Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eleonora Barilli | -- | 3860 | 2023-03-23 10:34:51 | | | |
| 2 | Jason Zhu | Meta information modification | 3860 | 2023-03-24 02:53:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Agudo-Jurado, F.J.; Reveglia, P.; Rubiales, D.; Evidente, A.; Barilli, E. Phytotoxic Metabolites from Necrotrophic Pathogenic Fungi. Encyclopedia. Available online: https://encyclopedia.pub/entry/42463 (accessed on 07 February 2026).
Agudo-Jurado FJ, Reveglia P, Rubiales D, Evidente A, Barilli E. Phytotoxic Metabolites from Necrotrophic Pathogenic Fungi. Encyclopedia. Available at: https://encyclopedia.pub/entry/42463. Accessed February 07, 2026.
Agudo-Jurado, Francisco J., Pierluigi Reveglia, Diego Rubiales, Antonio Evidente, Eleonora Barilli. "Phytotoxic Metabolites from Necrotrophic Pathogenic Fungi" Encyclopedia, https://encyclopedia.pub/entry/42463 (accessed February 07, 2026).
Agudo-Jurado, F.J., Reveglia, P., Rubiales, D., Evidente, A., & Barilli, E. (2023, March 23). Phytotoxic Metabolites from Necrotrophic Pathogenic Fungi. In Encyclopedia. https://encyclopedia.pub/entry/42463
Agudo-Jurado, Francisco J., et al. "Phytotoxic Metabolites from Necrotrophic Pathogenic Fungi." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Fungal phytotoxins can be defined as secondary metabolites toxic to host plants and are believed to be involved in the symptoms developed of a number of plant diseases by targeting host cellular machineries or interfering with host immune responses. As any crop, legumes can be affected by a number of fungal diseases, causing severe yield losses worldwide.
necrotrophic fungi
fungal phytotoxins
secondary metabolites
1. Phytotoxic Compounds Produced by Ascochyta spp.
Ascochyta species cause diseases globally called Ascochyta blights, whose symptoms typically develop in the aerial parts of the plants under high humidity and average temperature conditions, producing necrotic lesions on the leaves and stems [1]. Leaves with many lesions wither before the lesions become large, especially on the lower portion of the plants. On the stems, these fungi cause deep necrotic lesions that can lead to the breaking of stems and the death of plant parts above the affected zone. Infected grains and pods can spread disease through seeds, causing their use in the following crops to be harmful since they can drown growing plants. Ascochyta blights are incited by different pathogens in the various legumes such as Ascochyta lentis in lentil; A. pisi and A. pinodes in pea; A. lentis var. lathyri in grass pea; A. fabae in faba bean; and A. rabiei in chickpea [2]. Ascochyta blight remains an extremely difficult pathogen to control, primarily due to the limited levels of host resistance available, and secondarily, because fungicides are often uneconomic [3], forcing the integration of the use of genetic resistance with cultural practices. Therefore, the main disease control strategy has been to avoid sowing close to infested field stubbles and/or to delay the sowing of field crops for as long as possible. This minimizes inoculum carry-over and its survival on crop residues and in soil, avoiding the initial infection of the crop from aerial inoculum arising from infested residues [4][5][6]. Nevertheless, late sowing is not an option in some countries due to the short crop season, and this practice incurs unsustainable yield penalties in many instances.
Different metabolites with pathogenesis-determining cytotoxic capacity have been reported in several Ascochyta species. For instance, 10 metabolites have been isolated and identified from A. lentis, being lentiquinones A–C, lentisone, ω-hydroxypachybasin, 1,7-dihydroxy-3-methylanthracene-9,10-dione, phomarin, pachybasin, tyrosol, and pseurotin A (1–10, Figure 1). However, between them, only compounds 1–4, 8, and 9 showed phytotoxic activity on the lentil plants, being also capable of reducing the root growth and seed germination; in contrast, the reported activity of metabolites 5–7 was almost null in the bioassay condition [7][8], and the role they play in plant–pathogen interaction is not well-defined yet [9][10].
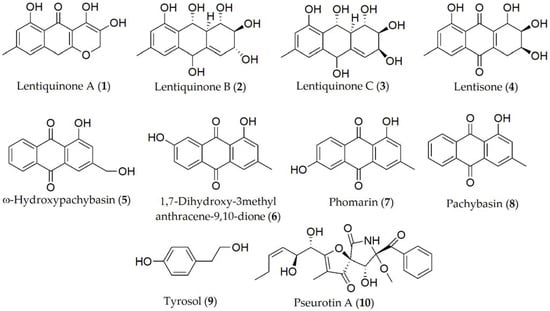
Figure 1. Phytotoxins isolated from Ascochyta lentis.
The so-called Ascochyta blight of peas is in fact a disease complex that can be caused by several fungi including A. pisi, A. pinodes, and Phoma medicaginis. Compounds described so far in A. pinodes with noteworthy phytotoxic activity have been pinolidoxin, 7-epi-pinolidoxin, 5,6-dihydropinolidoxin, 5,6-epoxypinolidoxin, herbarumin II, 2-epi-herbarumin II, and pinolide (11–17, Figure 2) [11][12][13]. Between them, pinolidoxin (11) showed the highest phytotoxic activity measured in pea plants such as lesion size (mm2) on both the pods and in leaves as well as in other grain legumes such as faba bean. In contrast, the other metabolites found only produced reduced symptoms [11][13]. When the compounds were tested on other legumes, it was observed that herbarumin II, 2-epi-herbarumin II, and pinolide (15 and 17) did not display significant phytotoxic activity. The importance of the stereochemistry of the hydroxy group at C-7 of these compounds on phytotoxicity was deduced by the authors [13].
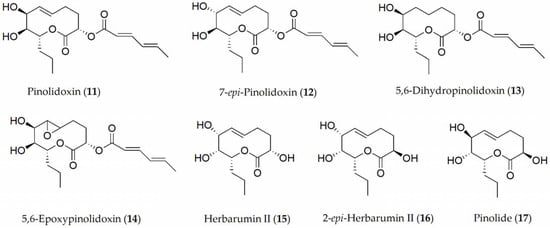
Figure 2. Phytotoxins isolated from Ascochyta pinodes.
Ascosalitoxin (18, Figure 3), which is a derivative of salycilic aldehyde, was isolated as the main phytotoxin from A. pisi [14]. Ascosalitoxin (18) displayed phytotoxic activity on pea and faba bean leaves and pods, and on tomato seedlings [15]. Ascochitine, an o-quinone methide, is an abundantly produced phytotoxin that was first discovered in culture extracts of A. pisi [16], and later in A. fabae [17] (19, Figure 3), where it displayed antibiotic activity.
