Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicolaj Kaden | -- | 1700 | 2023-03-23 11:50:12 | | | |
| 2 | Jason Zhu | Meta information modification | 1700 | 2023-03-24 02:42:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kaden, N.; Schlimbach, R.; Rohde García, �.; Dröder, K. Electrolyte Filling and Wetting in Lithium-Ion Battery Production. Encyclopedia. Available online: https://encyclopedia.pub/entry/42473 (accessed on 07 February 2026).
Kaden N, Schlimbach R, Rohde García �, Dröder K. Electrolyte Filling and Wetting in Lithium-Ion Battery Production. Encyclopedia. Available at: https://encyclopedia.pub/entry/42473. Accessed February 07, 2026.
Kaden, Nicolaj, Ricarda Schlimbach, Álvaro Rohde García, Klaus Dröder. "Electrolyte Filling and Wetting in Lithium-Ion Battery Production" Encyclopedia, https://encyclopedia.pub/entry/42473 (accessed February 07, 2026).
Kaden, N., Schlimbach, R., Rohde García, �., & Dröder, K. (2023, March 23). Electrolyte Filling and Wetting in Lithium-Ion Battery Production. In Encyclopedia. https://encyclopedia.pub/entry/42473
Kaden, Nicolaj, et al. "Electrolyte Filling and Wetting in Lithium-Ion Battery Production." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Electrolyte filling and wetting is a quality-critical and cost-intensive process step of battery cell production. Due to the importance of this process, a steadily increasing number of publications is emerging for its different influences and factors.
lithium-ion battery
battery production
electrolyte filling
electrolyte wetting
1. Introduction
Given the irreversible effects on the global climate, there is a collective societal challenge to reduce CO2 emissions. Research in battery technology has the potential to provide a solution for carbon-neutral mobility by increasing the efficiency as well as expanding the utility of storage options for electrical energy [1]. In this context, high capacity lithium-ion batteries have the potential to transform the mobility industry in the short and medium term by replacing fossil fuels. As a result, the demand for large-format battery cells with high specific capacities and power has increased rapidly in recent years [2]. The implementation of the EU regulation for CO2 reduction in the automotive sector indicates that this trend will likely continue [3].
Thus, this rising demand necessitates more efficient battery production in order to save time, material, and the associated costs. Battery cost drivers include, in addition to raw material acquisition costs, inefficient manufacturing processes [4]. The process of electrolyte filling, due to long storage times of cells, represents a promising entry point for cost savings [5][6]. This process step is critical for quality, as insufficient wetting of the cell stack materials can result in poor cell performance [7].
Although the potential for savings has been widely recognized in both research and industry, findings covering electrolyte filling remain fragmented. There are complicated interactions between process–structure–characteristics and their relationships, which are often related to prior process steps. Existing research focuses on highly application-specific industrial processes, covers simulation studies without experimental foundation, or investigates isolated aspects such as material or process influences separately. However, a comprehensive scientific analysis of experimental results on material and process parameters is lacking.
2. Trends
Figure 1 shows an increase in publications over the last ten years, with a peak in 2020, reflecting the growing importance of battery production over the last decade. This is particularly due to increasing efforts and political measures towards sustainability, which have a strong impact on the automotive industry. It is also notable that there has been an increasing number of contributions focusing on improved process parameters, as their efficient and effective design is crucial for the implementation of large-scale industrial production.
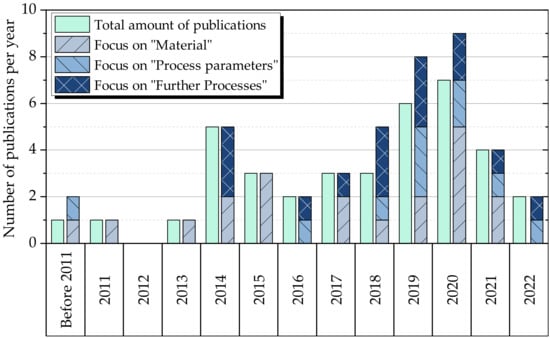
Figure 1. Illustration of the examined publications sorted by years. In addition, the publications to the corresponding main group labels. Since one publication can be assigned to various foci groups (e.g., material and further processes), its cumulation (right bar) sometimes exceeds the total amount of papers (left bar). The publications from 2022 include only those published up to the month of June.
