. [6], thus updated records were included. The reader is invited to refer to the abovementioned review for a more comprehensive overview with the corresponding references. The table also includes semi-synthetic derivatives of natural compounds that showed antiproliferative activity.
| Compound | Source | Chemical Structure of the Representative Component | Classification | Model | Mechanism of Action | References |
|---|---|---|---|---|---|---|
| Aminoalkyl derivatives of cycleanine | Triclisia subcordata | 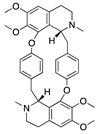 (Cycleanine) |
Bisbenzylisoquinoline macrocyclic alkaloid | Cell lines | activation of caspases 3/7, cleavage of PARP | [73] |
| Berberine | European barberry, goldenseal, goldthread, Oregon grape, phellodendron, and tree turmeric | 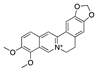 |
Alkaloid | A2780, HEY, HO8910 | Triggering oxidative DNA damage, targeting of cancer stem cells | [38][74] |
| Epigallocatechin gallate (EGCG) | Green tea | 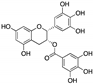 |
Flavonoid | SKOV3-ip1, SKOV3TR-ip2 | Reduction of hTERT and Bcl-2, alteration of the metabolism of stromal cells | [22][50][75] |
| FBA-TPQ (derivative of makaluvamines) | Zyzzya sponges | 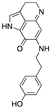 (Makaluvamine scaffold) |
Pyrroloiminoquinone alkaloid | in vitro and in vivo (xenograft) | ROS species, p53-MDM2 and PI3K-Akt pathways | [76] |
| Phloretin | Apple tree leaves | 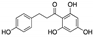 |
Dihydrochalcone | in vitro | Alteration of the metabolism of stromal cells | [50] |
| Semi-synthetic derivatives of celastrol | Tripterygium species | 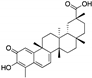 (Celastrol) |
Nortriterpen quinone | in vitro | STAT-3 pathway, induction of apoptosis, reduction of cell migration | [77] |
| Shikonin | Alkanna tinctoria | 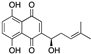 |
Naphthoquinone | A278 cells, in vitro | Alteration of the metabolism of stromal cells | [50][78] |
| Tanshinones | Salvia miltiorrhiza | 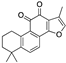 (Tanshinone IIA) |
Terpenoid/Abietane | A-549, TOV-21G | Growth capacity is inhibited by reducing cell viability, alteration of the microenvironment | [15][46][51][67] |
| Verticillin H esters | Fungi | 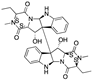 |
Verticillins | OVCAR-3 | Reduced cell proliferation | [79] |
| β-escin | horse chestnut seed | 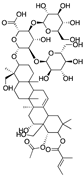 |
Pentacyclic triterpenoid saponin | in vitro and in vivo | Alteration of the microenvironment | [52] |
References
- Rais, J.; Jafri, A.; Siddiqui, S.; Tripathi, M.; Arshad, M. Phytochemicals in the Treatment of Ovarian Cancer. Front. Biosci. 2017, 9, 67–75.
- Hosein Farzaei, M.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as Adjunctive with Conventional Anticancer Therapies. Curr. Pharm. Des. 2016, 22, 4201–4218.
- Shafabakhsh, R.; Asemi, Z. Quercetin: A Natural Compound for Ovarian Cancer Treatment. J. Ovarian Res. 2019, 12, 55.
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32.
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int. J. Mol. Sci. 2021, 22, 13659.
- Wu, J.; Zhou, T.; Wang, Y.; Jiang, Y.; Wang, Y. Mechanisms and Advances in Anti-Ovarian Cancer with Natural Plants Component. Molecules 2021, 26, 5949.
- Pistollato, F.; Calderón Iglesias, R.; Ruiz, R.; Aparicio, S.; Crespo, J.; Dzul Lopez, L.; Giampieri, F.; Battino, M. The Use of Natural Compounds for the Targeting and Chemoprevention of Ovarian Cancer. Cancer Lett. 2017, 411, 191–200.
- Cheng, X.; Ferrell, J.E. Apoptosis Propagates through the Cytoplasm as Trigger Waves. Science 2018, 361, 607–612.
- Rajabi, S.; Maresca, M.; Yumashev, A.V.; Choopani, R.; Hajimehdipoor, H. The Most Competent Plant-Derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules 2021, 11, 534.