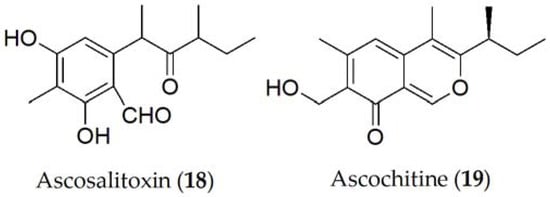
Figure 3. Phytotoxins isolated from Ascochyta pisi.
More recently, ascochitine (19, Figure 3) was found in the culture extracts of many wild vetch-infecting Ascochyta and Ascochyta-like species including A. viciae-villosae [17]. The widespread distribution of ascochitine (19) production indicates its ancient origin in these related taxa. Ascochitine (19) production is not restricted to the legume-associated Ascochyta species, but also to some Phoma species. In phytotoxicity studies performed on faba bean plants, ascochitine (19) was shown to produce electrolyte leakage when tested on leaf discs as well as necrosis and wilting in whole plants assays [18].
Concerning A. lentis var. lathyri, the compounds described have been lathyroxins A and B, p-hydroxybenzaldehyde, p-methoxyphenol (20–23, Figure 4), and tyrosol (9, Figure 1) [19]. The latter has also been isolated in A. pinodes [7]. Lathyroxin B (21) showed activity in a panel of legumes tested including lupine, lentil, and beans. In contrast, lathyroxin A (20) only showed activity on lupin and Sonchus oleraceus, while p-hydroxybenzaldehyde (22) was toxic only on lupin and lentil [19].
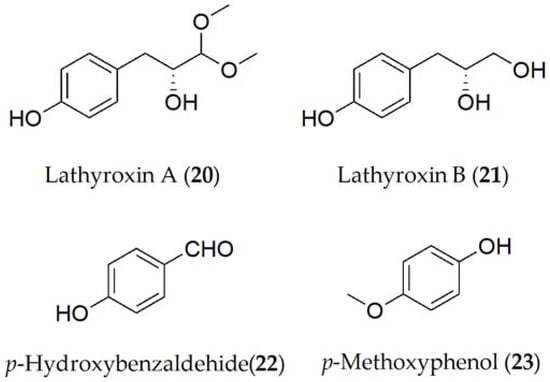
Figure 4. Phytotoxins isolated from Ascochyta pisi var. lathyri.
Finally, compounds described for Ascochyta rabiei were solanapyrone A [20], solanapyrone B [21], solanapyrone C [20], and cytochalasin D [22][23] (24–27, Figure 5). The solanapyrones A–C (24–26) are structural isomers that act as HSTs [13]. When tested on whole plant assays in chickpea plants, solanapyrones A, B, and C were shown to be active individually as well as in combination, being able to reduce root development as well as the seed germination [24] of the host plant.
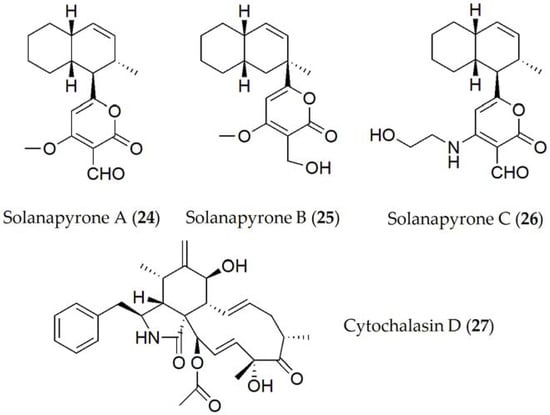
Figure 5. Phytotoxins isolated from Ascochyta rabiei.
Structure–toxicity relationship studies of phytotoxins produced by Ascochyta spp.
Although a significant number of compounds produced by Ascochyta spp. have been identified, only a few toxicity relationship studies have been carried out with them. For example, the nonenolide pinolidoxin (11) is the main phytotoxin produced by both Ascochyta spp., which is closely related to putaminoxin, having a similar nonenolide ring system and some substituent groups. Phoma putaminum is a fungus proposed for the biocontrol of the dangerous weed Erigeron annus. The two nonenolides, pinolodoxin and putaminoxin, along with some of their natural analogues and some hemisynthetic derivatives, were assayed for their phytotoxic, antifungal, and zootoxic activities. The results obtained by testing all of the compounds on the weeds and crops showed that the phytotoxic activity was related to the integrity of the nonenolide ring, to the presence of two hydroxyl groups, and to an unmodified propyl side chain. Likewise, pinolidoxin (11) was detected in A. pinodes in front of the hyphae as it developed, which suggests that this compound has a fundamental role in modulating the defense response in plants [25]. Furthermore, among a set of phytotoxins with different carbon skeletons and produced by different pathogenic fungi, only the nonenolides pinolidoxin and putaminoxin appeared to be inhibitors of the first steps in the phenylpropanoid pathway. This is the route in charge of generating compounds such as phytoalexins or lignin in the defense against parasitic attacks [26].
Structure–activity relationship studies have been also performed with compounds belonging to the Solanapyrone group (24–26). In fact, solanapyrone A (24) was shown to be active against Bacillus subtilis and Micrococcus tetragenus, in addition to certain saprobe fungi [27], while solanapyrone C (26) only acted against B. megaterium, and a unicellular alga [27][28]. Solanapyrone A (24) binds specifically to DNA polymerases [29], acting in cell control during mitosis and meiosis, postulating that this compound could inhibit DNA repair processes, unbalancing the cell cycle, and finally causing apoptosis; or by affecting defense signaling induced by DNA damage and the subsequent repair process [30][31].
2. Phytotoxic Compounds Produced by Botrytis spp.
Chocolate spot can be elicited by both pathogens, Botrytis fabae and B. cinerea, but B. fabae is more harmful to faba bean [32]. Chocolate spot is an important disease, having a worldwide distribution and causing a series of dark brown spots on the aerial parts of plants [33]. When the humidity reaches high levels and there is an average temperature of around 22 °C, the fungus begins an aggressive phase where it spreads very quickly, significantly increasing the number of necrotic spots and withering the plant completely in a period of two days in some cases [34]. When the plant is affected during the flowering period, the flowers fall, decreasing the final yield production and favoring the spread of the fungus to the lower parts of the host or to other neighboring plants. In addition, if the pathogen affects the pod during its formation, the seed quality decreases, and often, their commercialization is unviable [35].
The pathogen should be limited by applying both agronomic control including removing the crop infected remains and their destruction as well as by chemical control techniques through the utilization of fungicides with different chemical characteristics such as benzimidazoles (benomyl, carbendazim) or dithiocarbamates (mancozeb) among others [5][36]. However, these methods are usually very costly, which is why other strategies have been formulated such as the use of resistant varieties obtained through the development of plant breeding programs. Although in the last 20 years some resistant materials have been described [37], more effort is needed in order to incorporate resistance into commercial varieties as well as testing the stability of sources of resistance through time and space [38].
