Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Faheem Khan | -- | 3980 | 2023-03-23 07:22:27 | | | |
| 2 | Peter Tang | + 1 word(s) | 3981 | 2023-03-23 07:45:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Khan, M.F.; Khan, M.A. Plant-Derived Metal Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/42456 (accessed on 07 February 2026).
Khan MF, Khan MA. Plant-Derived Metal Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/42456. Accessed February 07, 2026.
Khan, Mohammad Faheem, Mohd Aamish Khan. "Plant-Derived Metal Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/42456 (accessed February 07, 2026).
Khan, M.F., & Khan, M.A. (2023, March 23). Plant-Derived Metal Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/42456
Khan, Mohammad Faheem and Mohd Aamish Khan. "Plant-Derived Metal Nanoparticles." Encyclopedia. Web. 23 March, 2023.
Copy Citation
Plant-derived metal nanoparticles (PDMNPs) have gained considerable interest because of their tremendous and remarkable potential as therapeutic agents as well as development of less expensive, safer, and easier biomedical equipment. PDMNPs are synthesized from metal salts or oxides by using plant extracts because plants have diversified bioactive compounds that can act as reducing and stabilizing agents at the time of nanoparticle synthesis. Besides, PDMNPs take advantages over the nanoparticles synthesized by other methods because of their low cost, environmental friendliness, and sustainability.
plant-derived metal nanoparticles
reactive oxygen species (ROS)
antioxidant
antimicrobial
anticancer
1. Introduction
The burgeoning branch of science based on nanoparticles (NPs), known as nanoscience and nanotechnology, has attracted a lot of study attention over the past few decades. NPs have been developed widely with unique and dazzling properties ranging in size from 1.0 to 100 nm because they provide the way for advancing multidisciplinary research ranging from medicine to engineering, physics, and chemistry [1]. For several years, NPs have been employed in the manufacture of nanodevices, nanotherapeutics, nanoelectronics, and engineered biological structures, with applications in pharmaceuticals, foods, agriculture, energy, environment, cosmetics, electronics, and catalysis [2]. Because of their tiny size, diverse structure, and numerous biological and physicochemical features, NPs have increasingly caught the attention of the research community over the past two decades [3]. In most of the cases, metal salts/oxides-mediated NPs outperform other nanoparticles due to their huge surface area to volume ratio, excellent biocompatibility, adjustable production, and high stability, making them appropriate for a wide range of applications [3]. Depending on the synthesis methods, NPs may be obtained in various forms, including nanotubes, nanorods, nanoparticles, and nano-sheets, which differ in morphology, size, and form along with optical properties, but they excel in a variety of applications in diverse sectors [4]. The top-down strategy and bottom-up approach are two methods used to create nanoparticles. The techniques that use chemical reactions to generate nanoparticles are commonly known as bottom-up approaches. These processes can also be used to grow thin films in some cases. For generating thin films when bulk material is used for depositions, top-down techniques are preferable. Radio frequency sputtering, pulsed laser deposition, molecular beam epitaxy, and e-beam evaporation are the techniques that are often utilized. NPs are sometimes grown using techniques like mechanical milling and high-energy ball milling [5].
Furthermore, due to significant energy consumption, environmental contamination, and detrimental impacts on humans, physical and chemical procedures are not regarded as an optimal and significant strategy for manufacturing NPs [6]. To address these inadequacies, green methods are preferable since they are environmentally friendly, save energy, and have no hazardous side effects. There are numerous green and sustainable methods for producing NPs, including microorganisms (bacteria, fungi, and yeast), biomolecules, and plants [7]. Plant-derived metal nanoparticles (PDMNPs) are among those that have gained recognition as nanomedicine in recent years and have advantages over synthetic NPs. Plants’ bioactive compounds serve as reducing, capping, and stabilizing agents by converting the metal sources into NPs [8]. Although PDMNPs are biologically efficient as therapeutics at the target sites of protein receptors, they may exhibit several types of toxicity influenced by their size, shape, surface area, crystallinity, agglomeration as well as dissolution [9]. However, they are quite effective for a variety of therapeutic applications [10]. Thus, PDMNPs’ ability to turn bioactive compounds that are poorly soluble and poorly absorbed into promising deliverable substances has enormous potential in the pharmaceutical and biomedical sectors. PDMNPs could lead to the development of cost-effective and safer therapeutic as well as drug-delivery agents against various diseases.