From 2011 to 2017, the focus was on material-centered publications related to electrolyte filling, such as studies on the wetting properties of newly developed materials and the effects of upstream process steps in electrode production, such as calendering or laser patterning, on the wetting properties of electrodes [8][9][10]. Since 2017 there has been a noticeable increase in publications investigating the electrolyte filling process itself, in addition to the development of new materials and the investigation of their wetting properties. Production-related issues are now also becoming a focus of scientific investigation.
Researchers mapped the distribution of the labeled contents in a diagram (cf. Figure 2) according to their frequency. From the inside out, the sub-labels became more and more specific. Several labels of a contribution were therefore represented in several different labelings, so the sum of all labels exceeded the total number of examined publications by far. Approximately half of the publications (24/52) were related to materials, while roughly 20–25% were related to process parameters (13/52) and other processes (15/52).
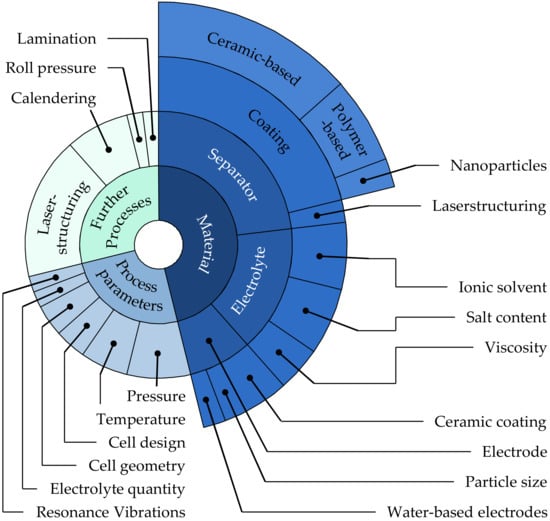
Figure 2. Representation of the labels and sub labels used in relation to their frequency of occurrence.
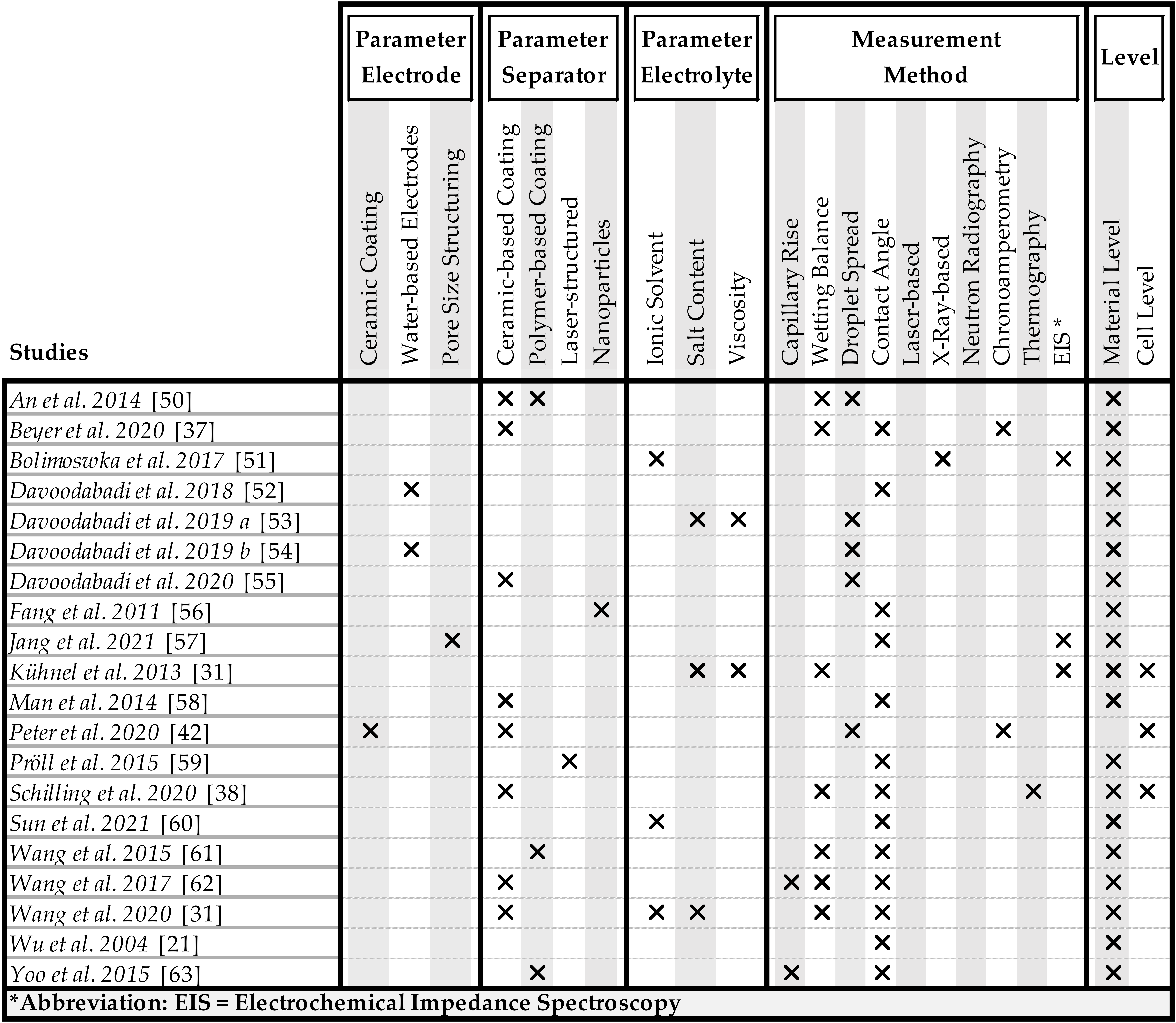
The category of Materials mainly referred to contributions that investigate the wetting behavior of different cell composite materials. The wetting behavior often characterized only one of many parameters used to dimension the cell composite materials (electrode, separator, and electrolyte). In most cases, however, the focus was on modifying the cell composite materials. The Process Parameters topic block included articles that specifically examined the electrolyte-filling process. Here, process parameters and certain product parameters such as cell design (hardcase