- Liu, C.; Zeng, Y.; Wen, Y.; Huang, X.; Liu, Y. Natural Products Modulate Cell Apoptosis: A Promising Way for the Treatment of Ulcerative Colitis. Front. Pharmacol. 2022, 13, 806148.
- Taparia, S.S.; Khanna, A. Procyanidin-Rich Extract of Natural Cocoa Powder Causes ROS-Mediated Caspase-3 Dependent Apoptosis and Reduction of pro-MMP-2 in Epithelial Ovarian Carcinoma Cell Lines. Biomed. Pharmacother. 2016, 83, 130–140.
- Xu, X.; Shi, J.; Gao, H.; Li, Q. Zeylenone Inhibits Proliferation and Promotes Apoptosis in Ovarian Carcinoma Cells via Janus Kinase 2 / Signal Transducers and Activators of Transcription 3 Pathways. J. Obstet. Gynaecol. Res. 2018, 44, 1451–1457.
- Lee, D.; Ko, H.; Kim, Y.J.; Kim, S.N.; Choi, K.C.; Yamabe, N.; Kim, K.H.; Kang, K.S.; Kim, H.Y.; Shibamoto, T. Inhibition of A2780 Human Ovarian Carcinoma Cell Proliferation by a Rubus Component, Sanguiin H-6. J. Agric. Food Chem. 2016, 64, 801–805.
- Yoon, J.H.; Shin, J.W.; Pham, T.H.; Choi, Y.J.; Ryu, H.W.; Oh, S.R.; Oh, J.W.; Yoon, D.Y. Methyl Lucidone Induces Apoptosis and G2/M Phase Arrest via the PI3K/Akt/NF-ΚB Pathway in Ovarian Cancer Cells. Pharm. Biol. 2020, 58, 51–59.
- Chang, C.-C.; Kuan, C.-P.; Lin, J.-Y.; Lai, J.-S.; Ho, T.-F. Tanshinone IIA Facilitates TRAIL Sensitization by Up-Regulating DR5 through the ROS-JNK-CHOP Signaling Axis in Human Ovarian Carcinoma Cell Lines. Chem. Res. Toxicol. 2015, 28, 1574–1583.
- Taheri, M.; Roudbari, N.H.; Amidi, F.; Parivar, K. The Protective Effect of Sulforaphane against Oxidative Stress in Granulosa Cells of Patients with Polycystic Ovary Syndrome (PCOS) through Activation of AMPK/AKT/NRF2 Signaling Pathway. Reprod. Biol. 2021, 21, 100563.
- Ribaudo, G.; Coghi, P.; Yang, L.J.; Ng, J.P.L.; Mastinu, A.; Memo, M.; Wong, V.K.W.; Gianoncelli, A. Computational and Experimental Insights on the Interaction of Artemisinin, Dihydroartemisinin and Chloroquine with SARS-CoV-2 Spike Protein Receptor-Binding Domain (RBD). Nat. Prod. Res. 2022, 36, 5358–5363.
- Coghi, P.; Yang, L.J.; Ng, J.P.L.; Haynes, R.K.; Memo, M.; Gianoncelli, A.; Wong, V.K.W.; Ribaudo, G. A Drug Repurposing Approach for Antimalarials Interfering with SARS-CoV-2 Spike Protein Receptor Binding Domain (RBD) and Human Angiotensin-Converting Enzyme 2 (ACE2). Pharmaceuticals 2021, 14, 954.
- Abate, G.; Zhang, L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia Annual. Biomolecules 2021, 11, 975.
- Liu, Y.; Gao, S.; Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H. Dihydroartemisinin Induces Apoptosis and Inhibits Proliferation, Migration, and Invasion in Epithelial Ovarian Cancer via Inhibition of the Hedgehog Signaling Pathway. Cancer Med. 2018, 7, 5704–5715.
- Zhang, H.; Jiao, Y.; Shi, C.; Song, X.; Chang, Y.; Ren, Y.; Shi, X. Berbamine Suppresses Cell Proliferation and Promotes Apoptosis in Ovarian Cancer Partially via the Inhibition of Wnt/β-Catenin Signaling. Acta Biochim. Biophys. Sin. 2018, 50, 532–539.
- Wang, F.; Chang, Z.; Fan, Q.; Wang, L. Epigallocatechin-3-Gallate Inhibits the Proliferation and Migration of Human Ovarian Carcinoma Cells by Modulating P38 Kinase and Matrix Metalloproteinase-2. Mol. Med. Rep. 2014, 9, 1085–1089.