Oxidative stress, a global health concern, is a redox imbalance in between the generation and accumulation of reactive oxygen species (ROS) during the metabolic processes [11]. Oxidative stress is primarily caused by oxygen free radicals such as hydrogen peroxide, superoxide, and hydroxyl groups, which are produced as byproducts of molecular oxygen through oxidative reactions, catalyzed by endothelial nitric oxide synthase (eNOS), xanthine oxidase (XO), and NADPH oxidases enzymes [12]. Based on types and sources, oxidative stress signaling causes metabolic dysfunctions and inflammatory responses. Consequently, increased inflammatory signaling is linked to altered redox balance, which leads to metabolic dysfunctions such as cardiovascular stroke, atherosclerosis, and diabetes [13]. On the other side, ROS overproduction causes oxidative damage to biomembranes, lipids, fats, proteins, and nucleic acids, which leads to aging, inflammation, pain, and the onset of various diseases such as diabetes, cancer, cardiovascular, neurodegenerative, and metabolic disorders [14]. Naturally, the human body has a number of independent cellular mechanisms that work together to counteract the negative effects of oxidative stress. Antioxidants, whether produced endogenously or taken as supplements, are helpful in reducing oxidative stress [15]. Thus, managing ROS may be an important therapeutic pursuit to prevent various diseases. PDMNPs have a well-established role in the prevention of oxidative stress-related diseases [16]. PDMNPs exposure may significantly increase ROS generation because PDMNPs release metal ions, which promote ROS overexpression by impairing mitochondrial function via targeting electron transport chain (ETC) reactions [17]. Additionally, at higher doses, PDMNPs may cause pathophysiological toxicity, including genotoxicity, inflammation, carcinogenesis, and cell necrosis [18]. PDMNPs, on the other side, release metal ions at very low doses, which interfere with the redox reaction and produce the Fenton reaction, e.g., H2O2 + Mn+ → Mn+ + HO− + •OH. This entire cascade suppresses the mitochondrial membrane potential and the electron shuttle chain, as well as blocking the enzymes that denature the proteins of the cell membrane [19]. Because of this process, PDMNPs exhibit antioxidant, antibacterial, and anticancer properties.
Thus, keeping all these points in mind, the researchers envisioned the PDMNPs in a concise manner through this research which is focused on their synthesis, characterization techniques, and pharmacological properties in a lucid approach (Figure 1).