vs. pouch cells) or cell geometry played a dominant role. The topic block of Further Processes dealt with research contributions on the influence of processes other than electrolyte filling on wetting. In addition to the divisions into topic blocks with corresponding sub-labels, it is also indicated which level the research addressed: the Material Level, which included analyses of individual materials of the cell stack without a cell composite present; or the Cell Level, where analyses were performed on assembled cell composites. Table 1, Table 2 and Table 3 below illustrate the categorization made of the research articles selected and analyzed in full text.
Table 2. Overview of the publications assigned to the Process Parameters category with a classification of the contents [11][16][30][31][32][33][34][35] [20][36].
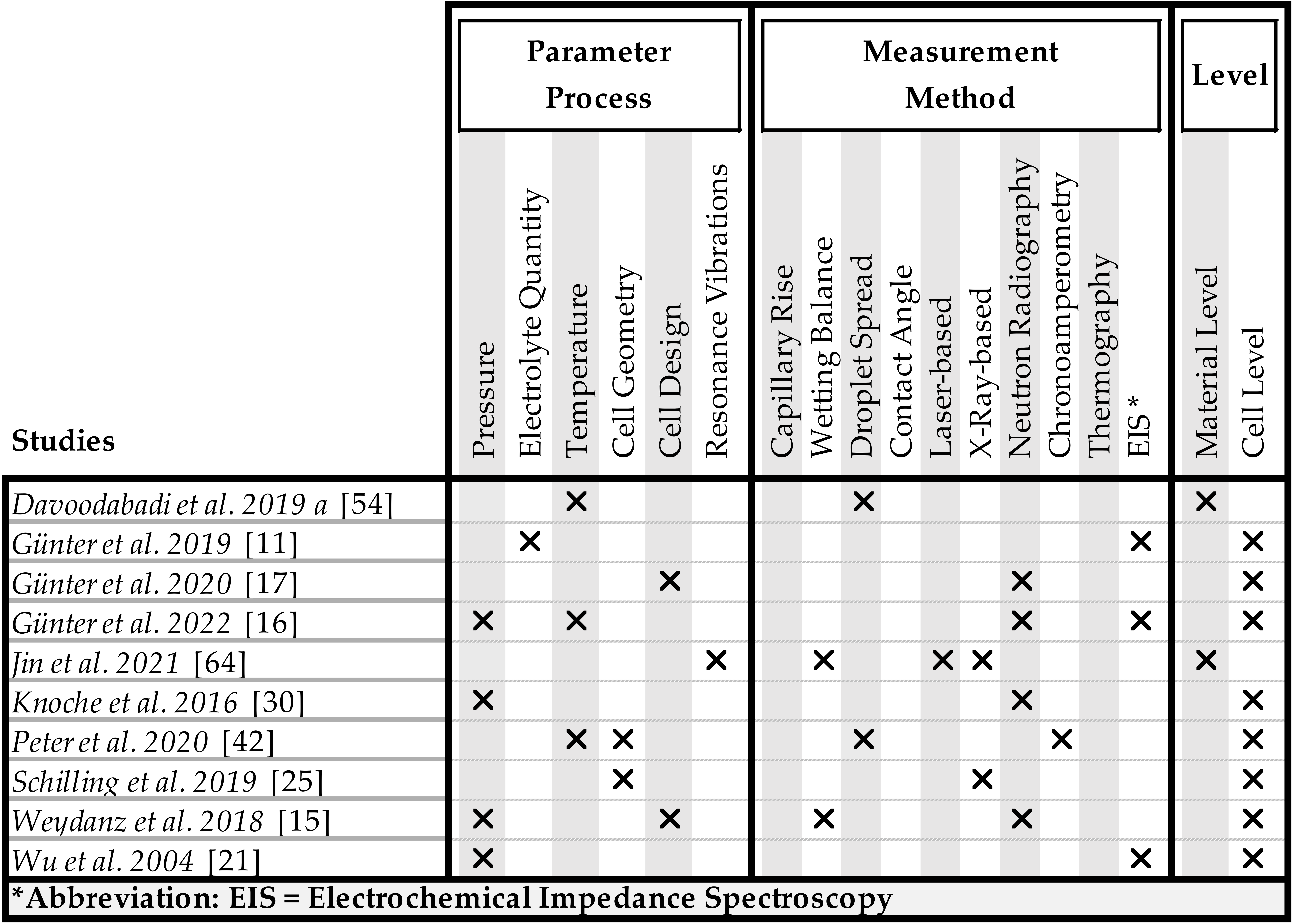 |
Table 3. Overview of publications assigned to the category Further Processes with a classification of the contents [7][9][16][18][20][31][37][38] [39][40][41][42][43][44].
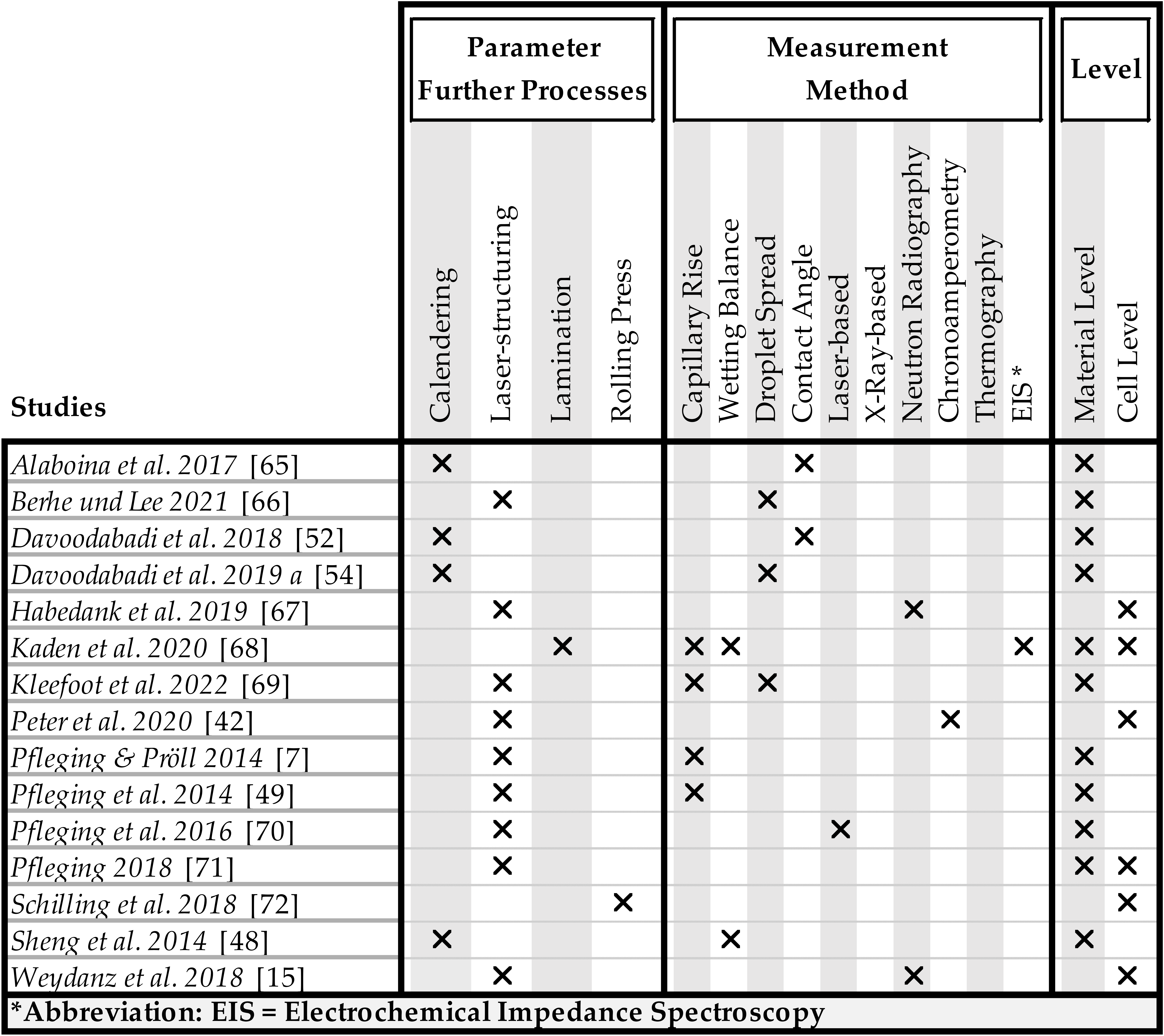 |
The analysis showed that a significant proportion of the publications focus on the wetting properties of materials at the material level, particularly the modification of poorly wettable polyolefin separators. These modifications in the majority of coatings made of ceramics or other polymers are intended to improve the properties of separators in general, such as thermal or mechanical durability. These changes to the surface often also result in an improvement in wetting ability, as the polar and disperse components of the free surface energies of these coatings better match those of the electrolyte solvents.