- Ahmed, O.H.; Hamad, M.N.; Jaafar, N.S. Phytochemical Investigation of Chenopodium Murale (Family: Chenopodiaceae) Cultivated in Iraq, Isolation and Identification of Scopoletin and Gallic Acid. Asian J. Pharm. Clin. Res. 2017, 10, 70–77.
- Jeong, M.; Kim, H.M.; Kim, H.J.; Choi, J.-H.; Jang, D.S. Kudsuphilactone B, a Nortriterpenoid Isolated from Schisandra Chinensis Fruit, Induces Caspase-Dependent Apoptosis in Human Ovarian Cancer A2780 Cells. Arch. Pharmacal Res. 2017, 40, 500–508.
- Vergara, D.; Simeone, P.; Toraldo, D.; Del Boccio, P.; Vergaro, V.; Leporatti, S.; Pieragostino, D.; Tinelli, A.; De Domenico, S.; Alberti, S.; et al. Resveratrol Downregulates Akt/GSK and ERK Signalling Pathways in OVCAR-3 Ovarian Cancer Cells. Mol. Biosyst. 2012, 8, 1078–1087.
- Seo, J.-A.; Kim, B.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Curcumin Induces Apoptosis by Inhibiting Sarco/Endoplasmic Reticulum Ca2+ ATPase Activity in Ovarian Cancer Cells. Cancer Lett. 2016, 371, 30–37.
- Manivannan, E.; Amawi, H.; Hussein, N.; Karthikeyan, C.; Fetcenko, A.; Narayana Moorthy, N.S.H.; Trivedi, P.; Tiwari, A.K. Design and Discovery of Silybin Analogues as Antiproliferative Compounds Using a Ring Disjunctive—Based, Natural Product Lead Optimization Approach. Eur. J. Med. Chem. 2017, 133, 365–378.
- Jiao, R.; Liu, Y.; Gao, H.; Xiao, J.; So, K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am. J. Chin. Med. 2016, 44, 463–488.
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. IJMS 2017, 18, 96.
- Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants 2020, 9, 714.
- Kwon, Y. Food-Derived Polyphenols Inhibit the Growth of Ovarian Cancer Cells Irrespective of Their Ability to Induce Antioxidant Responses. Heliyon 2018, 4, e00753.
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166, 112066.
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-Synthetic Isoflavones as BACE-1 Inhibitors against Alzheimer’s Disease. Bioorg. Chem. 2019, 87, 474–483.
- Park, S.; Bazer, F.W.; Lim, W.; Song, G. The O-Methylated Isoflavone, Formononetin, Inhibits Human Ovarian Cancer Cell Proliferation by Sub G0/G1 Cell Phase Arrest through PI3K/AKT and ERK1/2 Inactivation. J. Cell. Biochem. 2018, 119, 7377–7387.
- Ai, Z.; Lu, Y.; Qiu, S.; Fan, Z. Overcoming Cisplatin Resistance of Ovarian Cancer Cells by Targeting HIF-1-Regulated Cancer Metabolism. Cancer Lett. 2016, 373, 36–44.
- Park, S.; Lim, W.; Jeong, W.; Bazer, F.W.; Lee, D.; Song, G. Sideroxylin (Callistemon lanceolatus) Suppressed Cell Proliferation and Increased Apoptosis in Ovarian Cancer Cells Accompanied by Mitochondrial Dysfunction, the Generation of Reactive Oxygen Species, and an Increase of Lipid Peroxidation. J. Cell. Physiol. 2018, 233, 8597–8604.
- Ribaudo, G.; Zanforlin, E.; Canton, M.; Bova, S.; Zagotto, G. Preliminary Studies of Berberine and Its Semi-Synthetic Derivatives as a Promising Class of Multi-Target Anti-Parkinson Agents. Nat. Prod. Res. 2018, 32, 1395–1401.
- Hou, D.; Xu, G.; Zhang, C.; Li, B.; Qin, J.; Hao, X.; Liu, Q.; Zhang, X.; Liu, J.; Wei, J.; et al. Berberine Induces Oxidative Dna Damage and Impairs Homologous Recombination Repair in Ovarian Cancer Cells to Confer Increased Sensitivity to Parp Inhibition. Cell Death Dis. 2017, 8, e3070.
- Kakar, S.S.; Jala, V.R.; Fong, M.Y. Synergistic Cytotoxic Action of Cisplatin and Withaferin A on Ovarian Cancer Cell Lines. Biochem. Biophys. Res. Commun. 2012, 423, 819–825.