Reported compounds produced by B. fabae are botrytone, regiolone, cis-2,4,8- and trans-2,4,8-trihydro-1-tetralone, (4S)-(+)-isosclerone, scytalone, and 3-hydroxyjuglone (28–34, Figure 6). Out of these metabolites, botrytone (28) has shown some phytotoxicity on the host plant, with regiolone, cis-2,4,8, and trans-2,4,8 trihydro-1-tetralone (29–31) being the most toxic [39].
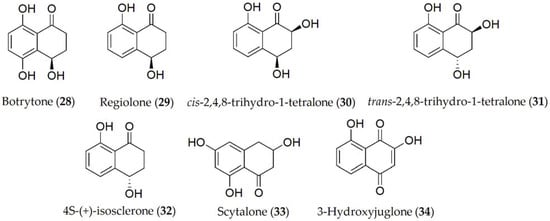
Figure 6. Phytotoxins isolated from Botrytis fabae.
Because of the diverse host range that B. cinerea presents, most of the phytotoxins that have been identified from this fungus have been extracted from other hosts including legumes such as the common bean (P. vulgaris), or other different species such as sweet pepper (Capsicum annuum), although later tests have been extrapolated to legumes. Compounds identified in B. cinerea (Table 1) include botrydial, botryendial, botrydienal, 8,9-epi-botrydial, 1-epi-botrydial, dihydrobotrydial, norbotrydialone acetate; 10-oxodihydrobotry-1(9),4(5)-diendial, 10-oxodehydrodihydrobotrydial, 4β-acetoxytetrahydrobotryslactone, botryenalol, β-O-methyldihydrobotridialone, botrylactone, botcinic acid, 3-acetylbotcinic acid, and botcinin A (35–50, Figure 7). Out of these compounds, botrytone, regiolone, cis- and trans-2,4,8-trihydroxy-1-tetralone botrydial, botryendial, botrydienal, 8,9-epi-botrydial, and 4β-acetoxytetrahydrobotryslactone (28–31; 35–38, and 44) showed phytotoxic activity when tested on the host both in the cut leaf and in the whole plant. Bioassays using B. cinerea mutants deficient in the production of botrydial and botcinin A (35 and 50) showed no reduction in pathogenicity, being capable of damaging cells of the host plant tissue. Moreover, a marked lower virulence of the fungus was demonstrated [40]. Phytotoxic activity of some metabolites such as botrydial (35) has been shown to be influenced by external factors such as light intensity [41].
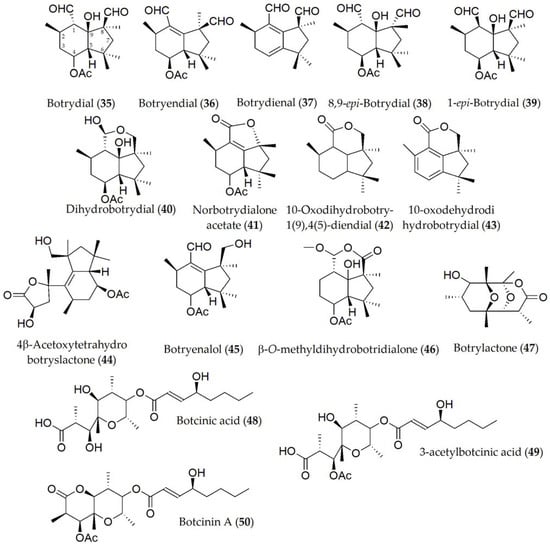
Figure 7. Phytotoxins isolated from Botrytis cinerea.
Structure–toxicity relationship studies of Phytotoxins from Botrytis spp.
Botrydial (35) was the only phytotoxin produced by Botrytis cinerea for which structure–activity relationship studies were conducted. This compound induces a hypersensitive response in the host, which is regulated via the salicylic acid and jasmonic acid pathways [42]. It has been shown that the activity of this compound and its epimers is closely related to the C-1 and C-8 carbons, depending on the oxidation states of the aldehyde substituents as well as the C-9 carbon, observing a lower activity, for example, in botryendial and botrydienal (36 and 37) with respect to botrydial (35). Likewise, the configuration (S) at the C-1 carbon has been observed to be critical in the substrate–receptor role [43].
3. Phytotoxic Compounds Produced by Macrophomina spp.
The management of this disease is quite complex, since an integrated approach is necessary to reduce the number of viable spores in the soil or in the material that is used, sowing clean seeds or adopting crop rotations with resistant material, because fungicides are not fully effective against the pathogen [44][45]. A recent meta-analysis on biological control methods highlighted that Trichoderma gamsii, Gliocladium virens, Trichoderma viride, and Pseudomonas fluorescence have a higher control efficiency [46]. Nevertheless, the search for genetic resistance in the crop is still scarce [47].
Phytotoxic metabolites produced by M. phaseolina have been described including phaseolinone, (-)-botryodiplodin, phaseocyclopentenones A and B, and guignardone A [48][49][50] (51–55, Figure 8). Phaseolinone (51) and (-)-botryodiplodin (52) are believed to play a role in the initial stages of infection, causing the wilting of seedlings and the formation of necrotic lesions on the leaves and roots [48]. This increases the virulence of M. phaseolina and may help to explain the highly efficient mechanism to infect different hosts and tissues. Likewise, although it has not yet been specified in a concrete way, it is speculated that the variation in the production of (51) and (52) between different isolates may be due to the geographical variation in the isolates due to different environmental conditions or the production and interaction with other phytotoxins [49].
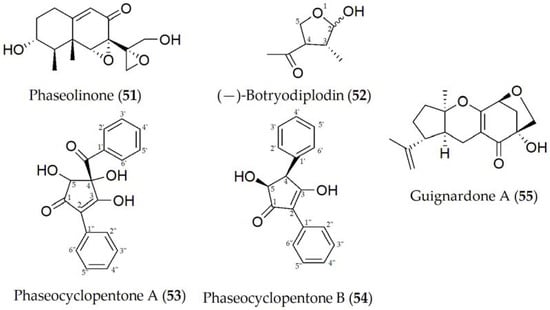
Figure 8. Phytotoxins isolated from Macrophomina phaseolina.
Phaseocyclopentenones A and B (53 and 54) were recently described together with guignardone A (56), extracted from a strain of M. phaseolina isolated in infected soybean tissues from Argentina. Compounds 53–55 showed phytotoxic activity assayed on tomato plants, used as a non-host control by the leaf puncture assay, while only 53 and 54 were toxic when tested on cuttings of the same plant. No antifungal activity was detected for the three metabolites against some fungal pathogens such as Cercospora nicotianae and Colletotrichum truncatum, which are two severe pathogens both isolated from infected soybean plants in Argentina [50].
Structure–toxicity relationship studies of phytotoxins from Macrophomina spp.