Research also focused on the modification of electrolyte composition, primarily involving carbonate-based electrolytes with LiPF6 as the conducting salt [12][13][20]. Only a few studies, such as the work of Davoodabadi et al., investigated specific variations in electrode formulation, using a water-based binder in their formulation [18][20]. Simple material characterization methods, such as the droplet spread test or contact angle measurement, were commonly used. In contrast, there was a lack of investigation into the transfer of findings to the cell level through cell performance tests, indicating a research gap.
The analysis also revealed several publications that addressed the main process parameters of temperature and pressure [20][31][32]. While temperature experiments were conducted at both the material and cell levels, they were never conducted in the same experiment, leaving a lack of information on the possible mutual influence of the process parameters on each other and the influence of different material parameters. Again, the material and cell levels were only investigated in isolation without analyzing the transfer of findings from the material level to the cell level.
The most commonly used measurement methods were electrochemical impedance spectroscopy and neutron radiography. Cell design and geometry also played a role in the dispensing and wetting conditions between pouch and hardcase cells. However, the evacuation process and the pressure it set were not fully considered in the literature. It is unclear whether the pressure applied on the process side also affects the pore system or just the dead volume of the cell. This is especially important for hardcase cells, which have narrow, coiled designs, where the diffusion paths for gas from the pores correspond to half the cell composite height.
The cell geometries discussed in the literature showed little variation. While the size and number of compartments may vary, the cell geometries often have similar aspect ratios with the same length-to-width ratio. With the trend in the automotive industry is towards blade cells for use in the cell-to-pack battery, the impact of varying the electrode area and shape, particularly the aspect ratio, has not been explored.
The analyzed literature covered various studies on upstream and downstream processes. Some contributions focused on the influence of calendering and laser structuring processes on the wetting properties of electrodes. Calendering, as the final process step in electrode manufacturing, significantly affects the pore morphology of the electrode. However, there is a lack of a comprehensive view that takes into account the influence of mixing, coating, and drying parameters on the resulting pore morphology in addition to calendering parameters. This would allow for more general conclusions to be drawn about the relationship between the electrode manufacturing process parameters, pore morphology, and wetting behavior of the electrode. Additionally, it would be useful to consider the materials used, as they have a major influence on both the resulting pore morphology and wetting properties.
Laser structuring is an additional or alternative process step in the manufacturing process chain that is used to improve ion diffusion into electrodes, such as for fast charging processes. However, this process step also leads to a decrease in specific volumetric capacity [39]. The introduced structures also provide additional channels for electrolyte to spread better in the direction of the electrode thickness and create punctual starting points for wetting in otherwise remote regions of the electrode. Many publications studied the influence of this process step on wetting at both the material and cell levels. Other articles occasionally addressed other processes, such as lamination of electrode-separator composites, or process improvements for electrolyte filling processes such as roll pressing, in which the electrolyte is distributed in the filled and sealed cell by a roller [40][44].