- Guo, Y.; Zhang, Z.; Wang, Z.; Liu, G.; Liu, Y.; Wang, H. Astragalus Polysaccharides Inhibit Ovarian Cancer Cell Growth via MicroRNA-27a/FBXW7 Signaling Pathway. Biosci. Rep. 2020, 40, BSR20193396.
- Fernandes, J.V.; Cobucci, R.N.O.; Jatobá, C.A.N.; de Medeiros Fernandes, T.A.A.; de Azevedo, J.W.V.; de Araújo, J.M.G. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. 2015, 21, 527–534.
- Kim, M.K.; Kim, K.; Han, J.Y.; Lim, J.M.; Song, Y.S. Modulation of Inflammatory Signaling Pathways by Phytochemicals in Ovarian Cancer. Genes Nutr. 2011, 6, 109–115.
- Yin, J.; Yu, C.; Yang, Z.; He, J.L.; Chen, W.J.; Liu, H.Z.; Li, W.M.; Liu, H.T.; Wang, Y.X. Tetramethylpyrazine Inhibits Migration of SKOV3 Human Ovarian Carcinoma Cells and Decreases the Expression of Interleukin-8 via the ERK1/2, P38 and AP-1 Signaling Pathways. Oncol. Rep. 2011, 26, 671–679.
- Lu, J.; Xu, Y.; Wei, X.; Zhao, Z.; Xue, J.; Liu, P. Emodin Inhibits the Epithelial to Mesenchymal Transition of Epithelial Ovarian Cancer Cells via ILK/GSK-3 β /Slug Signaling Pathway. BioMed Res. Int. 2016, 2016, 6253280.
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Rojanasakul, Y.; Ren, N.; Ye, X.; Chen, Y.C. Dietary Compound Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves Inhibit Angiogenesis and Regulate Cell Cycle of Cisplatin-Resistant Ovarian Cancer Cells via Targeting Akt Pathway. J. Funct. Foods 2018, 40, 573–581.
- Zhou, J.; Jiang, Y.-Y.; Wang, X.-X.; Wang, H.-P.; Chen, H.; Wu, Y.-C.; Wang, L.; Pu, X.; Yue, G.-Z.; Zhang, L. Tanshinone IIA Suppresses Ovarian Cancer Growth through Inhibiting Malignant Properties and Angiogenesis. Ann. Transl. Med. 2020, 8, 1295.
- Liskova, A.; Koklesova, L.; Samec, M.; Varghese, E.; Abotaleb, M.; Samuel, S.M.; Smejkal, K.; Biringer, K.; Petras, M.; Blahutova, D.; et al. Implications of Flavonoids as Potential Modulators of Cancer Neovascularity. J. Cancer Res. Clin. Oncol. 2020, 146, 3079–3096.
- Gao, J.; Zhu, H.; Wan, H.; Zou, X.; Ma, X.; Gao, G. Harmine Suppresses the Proliferation and Migration of Human Ovarian Cancer Cells through Inhibiting ERK/CREB Pathway. Oncol. Rep. 2017, 38, 2927–2934.
- Kim, K.K.; Singh, A.P.; Singh, R.K.; DeMartino, A.; Brard, L.; Vorsa, N.; Lange, T.S.; Moore, R.G. Anti-Angiogenic Activity of Cranberry Proanthocyanidins and Cytotoxic Properties in Ovarian Cancer Cells. Int. J. Oncol. 2012, 40, 227–235.
- Dias, A.S.; Helguero, L.; Almeida, C.R.; Duarte, I.F. Natural Compounds as Metabolic Modulators of the Tumor Microenvironment. Molecules 2021, 26, 3494.
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling Tumor Microenvironment with Natural Products to Overcome Drug Resistance. Front. Immunol. 2022, 13, 1051998.
- Kenny, H.A.; Hart, P.C.; Kordylewicz, K.; Lal, M.; Shen, M.; Kara, B.; Chen, Y.-J.; Grassl, N.; Alharbi, Y.; Pattnaik, B.R.; et al. The Natural Product β-Escin Targets Cancer and Stromal Cells of the Tumor Microenvironment to Inhibit Ovarian Cancer Metastasis. Cancers 2021, 13, 3931.