Phaseolinone (51) is considered a mutagenic compound due to its primary and secondary alcoholic groups. When these hydroxyl groups are modified, a reduction in mutagenic activity is observed when one of them is replaced (with a ketone group), and the complete loss of this activity when a complete substitution of the hydroxyl groups is performed. In the same way, a reduction in the toxicity of the molecule was also observed as more hydroxyl groups are substituted, suggesting that side-chain epoxide and alcoholic groups are essential for its activity [51]. Additionally, (-)-botryodiplodin (52), which is a natural analogue of ribose, interferes with different cellular mechanisms such as transporters or enzymes, although it is not entirely clear which. An example of this interference may be the absence of a hydroxyl group at C-5, causing it not to be an optimal substrate for the ribose 5-kinase enzyme, or it may exert another function when present in the cell cytoplasm in its phosphorylated form [52].
4. Phytotoxic Compounds Produced by Colletotrichum spp.
Species of the anamorphic genus Colletotrichum (teleomorph Glomerella) are implicated in plant diseases, generally referred to as anthracnoses, which are found throughout the world. The various Colletotrichum species include some of the most destructive post-harvest pathogens that can affect a multitude of hosts including cereals, legumes, fruits, and vegetables [53]. Colletotrichum spp. can survive for several years on plant debris that remains in the field after harvest [54]. The pathogen requires more than 16 h of leaf wetness in combination with temperatures between 20 and 30 °C to infect the host plant [53]. Initial symptoms on leaves are small yellow spots that enlarge into brown-colored lesions with a distinct dark margin. This might result in premature leaf drop.
C. truncatum is the main causal agent of soybean anthracnose, which is characterized by pre- and postemergence damage on cotyledons, pods, petioles, and stems. The metabolites produced by C. truncatum are colletruncoic acid methyl ester and meso-2,3-butane-2,3-diol (56–57, Figure 9), together with the isomers of 57, which are (2R,3R)-butane-2,3-diol and (2S,3S)-butane-2,3-diol (58–59, Figure 9), although no phytotoxic activity has yet been determined [55][56]. Recently, a bioactive disubstituted nonenolide, named truncatenolide (60), and a new trisubstituted oct-2-en-4-one, named truncatenone (61), and the well-known tyrosol and N-acetyltyramine (9 and 62) have also been described (Figure 9).
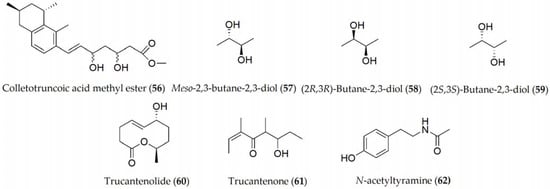
Figure 9. Phytotoxins isolated from Colletotrichum truncatum.
Truncatenolide (60) showed the strongest phytotoxic activity when tested on soybean seeds while tyrosol and N-acetyltyramine (9 and 62) exhibited phytotoxicity to a lesser extent. Furthermore, truncatenone (61) weakly stimulated the growth of the seed root in the condition tested [57]. When the same metabolites were assayed against M. phaseolina and C. nicotianae, truncatenolide (60) showed significant antifungal activity against M. phaseolina and the total inhibition of C. nicotianae. Thus, some other fungal nonenolides and their derivatives were assayed for their antifungal activity against both fungi in comparison with truncatenolide for a structure–activity relationship study.
Lupindolinone, lupinlactone, (3R)-mevalonolactone, and tyrosol (63–65 and 9, Figure 10) were isolated from Colletotrichum lupini, which is the causal agent of anthracnose in lupin (Lupinus albus) [58]. When these metabolites were tested for their toxicity through different experiments including the effect on root elongation in cress (Nasturtium officinale), lupine and duckweed (Lemma minor) leaves, or on the seed germination of parasitic plants such as broomrape (Phelipanche ramosa), only lupinlactone (64) and tyrosol (9) showed the greatest activity out of all of them [58].
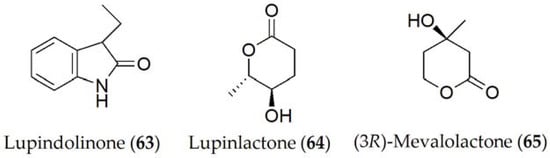
Figure 10. Phytotoxins isolated from Colletotrichum lupini.
Colletotrichin and colletopyrone (66 and 67, Figure 11) have been isolated from Colletotrichum lindemuthianum, the causal agent of anthracnose on common bean (Phaselous vulgaris). The exudate filtrates from the fungal culture have been shown to cause necrotic spots on common bean leaves [59] and to inhibit the seed germination of cowpea (Vigna unguiculata), soybean (Glycine max), maize (Zea mays), sorghum (Sorghum spp.), and millet (Panicum miliaceum) [60].
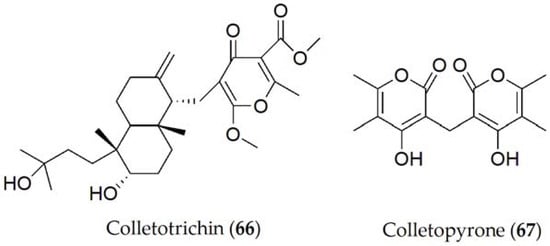
Figure 11. Phytotoxins isolated from Colletotrichum lindemuthianum.
Structure–toxicity relationship studies of phytotoxins from Colletotrichum
A structure–activity relationship study was carried out using truncatenolide and pinolidoxin, 7-epi-pinolidoxin, 7,8-O,O′-diacetylpinolidoxin [11], stagonolide C [61], modiolide A, and stagonolide H [62]. The last three nonenolides were obtained from Stagonospora cirsii and were previously proposed as a mycoherbicide to the biocontrol of Cirsium arvense and Sonchus arvensis, which are two common weeds limiting the growth of several cereal cultures. Among all of the tested nonenolides, pinolidoxin (11) showed low antifungal activity against both fungi, while modiolide A selectively and totally inhibited only the growth of C. nicotianae. These results show that their activity could be linked to the nonenolide ring [57].
5. Phytotoxic Compounds Produced by Cercospora spp.
Cercospora is a genus of fungi that causes pink-violet spots on the seeds and spreads as the plant develops [63], penetrating through the stomata of the leaf surface and colonizing the intercellular spaces. Initially, the necrotic red-violet lesions mainly affect the leaves, expanding rapidly to coalesce with adjacent lesions, resulting in severe blighting of the leaves, and conidia protrude in fasciculate bundles in moist conditions from the center. The symptoms were often confused with those developed from the fungi of the genus Ascochyta [64]. The spores can be dispersed by environmental agents such as rain or wind, although these can flourish in later or nearby crops if the infected remains are not removed. Environmental conditions such as high humidity and warm temperature are required for spore germination and fungal development [63]. Crop rotation, the usage of resistant varieties, and seeds treated to suppress spore development are useful practices applied to control the pathogen.