The bar chart in Figure 3 illustrates the frequency with which different measurement methods were used in the publications studied. It distinguishes between investigations at the cell level (in dark blue) and those at the material level (in light blue). While contact angle measurement and wetting balance tests are commonly used at the material level, electrochemical impedance spectroscopy and neutron radiography are most frequently used at the cell level. The choice of measurement method is often dictated by the level at which it operates; contact angle measurement can only be performed between a solid and a liquid, so it cannot be used to image the cell level. Similarly, chronoamperometry and electrochemical impedance spectroscopy require a complete cell, which precludes measurements on individual cell stack materials. Therefore, no dominant measurement method emerged.
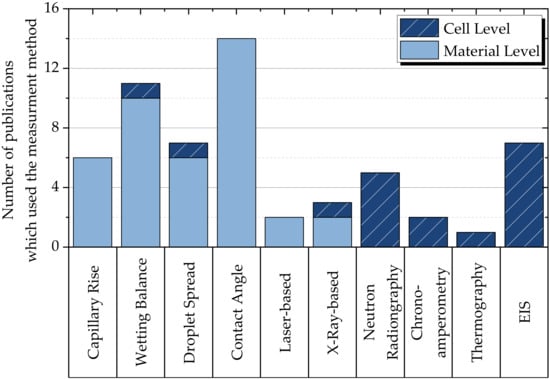
Figure 3. Illustration of the number of measurement methods used in the publications examined, with a classification of the measurements into material and cell levels.
References
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430.
- International Energy Agency. World Energy Outlook 2022; IEA: Paris, France, 2022.
- The European Parliament and the Council of the European Union. Regulation (EU) 2018/858 of the European Parliament and of the Council on the Approval and Market Surveillance of Motor Vehicles and Their Trailers, and of Systems, Components and Separate Technical Units Intended for Such Vehicles, amending Regulations (EC) No 715/2007 and (EC) No 595/2009 and Repealing Directive 2007/46/EC: Regulation (EU) 2018/858; The European Parliament and the Council of the European Union: Strasbourg, France, 2018.
- Kwade, A.; Haselrieder, W.; Leithoff, R.; Modlinger, A.; Dietrich, F.; Droeder, K. Current status and challenges for automotive battery production technologies. Nat. Energy 2018, 3, 290–300.
- Daniel, C. Materials and processing for lithium-ion batteries. J. Miner. Met. Mater. Soc. 2008, 60, 43–48.
- Wood, D.L.; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium ion batteries. J. Power Sources 2015, 275, 234–242.
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926.
- Sheng, Y.; Fell, C.R.; Son, Y.K.; Metz, B.M.; Jiang, J.; Church, B.C. Effect of Calendering on Electrode Wettability in Lithium-Ion Batteries. Front. Energy Res. 2014, 2, 56.
- Pfleging, W.; Kohler, R.; Pröll, J. Laser generated microstructures in tape cast electrodes for rapid electrolyte wetting: New technical approach for cost efficient battery manufacturing. In Laser-Based Micro- and Nanoprocessing VIII; Klotzbach, U., Washio, K., Arnold, C.B., Eds.; SPIE LASE: San Francisco, CA, USA, 2014; 89680B.
- An, M.-Y.; Kim, H.-T.; Chang, D.-R. Multilayered separator based on porous polyethylene layer, Al2O3 layer, and electro-spun PVdF nanofiber layer for lithium batteries. J. Solid State Electrochem. 2014, 18, 1807–1814.
- Wu, M.-S.; Liao, T.-L.; Wang, Y.-Y.; Wan, C.-C. Assessment of the Wettability of Porous Electrodes for Lithium-Ion Batteries. J. Appl. Electrochem. 2004, 34, 797–805.
- Kühnel, R.-S.; Obeidi, S.; Lübke, M.; Lex-Balducci, A.; Balducci, A. Evaluation of the wetting time of porous electrodes in electrolytic solutions containing ionic liquid. J. Appl. Electrochem. 2013, 43, 697–704.
- Wang, J.; Yuan, B.; Pan, F.; Qiao, L.; Guo, J.; Duan, C.; Wu, W.; Chen, Z.; Su, Y. Nano-silica-decorated Poly(m-Phenylene Isophthalamide) Separator with Enhanced Mechanical and Electrolyte Wetting Properties for Lithium-Ion Batteries. Trans. Tianjin Univ. 2020, 26, 256–264.