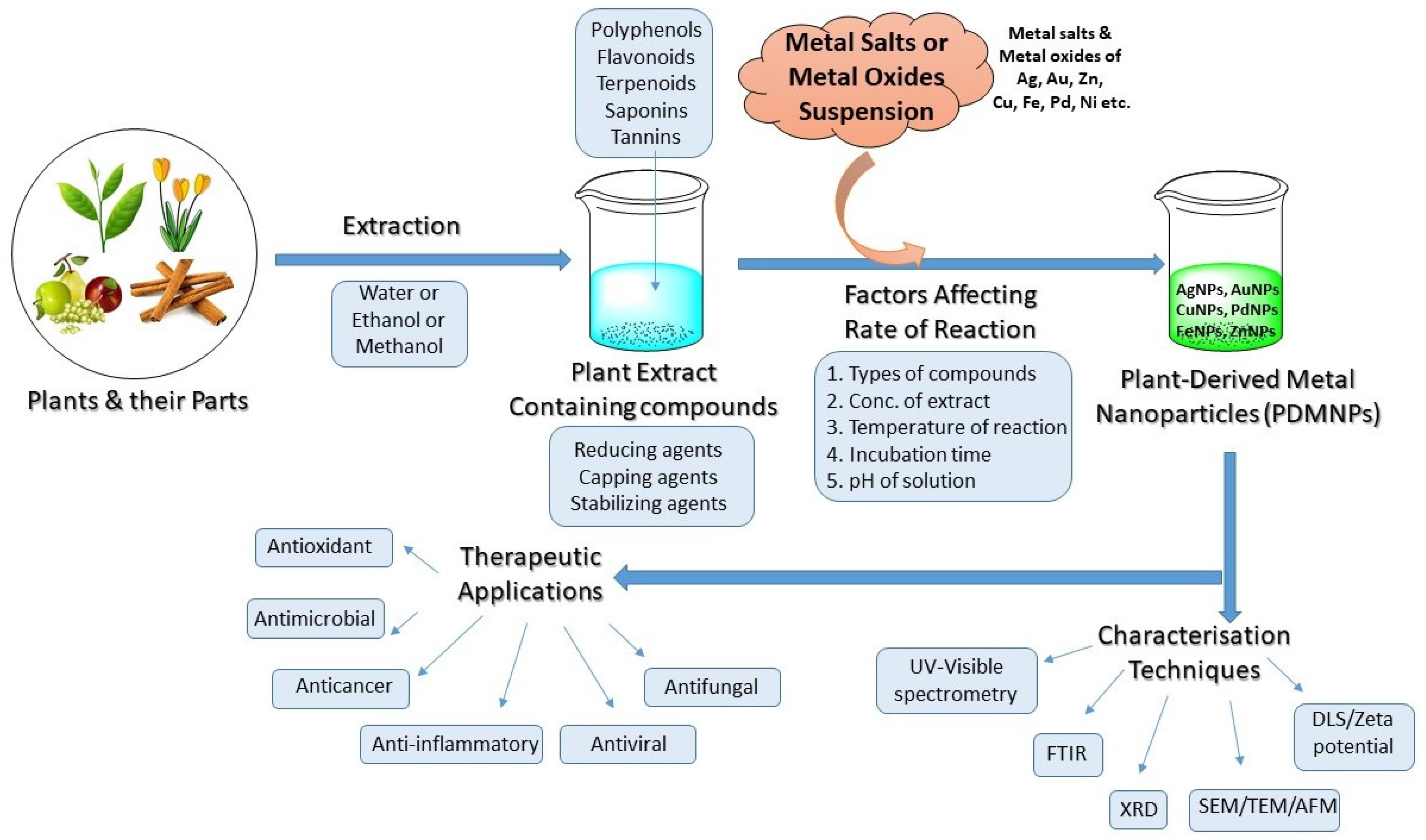
Figure 1. Schematic diagram showing the strategies for the synthesis, optimization of reactions, characterization, and therapeutic applications of PDMNPs.