Cercosporin (68, Figure 12) is the only compound elucidated by Cercospora kikuchii [65]. Cercosporin (68) is a non-specific phytotoxin, which is reported to have a role in the pathogenicity of the fungus, as it has been tested on different hosts (such as Ricinus communis or Phaseolus vulgaris among others), causing chlorosis and necrosis in most of them [66]. Additionally, the production of cercosporin (68) varied between different species or strains, being produced through a polyketide pathway and regulated by the calcium/calmodulin complex or mitogen-activated protein kinase signaling pathways (MAPKs). Likewise, this production is also affected by many other physiological and environmental factors such as the availability of nutrients, the ratio between C:N, and the amount of light or temperature [67].
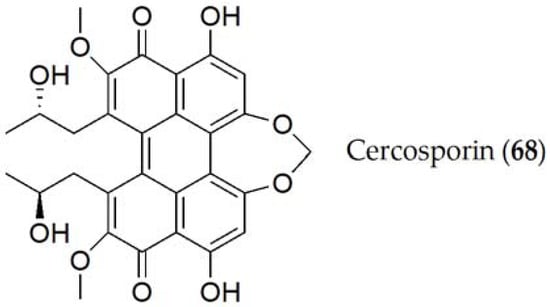
Figure 12. Phytotoxins isolated from Cercospora kikuchii.
Structure–toxicity relationship studies of phytotoxins from Cercospora spp.
Cercosporin (68) has been classified as a photosensitizer, since the phytotoxicity of this compound depends on the intensity of light. This compound reacts with light, producing free radicals and active oxygen species, particularly singlet oxygen. These reactive compounds are those that induce degradation in the acids of the cell wall of the host, thus increasing the virulence of the disease [68].
6. Phytotoxic Compounds Produced by Fungi Pleiochaeta setosa
Brown spot disease induced by Pleiochaeta setosa can affect legumes in general, although its most common host is lupine (L. albus), producing a disease known as brown spot disease, characterized by the appearance of brown spots on the aerial parts of plants such as the leaves and stems and can even affect the root, causing leaf necrosis and finally total wilt [69]. Although there are chemical methods of control including fungicide application, their use has not been proven effective yet. Instead, there are effective physical methods such as the use of heat and low humidity to sterilize seeds [70].
Setosol (69, Figure 13) is the only compound produced by P. setosa, although its triacetylated derivative (70, Figure 13) has also been isolated in lesser quantity [71]. Setosol (69) was tested on four lupine variants against an unpurified fungal extract of P. setosa and it was observed that the same lesions occurred as an infection caused by the fungus, which led to the conclusion that this is the compound responsible for pathogenicity in the host [71]. However, when setosol is acetylated (70), the molecule significantly loses its effectiveness [72].
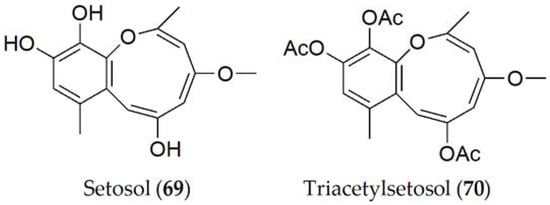
Figure 13. Phytotoxins isolated from Pleiochaeta setosa.
Structure–toxicity relationship studies of phytotoxins from Pleiochaeta spp.
It has been shown that the toxicity of setosol (69) is related to the hydroxyl groups of the carbons in positions 6, 10, and 11. Setosol (69) has been shown to be an unstable molecule, so natural acetylation increases its stability. However, as this acetylation increases, its phytotoxic activity decreases. After studying the phytotoxicity of these compounds and their structure, it has been hypothesized that the introduction of chlorine and bromine at these sites may increase the activity of the molecule [72].
7. Phytotoxic Compounds Produced by Sclerotinia spp.
Sclerotinia disease can cause serious yield loss and seed quality problems. This genus of fungi is characterized by the formation of an apothecium in which ascospores are formed, thus differing in the regulation of sexual reproduction [73]. Symptoms are similar to those induced by Botrytis cinerea, starting with white and hairy mycelium developing in the aerial parts of the plant that later darken and harden, being more common during the inflorescence period. Later, when the wilted parts fall to the ground or are handled during the farming operations, the spores spread through the soil, favoring the beginning of a new infection cycle [74]. The effective control of this disease depends on various factors such as irrigation, avoiding an excess of water, and the application of fungicides, which are more effective when applied in the full bloom of primary inflorescences. Among the tested fungicides, benomyl, thiophanate methyl, and vinclozolin prevented the appearance of symptoms in leaf tissue on greenhouse-grown soybean plants. Furthermore, vinclozolin was also effective in reducing the mycelium growth of the pathogen Botrytis cinerea when added to PDA culture medium [75][76].
Ethanedioic acid, better known as oxalic acid (71, Figure 14), is the only metabolite reported for Sclerotinia sclerotiorum [77]. It acts during the pathogenesis process of the fungus by breaking the host cell wall, maximizing the efficacy of the different enzymes produced by the pathogen [78]. Its cytotoxic activity has been tested on sunflower and tomato, showing its involvement during the process, but its role against other crops still needs to be evaluated [79].
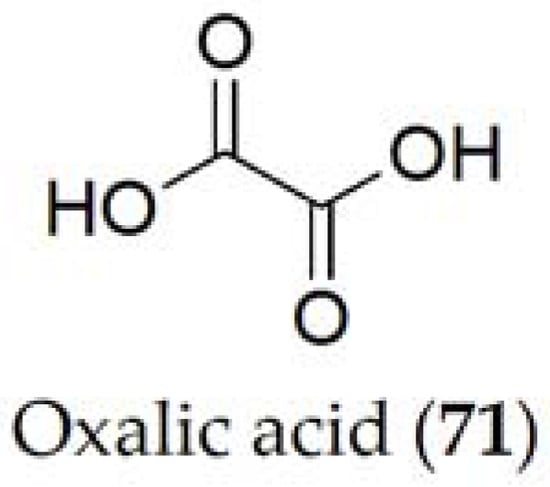
Figure 14. Phytotoxin isolated from Sclerotinia sclerotiorum.
In studies related to oxalic acid (71), it has been observed that it is a determining compound in the pathogenicity of the Sclerotinia fungus, seeing that increasing production of this compound causes greater damage to the host [77]. Likewise, it has been seen that this compound is metabolized with oxygen and carbon dioxide from the medium at the time of pathogenicity. However, the pathogenicity of oxalic acid (72), generated through the glyoxylate acid pathway [80], is closely related to its ability to manipulate enzymes involved in the plant defense mechanism. Alteration of these processes reduces the pathogenicity because the fungus is not able to extract nutrients involved in plant colonization [81].
References
- Shahid, A.A.; Husnain, T.; Riazuddin, S. Ascochyta blight of chickpea: Production of phytotoxins and disease management. Biotechnol. Adv. 2008, 26, 511–515.