- Beyer, S.; Kobsch, O.; Pospiech, D.; Simon, F.; Peter, C.; Nikolowski, K.; Wolter, M.; Voit, B. Influence of surface characteristics on the penetration rate of electrolytes into model cells for lithium ion batteries. J. Adhes. Sci. Technol. 2020, 34, 849–866.
- Schilling, A.; Wiemers-Meyer, S.; Winkler, V.; Nowak, S.; Hoppe, B.; Heimes, H.H.; Dröder, K.; Winter, M. Influence of Separator Material on Infiltration Rate and Wetting Behavior of Lithium-Ion Batteries. Energy Technol. 2020, 8, 1900078.
- Peter, C.; Nikolowski, K.; Reuber, S.; Wolter, M.; Michaelis, A. Chronoamperometry as an electrochemical in situ approach to investigate the electrolyte wetting process of lithium-ion cells. J. Appl. Electrochem. 2020, 50, 295–309.
- Bolimowska, E.; Morales-Ugarte, J.E.; Rouault, H.; Santos-Peña, J.; Santini, C.; Benayad, A. Influence of the Vinylene Carbonate on the Wetting and Interface Chemical Structure of Doped Ionic Liquid Electrolyte at Porous Graphite Surface. J. Phys. Chem. C 2017, 121, 16166–16173.
- Davoodabadi, A.; Li, J.; Liang, Y.; Wang, R.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Characterization of Surface Free Energy of Composite Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2018, 165, A2493–A2501.
- Davoodabadi, A.; Li, J.; Liang, Y.; Wood, D.L.; Singler, T.J.; Jin, C. Analysis of electrolyte imbibition through lithium-ion battery electrodes. J. Power Sources 2019, 424, 193–203.
- Davoodabadi, A.; Li, J.; Zhou, H.; Wood, D.L.; Singler, T.J.; Jin, C. Effect of calendering and temperature on electrolyte wetting in lithium-ion battery electrodes. J. Energy Storage 2019, 26, 101034.
- Davoodabadi, A.; Jin, C.; Wood III, D.L.; Singler, T.J.; Li, J. On electrolyte wetting through lithium-ion battery separators. Extrem. Mech. Lett. 2020, 40, 100960.
- Fang, J.; Kelarakis, A.; Lin, Y.-W.; Giannelis, E.; Tsai, L.-D. Nanoparticle Coated Separators for Lithium-Ion Batteries with Advanced Electrochemical Performance. ECS Meet. Abstr. 2011, MA2011-02, 1456.
- Jang, D.; Suh, S.; Yoon, H.; Kim, J.; Kim, H.; Baek, J.; Kim, H.-J. Enhancing rate capability of graphite anodes for lithium-ion batteries by pore-structuring. Appl. Surf. Sci. Adv. 2021, 6, 100168.
- Man, C.; Jiang, P.; Wong, K.; Zhao, Y.; Tang, C.; Fan, M.; Lau, W.; Mei, J.; Li, S.; Liu, H.; et al. Enhanced wetting properties of a polypropylene separator for a lithium-ion battery by hyperthermal hydrogen induced cross-linking of poly(ethylene oxide). J. Mater. Chem. A 2014, 2, 11980–11986.
- Pröll, J.; Schmitz, B.; Niemöeller, A.; Robertz, B.; Schäfer, M.; Torge, M.; Smyrek, P.; Seifert, H.J.; Pfleging, W. Femtosecond laser patterning of lithium-ion battery separator materials: Impact on liquid electrolyte wetting and cell performance. In Laser-Based Micro- and Nanoprocessing IX; Klotzbach, U., Washio, K., Arnold, C.B., Eds.; SPIE LASE: San Francisco, CA, USA, 2015; 93511F.
- Sun, Y.; Wang, J.; Prausnitz, J.M. Interfacial properties between ionic-liquid-based electrolytes and lithium-ion-battery separator. AIChE J. 2021, 67, e17208.
- Wang, Z.; Guo, F.; Chen, C.; Shi, L.; Yuan, S.; Sun, L.; Zhu, J. Self-assembly of PEI/SiO2 on polyethylene separators for Li-ion batteries with enhanced rate capability. ACS Appl. Mater. Interfaces 2015, 7, 3314–3322.
- Wang, Y.; Wang, S.; Fang, J.; Ding, L.-X.; Wang, H. A nano-silica modified polyimide nanofiber separator with enhanced thermal and wetting properties for high safety lithium-ion batteries. J. Membr. Sci. 2017, 537, 248–254.