- Di, W.; Zhang, L.; Yi, H.; Han, X.; Zhang, Y.; Xin, L. Exopolysaccharides Produced by Lactobacillus Strains Suppress HT-29 Cell Growth via Induction of G0/G1 Cell Cycle Arrest and Apoptosis. Oncol. Lett. 2018, 16, 3577–3586.
- Ren, L.; Cao, Q.X.; Zhai, F.R.; Yang, S.Q.; Zhang, H.X. Asiatic Acid Exerts Anticancer Potential in Human Ovarian Cancer Cells via Suppression of PI3K/Akt/MTOR Signalling. Pharm. Biol. 2016, 54, 2377–2382.
- Yu, S.; Yan, H.; Zhang, L.; Shan, M.; Chen, P.; Ding, A.; Li, S.F.Y. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules 2017, 22, 299.
- Zhang, Y.; Chen, S.; Wei, C.; Rankin, G.O.; Ye, X.; Chen, Y.C. Dietary Compound Proanthocyanidins from Chinese Bayberry (Myrica Rubra Sieb. et Zucc.) Leaves Attenuate Chemotherapy-Resistant Ovarian Cancer Stem Cell Traits via Targeting the Wnt/β-Catenin Signaling Pathway and Inducing G1 Cell Cycle Arrest. Food Funct. 2018, 9, 525–533.
- Nordin, N.; Fadaeinasab, M.; Mohan, S.; Hashim, N.M.; Othman, R.; Karimian, H.; Iman, V.; Ramli, N.; Ali, H.M.; Majid, N.A. Pulchrin A, a New Natural Coumarin Derivative of Enicosanthellum Pulchrum, Induces Apoptosis in Ovarian Cancer Cells via Intrinsic Pathway. PLoS ONE 2016, 11, e0154023.
- Kavandi, L.; Lee, L.R.; Bokhari, A.A.; Pirog, J.E.; Jiang, Y.; Ahmad, K.A.; Syed, V. The Chinese Herbs Scutellaria Baicalensis and Fritillaria Cirrhosa Target NFκB to Inhibit Proliferation of Ovarian and Endometrial Cancer Cells. Mol. Carcinog. 2015, 54, 368–378.
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica Charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555.
- Chen, H.Y.; Huang, T.C.; Shieh, T.M.; Wu, C.H.; Lin, L.C.; Hsia, S.M. Isoliquiritigenin Induces Autophagy and Inhibits Ovarian Cancer Cell Growth. Int. J. Mol. Sci. 2017, 18, 2025.
- Zhan, L.; Zhang, Y.; Wang, W.; Song, E.; Fan, Y.; Li, J.; Wei, B. Autophagy as an Emerging Therapy Target for Ovarian Carcinoma. Oncotarget 2016, 7, 83476–83487.
- De, A.; De, A.; Papasian, C.; Hentges, S.; Banerjee, S.; Haque, I.; Banerjee, S.K. Emblica Officinalis Extract Induces Autophagy and Inhibits Human Ovarian Cancer Cell Proliferation, Angiogenesis, Growth of Mouse Xenograft Tumors. PLoS ONE 2013, 8, e72748.
- Wang, H.Y.; Peng, Y.; Wang, J.; Gu, A.X.; Li, Q.; Mao, D.W.; Guo, L.Y. Effect of Autophagy on the Resveratrol-Induced Apoptosis of Ovarian Cancer SKOV3 Cells. J. Cell. Biochem. 2019, 120, 7788–7793.
- Fong, M.Y.; Jin, S.; Rane, M.; Singh, R.K.; Gupta, R.; Kakar, S.S. Withaferin a Synergizes the Therapeutic Effect of Doxorubicin through ROS-Mediated Autophagy in Ovarian Cancer. PLoS ONE 2012, 7, e42265.
- Che, X.; Yan, H.; Sun, H.; Dongol, S.; Wang, Y.; Lv, Q.; Jiang, J. Grifolin Induces Autophagic Cell Death by Inhibiting the Akt/MTOR/S6K Pathway in Human Ovarian Cancer Cells. Oncol. Rep. 2016, 36, 1041–1047.
- Lin, L.; Baehrecke, E.H. Autophagy, Cell Death, and Cancer. Mol. Cell. Oncol. 2015, 2, e985913.
- Zhou, J.; Jiang, Y.-Y.; Chen, H.; Wu, Y.-C.; Zhang, L. Tanshinone I Attenuates the Malignant Biological Properties of Ovarian Cancer by Inducing Apoptosis and Autophagy via the Inactivation of PI3K/AKT/MTOR Pathway. Cell Prolif. 2020, 53, e12739.