- Rubiales, D.; Fondevilla, S. Future prospects for ascochyta blight resistance breeding in cool season food legumes. Front. Plant Sci. 2012, 3, 27.
- Tivoli, B.; Banniza, S. Comparison of the epidemiology of ascochyta blights on grain legumes. Eur. J. Plant. Pathol. 2007, 119, 59–76.
- Gan, Y.T.; Siddique, K.H.M.; MacLeod, W.J.; Jayakumar, P. Management options for minimizing the damage by ascochyta blight (Ascochyta rabiei) in chickpea (Cicer arietinum L.). Field Crops Res. 2006, 97, 121–134.
- Stoddard, F.L.; Nicholas, A.H.; Rubiales, D.; Thomas, J.; Villegas-Fernández, A.M. Integrated pest management in faba bean. Field Crops Res. 2010, 115, 308–318.
- Khan, T.N.; Timmerman-Vaughan, G.M.; Rubiales, D.; Warkentin, T.D.; Siddique, K.H.M.; Erskine, W.; Barbetti, M.J. Didymella pinodes and its management in field pea: Challenges and opportunities. Field Crops Res. 2013, 148, 61–77.
- Andolfi, A.; Cimmino, A.; Villegas-Fernández, A.M.; Tuzi, A.; Santini, A.; Melck, D.; Rubiales, D.; Evidente, A. Lentisone, a new phytotoxic anthraquinone produced by Ascochyta lentis, the causal agent of Ascochyta Blight in Lens culinaris. J. Agric. Food Chem. 2013, 61, 7301–7308.
- Masi, M.; Nocera, P.; Zonno, M.C.; Tuzi, A.; Pescitelli, G.; Cimmino, A.; Boari, A.; Infantino, A.; Vurro, M.; Evidente, A. Lentiquinones A, B, and C, phytotoxic anthraquinone derivatives isolated from Ascochyta lentis, a pathogen of lentil. J. Nat. Prod. 2018, 81, 2700–2709.
- Duval, J.; Pecher, V.; Poujol, M.; Lesellier, E. Research advances for the extraction, analysis and uses of anthraquinones: A review. Ind. Crops Prod. 2016, 94, 812–833.
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64.
- Evidente, A.; Lanzetta, R.; Capasso, R.; Vurro, M.; Botralico, A. Pinolidoxin, a phytotoxic nonenolide from Ascochyta pinodes. Phytochemistry 1993, 3, 999–1003.
- Evidente, A.; Capasso, R.; Abouzeid, M.A.; Lanzetta, R.; Vurro, M.; Bottalico, A. Three new toxic pinolidoxins from Ascochyta pinodes. J. Nat. Prod. 1993, 56, 1937–1943.
- Cimmino, A.; Andolfi, A.; Fondevilla, S.; Abouzeid, M.A.; Rubiales, D.; Evidente, A. Pinolide, a new nonenolide produced by Didymella pinodes, the causal agent of Ascochyta blight on Pisum sativum. J. Agric. Food Chem. 2012, 60, 5273–5278.
- Evidente, A.; Capasso, R.; Vurro, M.; Bottalico, A. Ascosalitoxin, a phytotoxic trisubstituted salicylic aldehyde from Ascochyta pisi. Phytochemistry 1993, 34, 995–998.
- Bertini, S. Su di un composto ad azione antibiotica prodotto da Ascochyta pisi Lib. Ann. Stazione Chim. Agrar. Sper. Roma 1956, 11, 545–556.
- Oku, H.; Nakanishi, T. A toxic metabolite from Ascochyta fabae having antibiotic activity. Phytopathology 1963, 53, 1321–1325.
- Kim, W.; Chen, W. Phytotoxic metabolites produced by legume-associated Ascochyta and its related genera in the Dothideomycetes. Toxins 2019, 11, 627.
- Beed, F.D.; Strange, R.N.; Onfroy, C.; Tivoli, B. Virulence for faba bean and production of ascochitine by Ascochyta fabae. Plant Pathol. 1994, 43, 987–997.
- Masi, M.; Maddau, L.; Linaldeddu, B.T.; Scanu, B.; Evidente, A.; Cimmino, A. Bioactive metabolites from pathogenic and endophytic fungi of forest trees. Curr. Med. Chem. 2018, 25, 208–252.
- Alam, S.S.; Bilton, J.N.; Slawin, A.M.; Williams, D.J.; Sheppard, R.N.; Strange, R.N. Chickpea blight: Production of the phytotoxins solanapyrones A and C by Ascochyta rabiei. Phytochemistry 1989, 28, 2627–2630.
- Hamid, K.; Strange, R.N. Phytotoxicity of solanapyrones A and B produced by the chickpea pathogen Ascochyta rabiei (Pass.) Labr. and the apparent metabolism of solanapyrone A by chickpea tissues. Physiol. Mol. Plant Pathol. 2000, 56, 235–244.
- Latif, Z.; Strange, R.N.; Bilton, J.; Riazuddin, S. Production of the phytotoxins, solanapyrones A and C and cytochalasin D among nine isolates of Ascochyta rabiei. Plant Pathol. 1993, 42, 172–180.
- Türkkan, M.; Dolar, F.S.; Şenyuva, H.Z.; Özcan, S. Determination and identification of the solanapyrones and new metabolites from Ascochyta rabiei. Žemdirbystė (Agric.) 2011, 98, 439–444.
- Kaur, S. Phytotoxicity of solanapyrones produced by the fungus Ascochyta rabiei and their possible role in blight of chickpea (Cicer arietinum). Plant Sci. 1995, 109, 23–29.
- Evidente, A.; Capasso, R.; Andolfi, A.; Vurro, M.; Zonno, M.C. Structure–activity relationship studies of putaminoxins and pinolidoxins: Phytotoxic nonenolides produced by phytopathogenic Phoma and Ascochyta species. Nat. Toxins 1998, 6, 183–188.
- Vurro, M.; Ellis, B.E. Effect of fungal toxins on induction of phenylalanine ammonia-lyase activity in elicited cultures of hybrid poplar. Plant Sci. 1997, 126, 29–38.
- Wang, X.Z.; Luo, X.H.; Xiao, J.; Zhai, M.M.; Yuan, Y.; Zhu, Y.; Crews, P.; Yuang, C.; Wu, Q.X. Pyrone derivatives from the endophytic fungus Alternaria tenuissima SP-07 of Chinese herbal medicine Salvia przewalskii. Fitoterapia 2014, 99, 184–190.
- Jenkins, K.M.; Toske, S.G.; Jensen, P.R.; Fenical, W. Solanapyrones EG, antialgal metabolites produced by a marine fungus. Phytochem. 1998, 49, 2299–2304.