- Yoo, Y.; Kim, B.G.; Pak, K.; Han, S.J.; Song, H.-S.; Choi, J.W.; Im, S.G. Initiated Chemical Vapor Deposition (iCVD) of Highly Cross-Linked Polymer Films for Advanced Lithium-Ion Battery Separators. ACS Appl. Mater. Interfaces 2015, 7, 18849–18855.
- Günter, F.J.; Burgstaller, C.; Konwitschny, F.; Reinhart, G. Influence of the Electrolyte Quantity on Lithium-Ion Cells. J. Electrochem. Soc. 2019, 166, A1709–A1714.
- Weydanz, W.J.; Reisenweber, H.; Gottschalk, A.; Schulz, M.; Knoche, T.; Reinhart, G.; Masuch, M.; Franke, J.; Gilles, R. Visualization of electrolyte filling process and influence of vacuum during filling for hard case prismatic lithium ion cells by neutron imaging to optimize the production process. J. Power Sources 2018, 380, 126–134.
- Günter, F.J.; Keilhofer, J.; Rauch, C.; Rössler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Daub, R.; Reinhart, G. Influence of pressure and temperature on the electrolyte filling of lithium-ion cells: Experiment, model and method. J. Power Sources 2022, 517, 230668.
- Günter, F.J.; Rössler, S.; Schulz, M.; Braunwarth, W.; Gilles, R.; Reinhart, G. Influence of the Cell Format on the Electrolyte Filling Process of Lithium-Ion Cells. Energy Technol. 2020, 8, 1801108.
- Schilling, A.; Gümbel, P.; Möller, M.; Kalkan, F.; Dietrich, F.; Dröder, K. X-ray Based Visualization of the Electrolyte Filling Process of Lithium Ion Batteries. J. Electrochem. Soc. 2019, 166, A5163–A5167.
- Knoche, T.; Zinth, V.; Schulz, M.; Schnell, J.; Gilles, R.; Reinhart, G. In situ visualization of the electrolyte solvent filling process by neutron radiography. J. Power Sources 2016, 331, 267–276.
- Jin, D.; Kang, H.; Do, H.W.; Kim, G.; Kim, T.; Kim, S.; Choi, S.; Won, J.; Park, I.; Jung, K.; et al. Enhancing Li Ion Battery Performance by Mechanical Resonance. Nano Lett. 2021, 21, 5345–5352.
- Alaboina, P.K.; Cho, J.-S.; Cho, S.-J. Engineering and Optimization of Silicon-Iron-Manganese Nanoalloy Electrode for Enhanced Lithium-Ion Battery. Nanomicro. Lett. 2017, 9, 41.
- Berhe, M.G.; Lee, D. A Comparative Study on the Wettability of Unstructured and Structured LiFePO4 with Nanosecond Pulsed Fiber Laser. Micromachines 2021, 12, 582.
- Habedank, J.B.; Günter, F.J.; Billot, N.; Gilles, R.; Neuwirth, T.; Reinhart, G.; Zaeh, M.F. Rapid electrolyte wetting of lithium-ion batteries containing laser structured electrodes: In situ visualization by neutron radiography. Int. J. Adv. Manuf. Technol. 2019, 102, 2769–2778.
- Kaden, N.; Schlüter, N.; Leithoff, R.; Savas, S.; Grundmeier, S.; Dröder, K. Influence of the Lamination Process on the Wetting Behavior and the Wetting Rate of Lithium-Ion Batteries. Processes 2021, 9, 1851.
- Kleefoot, M.-J.; Enderle, S.; Sandherr, J.; Bolsinger, M.; Maischik, T.; Simon, N.; Martan, J.; Ruck, S.; Knoblauch, V.; Riegel, H. Enhancement of the wettability of graphite-based lithium-ion battery anodes by selective laser surface modification using low energy nanosecond pulses. Int. J. Adv. Manuf. Technol. 2022, 118, 1987–1997.
- Pfleging, W.; Zheng, Y.; Mangang, M.; Bruns, M.; Smyrek, P. Laser processes and analytics for high power 3D battery materials. In Frontiers in Ultrafast Optics: Biomedical, Scientific, and Industrial Applications XVI; Heisterkamp, A., Herman, P.R., Meunier, M., Nolte, S., Eds.; SPIE LASE: San Francisco, CA, USA, 2016; p. 974013.
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573.
- Schilling, A. Design of an Automated System to Accelerate the Electrolyte Distribution in Lithium-Ion Batteries. Int. J. Mech. Eng. Robot. Res. 2018, 8, 162–166.
More
Information
Subjects:
Engineering, Manufacturing
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No