- Gossner, G.; Choi, M.; Tan, L.; Fogoros, S.; Griffith, K.A.; Kuenker, M.; Liu, J.R. Genistein-Induced Apoptosis and Autophagocytosis in Ovarian Cancer Cells. Gynecol. Oncol. 2007, 105, 23–30.
- Engelke, L.H.; Hamacher, A.; Proksch, P.; Kassack, M.U. Ellagic Acid and Resveratrol Prevent the Development of Cisplatin Resistance in the Epithelial Ovarian Cancer Cell Line A2780. J. Cancer 2016, 7, 353–363.
- Pastorek, M.; Simko, V.; Takacova, M.; Barathova, M.; Bartosova, M.; Hunakova, L.; Sedlakova, O.; Hudecova, S.; Krizanova, O.; Dequiedt, F.; et al. Sulforaphane Reduces Molecular Response to Hypoxia in Ovarian Tumor Cells Independently of Their Resistance to Chemotherapy. Int. J. Oncol. 2015, 47, 51–60.
- Kim, Y.; Lee, S.M.; Kim, J.H. Unripe Rubus Coreanus Miquel Suppresses Migration and Invasion of Human Prostate Cancer Cells by Reducing Matrix Metalloproteinase Expression. Biosci. Biotechnol. Biochem. 2014, 78, 1402–1411.
- Kim, M.K.; Choi, H.S.; Cho, S.G.; Shin, Y.C.; Ko, S.G. Rubus Coreanus Miquel Extract Causes Apoptosis of Doxorubicin-Resistant NCI/ADR-RES Ovarian Cancer Cells via JNK Phosphorylation. Mol. Med. Rep. 2016, 13, 4065–4072.
- Uche, F.I.; McCullagh, J.; Claridge, T.W.D.; Richardson, A.; Li, W.-W. Synthesis of (Aminoalkyl)Cycleanine Analogues: Cytotoxicity, Cellular Uptake, and Apoptosis Induction in Ovarian Cancer Cells. Bioorg. Med. Chem. Lett. 2018, 28, 1652–1656.
- Taylor, W.F.; Jabbarzadeh, E. The Use of Natural Products to Target Cancer Stem Cells. Am. J. Cancer Res. 2017, 7, 1588–1605.
- Chen, H.; Landen, C.N.; Li, Y.; Alvarez, R.D.; Tollefsbol, T.O. Epigallocatechin Gallate and Sulforaphane Combination Treatment Induce Apoptosis in Paclitaxel-Resistant Ovarian Cancer Cells through HTERT and Bcl-2 down-Regulation. Exp. Cell Res. 2013, 319, 697–706.
- Chen, T.; Xu, Y.; Guo, H.; Liu, Y.; Hu, P.; Yang, X.; Li, X.; Ge, S.; Velu, S.E.; Nadkarni, D.H.; et al. Experimental Therapy of Ovarian Cancer with Synthetic Makaluvamine Analog: In Vitro and In Vivo Anticancer Activity and Molecular Mechanisms of Action. PLoS ONE 2011, 6, e20729.
- Li, N.; Li, C.; Zhang, J.; Jiang, Q.; Wang, Z.; Nie, S.; Gao, Z.; Li, G.; Fang, H.; Ren, S.; et al. Discovery of Semisynthetic Celastrol Derivatives Exhibiting Potent Anti-Ovarian Cancer Stem Cell Activity and STAT3 Inhibition. Chem. Biol. Interact. 2022, 366, 110172.
- Beretta, G.L.; Ribaudo, G.; Menegazzo, I.; Supino, R.; Capranico, G.; Zunino, F.; Zagotto, G. Synthesis and Evaluation of New Naphthalene and Naphthoquinone Derivatives as Anticancer Agents: Naphthalene and Naphthoquinone Derivatives as Anticancer Agents. Arch. Pharm. Chem. Life Sci. 2017, 350, e1600286.
- Amrine, C.S.M.; Huntsman, A.C.; Doyle, M.G.; Burdette, J.E.; Pearce, C.J.; Fuchs, J.R.; Oberlies, N.H. Semisynthetic Derivatives of the Verticillin Class of Natural Products through Acylation of the C11 Hydroxy Group. ACS Med. Chem. Lett. 2021, 12, 625–630.
More
Information
Subjects:
Medicine, Legal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