- Mizushina, Y.; Kamisuki, S.; Kasai, N.; Shimazaki, N.; Takemura, M.; Asahara, H.; Lin, S.; Yoshida, S.; Matsukage, A.; Koiwai, O.; et al. A plant phytotoxin, solanapyrone A, is an inhibitor of DNA polymerase β and λ. J. Biol. Chem. 2002, 277, 630–638.
- García-Díaz, M.; Domínguez, O.; López-Fernández, L.A.; de Lera, L.T.; Saníger, M.L.; Ruiz, J.F.; Párraga, M.; García-Ortiz, M.J.; Kirchhoff, T.; del Mazo, J.; et al. DNA polymerase lambda (Pol λ), a novel eukaryotic DNA polymerase with a potential role in meiosis. J. Mol. Biol. 2000, 301, 851–867.
- Yamtich, J.; Sweasy, J.B. DNA polymerase family X: Function, structure, and cellular roles. Biochim. Biophys. Acta Proteins Proteom. 2010, 1804, 1136–1150.
- Villegas-Fernández, A.M.; Sillero, J.C.; Emeran, A.A.; Winkler, J.; Raffiot, B.; Tay, J.; Flores, F.; Rubiales, D. Identification and multi-environment validation of resistance to Botrytis fabae in Vicia faba. Field Crops Res. 2009, 114, 84–90.
- Sahile, S.; Ahmed, S.; Fininsa, C.; Abang, M.M.; Sakhuja, P.K. Survey of chocolate spot (Botrytis fabae) disease of faba bean (Vicia faba L.) and assessment of factors influencing disease epidemics in northern Ethiopia. J. Crop Prot. 2008, 27, 1457–1463.
- Tivoli, B.; Baranger, A.; Avila, C.M.; Banniza, S.; Barbetti, M.; Chen, W.; Davidson, J.; Lindeck, K.; Kharrat, M.; Rubiales, D.; et al. Screening techniques and sources of resistance to foliar diseases caused by major necrotrophic fungi in grain legumes. Euphytica 2006, 147, 223–253.
- Hua, L.; Yong, C.; Zhanquan, Z.; Boqiang, L.; Guozheng, Q.; Shiping, T. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2018, 2, 111–119.
- Davidson, J.A.; Kimber, R. Integrated disease management of Ascochyta blight in pulse crops. In Ascochyta Blights of Grain Legumes; Tivoli, B., Baranger, A., Muehlbauer, F.J., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 99–110.
- Rubiales, D.; Khazaei, H. Advances in disease and pest resistance in faba bean. Theor. Appl. Genet. 2022, 135, 3735–3756.
- Schmidt, L.E.; Gloer, J.B.; Wicklow, D.T. Solanapyrone analogues from a Hawaiian fungicolous fungus. J. Nat. Prod. 2007, 70, 1317–1320.
- Cimmino, A.; Villegas-Fernández, A.M.; Andolfi, A.; Melck, D.; Rubiales, D.; Evidente, A. Botrytone, a new naphthalenone pentaketide produced by Botrytis fabae, the causal agent of chocolate spot disease on Vicia faba. J. Agric. Food Chem. 2011, 59, 9201–9206.
- Dalmais, B.; Schumacher, J.; Moraga, J.; Le Pecheur, P.; Tudzynski, B.; Collado, I.G.; Viaud, M. The Botrytis cinerea phytotoxin botcinic acid requires two polyketide synthases for production and has a redundant role in virulence with botrydial. Molecular Plant Pathol. 2011, 12, 564–579.
- Colmenares, A.J.; Aleu, J.; Duran-Patron, R.; Collado, I.G.; Hernandez-Galan, R. The putative role of botrydial and related metabolites in the infection mechanism of Botrytis cinerea. J. Chem. Ecol. 2002, 28, 997–1005.
- Ascari, J.; Boaventura, M.A.D.; Takahashi, J.A.; Duran-Patron, R.; Hernandez-Galan, R.; Macias-Sanchez, A.J.; Collado, I.G. Phytotoxic activity and metabolism of Botrytis cinerea and structure–activity relationships of isocaryolane derivatives. J. Nat. Prod. 2013, 76, 1016–1024.
- Durán-Patrón, R.; Hernández-Galán, R.; Rebordinos, L.G.; Cantoral, J.M.; Collado, I.G. Structure-activity relationships of new phytotoxic metabolites with the botryane skeleton from Botrytis cinerea. Tetrahedron 1999, 55, 2389–2400.
- Lodha, S.; Mawar, R. Population dynamics of Macrophomina phaseolina in relation to disease management: A review. J. Phytopathol. 2020, 168, 1–17.
- Naseri, B.; Veisi, M.; Khaledi, N. Towards a better understanding of agronomic and soil basis for possible charcoal root rot control and production improvement in bean. Arch. Phytopathol. Pflanzenschutz 2018, 51, 349–358.
- Naseri, B.; Younesi, H. Beneficial microbes in biocontrol of root rots in bean crops: A meta-analysis (1990–2020). Physiol. Mol. Plant Pathol. 2021, 116, 101712.
- Coser, S.M.; Chowda Reddy, R.V.; Zhang, J.; Mueller, D.S.; Mengistu, A.; Wise, K.A.; Allen, T.W.; Singh, A.; Singh, A.K. Genetic architecture of charcoal rot (Macrophomina phaseolina) resistance in soybean revealed using a diverse panel. Front. Plant Sci. 2017, 8, 1626.
- Siddiqui, K.A.I.; Gupta, A.K.; Paul, A.K.; Banerjee, A.K. Purification and properties of a heat-resistant exotoxin produced by Macrophomina phaseolina (Tassi) Goid in culture. Experientia 1979, 35, 1222–1223.
- Ramezani, M.; Shier, W.T.; Abbas, H.K.; Tonos, J.L.; Baird, R.E.; Sciumbato, G.L. Soybean charcoal rot disease fungus Macrophomina phaseolina in Mississippi produces the phytotoxin (-)-botryodiplodin but no detectable phaseolinone. J. Nat. Prod. 2007, 70, 128–129.
- Masi, M.; Sautua, F.; Zatout, R.; Castaldi, S.; Arrico, L.; Isticato, R.; Pescitelli, G.; Carmona, M.A.; Evidente, A. Phaseocyclopentenones A and B, phytotoxic penta- and tetrasubstituted cyclopentenones produced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in Argentina. J. Nat. Prod. 2021, 84, 459–465.
- Bhattacharya, G.; Dhar, T.K.; Bhattacharya, F.K.; Siddiqui, K.A. Mutagenic action of phaseolinone, a mycotoxin isolated from Macrophomina phaseolina. Aust. J. Biol. Sci. 1987, 40, 349–354.
- Shier, W.T.; Abbas, H.K.; Baird, R.E.; Ramezani, M.; Sciumbato, G.L. (-)-Botryodiplodin, a unique ribose-analog toxin. Toxin Rev. 2007, 26, 343–386.
- García-Pajón, C.M.; Collado, I.G. Secondary metabolites isolated from Colletotrichum species. Nat. Prod. Rep. 2003, 20, 426–431.
- Chongo, G.; Bernier, C.C. Effects of host, inoculum concentration, wetness duration, growth stage, and temperature on anthracnose of lentil. Plant Dis. 2000, 84, 544–548.
- Stoessl, A.; Stothers, J.B. Colletruncoic acid methyl ester, a unique meroterpenoid from Colletotrichum truncatum. Z. Naturforsch., C, J. Biosci. 1986, 41, 677–680.
- Evidente, A.; Cimmino, A.; Masi, M. Phytotoxins produced by pathogenic fungi of agrarian plants. Phytochem. Rev. 2019, 18, 843–870.
- Masi, M.; Castaldi, S.; Sautua, F.; Pescitelli, G.; Carmona, M.A.; Evidente, A. Truncatenolide, a bioactive disubstituted nonenolide produced by Colletotrichum truncatum, the causal agent of anthracnose of soybean in Argentina: Fungal antagonism and SAR Studies. J. Agric. Food Chem. 2022, 70, 9834–9844.
- Masi, M.; Nocera, P.; Boari, A.; Zonno, M.C.; Pescitelli, G.; Sarrocco, S.; Barroncelli, R.; Vannacci, G.; Vurro, M.; Evidente, A. Secondary metabolites produced by Colletotrichum lupini, the causal agent of anthachnose of lupin (Lupinus spp.). Mycologia 2020, 112, 533–542.
- Fernández, M.T.; Fernandez, M.; Centeno, M.L.; Cañal, M.J.; Rodriguez, R. Reaction of common bean callus to culture filtrate of Colletotrichum lindemuthianum: Differences in the composition and toxic activity of fungal culture filtrates. Plant Cell Tissue Organ Cult. 2000, 6, 41–49.
- Amusa, N.A. Production, partial purification and bioassay of toxic metabolites of three plant pathogenic species of Colletotrichum in Nigeria. Mycopathologia 1994, 128, 161–166.
- Evidente, A.; Cimmino, A.; Berestetskiy, A.; Mitina, G.; Andolfi, A.; Motta, A. Stagonolides B− F, nonenolides produced by Stagonospora cirsii, a potential mycoherbicide of Cirsium arvense. J. Nat. Prod. 2008, 71, 31–34.
- Evidente, A.; Cimmino, A.; Berestetskiy, A.; Andolfi, A.; Motta, A. Stagonolides G− I and modiolide A, nonenolides produced by Stagonospora cirsii, a potential mycoherbicide for Cirsium arvense. J. Nat. Prod. 2008, 71, 1897–1901.
- Albu, S.; Schneider, R.W.; Price, P.P.; Doyle, V.P. Cercospora cf. flagellaris and Cercospora cf. sigesbeckiae are associated with Cercospora leaf blight and purple seed stain on soybean in North America. Phytopathology 2016, 106, 1376–1385.
- Kimber, R.B.E.; Paull, J.G. Identification and genetics of resistance to cercospora leaf spot (Cercospora zonata) in faba bean (Vicia faba). Euphytica 2011, 177, 419–429.
- Newman, A.G.; Townsend, C.A. Molecular characterization of the cercosporin biosynthetic pathway in the fungal plant pathogen Cercospora nicotianae. J. Am. Chem. Soc. 2016, 138, 4219–4228.
- Fajola, A.O. Cercosporin, a phytotoxin from Cercospora spp. Physiol. Plant Pathol. 1978, 13, 157–164.
- You, B.J.; Lee, M.H.; Chung, K.R. Production of cercosporin toxin by the phytopathogenic Cercospora fungi is affected by diverse environmental signals. Can. J. Microbiol. 2008, 54, 259–269.
- Daub, M.E.; Ehrenshaft, M. The photoactivated Cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 2000, 38, 461–490.
- Gan, M.J.; Ash, G.J.; Cowley, R.B.; Savocchia, S.; Luckett, D.J. Genetic variation of Pleiochaeta setosa from Lupinus albus. Australasian Plant Pathol. 2009, 38, 518–524.
- Del Moral, J.; Arias, A.; De Arcos, R. Aparición en España del moteado Pleiochaeta setosa (Kichn) Hughes del altramuz. Bol. Serv. Plagas 1981, 7, 141–145.
- Okeke, B.; Kaouadji, M.; Seigle-Murandi, F.; Steiman, R. Setosol, a biologically active heptaketide-like metabolite from the Pleiochaeta setosa phytopathogen. Biosci. Biotechnol. Biochem. 1994, 58, 734–736.
- Okeke, B.; Seigle-Murandi, F.; Steiman, R. Biological activity of a heptaketide metabolite from Pleiochaeta setosa. Biosci. Biotechnol. Biochem. 1995, 59, 173–175.
- Ellis, M.B.; Waller, J.M. Sclerotinia fuckeliana (conidial state: Botrytis cinerea). CMI Descr. Pathog. Fungi Bact. 1974, 1974, 431.
- Purdy, L. Sclerotinia sclerotiorum: History, diseases and symptomatology, host range, geographic distribution, and impact. Phytopathology 1979, 69, 875–880.
- Steadman, J.R. White mold-a serious yield-limiting disease of bean. Plant Dis. 1983, 67, 346–350.
- Mueller, D.S.; Dorrance, A.E.; Derksen, R.C.; Ozkan, E.; Kurle, J.E.; Grau, C.R.; Gaska, J.M.; Hartman, G.L.; Pedersen, W.L. Efficacy of fungicides on Sclerotinia sclerotiorum and their potential for control of Sclerotinia stem rot on soybean. Plant Dis. 2002, 86, 26–31.
- Godoy, G.; Steadman, J.R.; Dickman, M.B.; Dam, R. Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol. Mol. Plant Pathol. 1990, 37, 179–191.
- Noyes, R.D.; Hancock, J.G. Role of oxalic acid in the Sclerotinia wilt of sunflower. Physiol.Plant Pathol. 1981, 18, 123–132.
- Magro, P.; Marciano, P.; Di Lenna, P. Oxalic acid production and its role in pathogenesis of Sclerotinia sclerotiorum. FEMS Microbiol. Lett. 1984, 24, 9–12.
- Gadd, G.M. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv. Microb. Physiol. 1999, 41, 47–92.
- Ziman, L.; Jędryczka, M.; Šrobárová, A. Relationship between morphological and biochemical characteristics of Sclerotinia sclerotiorum isolates and their aggressivity/Beziehung zwischen morphologischen und biochemischen Eigenschaften von Sclerotinia sclerotiorum Stämmen und ihrer Aggressivität. Z. Pflanzenkrankh. Pflanzenschutz/ JPDP 1998, 105, 283–288.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
882
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