2. Overview of Nanoparticles
2.1. History and Development of Nanoparticles
NPs made of carbon or other materials such as silver (Ag), gold (Au), or iron (Fe) metals have nanoscopic dimensions ranging from 1 to 100 nanometers in size [20]. They cannot be seen with the naked eye or even a standard microscope [21]. Because of their small size, NPs exhibit high surface energy, and huge surface area, and thus, they have unique and distinct physical and chemical properties from their bulk counterparts [22]. NPs can be made artificially as by-products or by modifying/engineering raw materials that have specific functions. The chemical synthesis of metal-based NPs, including Ag, Au, Fe, copper (Cu), zinc (Zn), palladium (Pd), platinum (Pt), and cobalt (Co) comes to mind when we discuss the different types of nanoparticles [23]. Materials like metal salts, metal oxide, silicates, polymers, organics, carbon, and others are also tuned into nano-size on a large scale using physical, mechanical, and chemical approaches [24]. Depending on the material from which they are made, nanomaterials can adopt a variety of morphologies such as spheres, cylinders, sheets, or tubes [25]. Nanomaterials can take many different forms, such as zero-dimensional (colloids, quantum dots, nanoclusters, etc.), one-dimensional (nanowires, nanotubes, nanobelts, and nanorod, etc.), two-dimensional (quantum wells, super lattices, and bio membranes, etc.), and three-dimensional (nanocomposites, filamentary composites, cellular materials, porous materials, hybrids, and nanocrystal array) [26][27][28]. The synthesis of metal nanoparticles in suspension was made by Faraday in 1857, and Mie characterized the quantitative color change of nanoparticles in suspension in 1908 [29]. Metal-based nanomaterials are superior to other nanomaterials due to their high area-to-volume ratio, high surface energy, and distinctive electronic structure, which are formed via simple electronic transitions between various energy levels [30]. Ag is one of the metals that is frequently used to create nanomaterials using a variety of techniques because it is simple to convert from Ag+ ion to zero valent Ag0. As a result, silver has many excellent uses in the fields of medicine, catalysis, biotechnology, the environment, and the textile industry. According to a literature review, Au is also used in the synthesis of nanomaterials to a significant level, followed by other metals, viz., Cu, Zn, Fe, Pd, Pt, and Co but to a lesser extent [31]. Nanomaterials based on metal oxide are used to create nano-polymers because they have an M-O-M bridge connecting the metal to the oxygen atom [32]. Alloy-based nanomaterials are also created with specialized qualities, like the preservation of atomic ordering, lusters, and more cluster shining, to improve the metal properties in electronic appliances. Additionally, magnetic nanoparticles have a magnetic component (such as Fe, Co, or Ni) and a chemical component to modify their magnetic characteristics, which are widely used in industries for catalysis [33].
2.2. Methodology
The electronic search engines like SciFinder, Google Search, Google Scholar, National Center for Biotechnology Information (NCBI), PubMed, EMBASE (biomedical and pharmacological bibliographic database of published literature), and clinicaltrials.org were used to collect the scientific data based on PDMNPs and their therapeutic applications. In order to get the published papers, the researchers also looked for publications including ScienceDirect, the American chemical society (ACS), Royal society of chemistry (RSC), Springer Nature, Elsevier, Taylor & Francis, and Bentham Sciences. The key terms, like Nanoparticles, plant-derived nanoparticles, plant-based nanoparticles, metal nanoparticles, therapeutic applications of nanoparticles, antioxidant properties of nanoparticles, antimicrobial properties of nanoparticles, and anticancer properties of nanoparticles, were used to search the literature for this research.
3. Synthesis of PDMNPs
3.1. General Synthesis
The properties and therapeutic applications of NPs or PDMNPs depend on their synthesis method. They are often synthesized using the top-down approach and the bottom-up approach [34][35]. As shown in Figure 2, to make nanoparticles, both approaches include physical, chemical, and biological processes [36]. The bulk material under study is broken down into fine particles using suitable techniques such as mechanical grinding, thermal evaporation, sputtering, and ball-milling in the top-down approach, whereas in the bottom-up approach, nanomaterials are synthesized by assembling atoms or molecules into new or novel material in the nano-size using chemical and biological methods [37]. Condensation and evaporation are two important physical processes that generate metal NPs in a furnace tube at atmospheric pressure. These processes mostly follow the top-down method, utilizing electrical or mechanical energy to grind materials into minute NPs. Even though physical techniques do not involve hazardous materials, they nonetheless cost more to produce nanoparticles since they need expensive and energy-intensive equipment [38]. Chemical methods include the reduction, precipitation, hydrothermal, sol-gel, emulsion, and micro-emulsion processes. The most widely used technology among them is sol-gel because of its flexible implementation and high yield. Chemical processes are faster at producing bulk materials, but they also require expensive, hazardous substances that are detrimental to the environment and our health [39]. The synthesis of NPs using salts or oxides of metals such as Ag, Au, Pt, and Pd has attracted a lot of attention in recent decades due to the need to create materials that are safe for the environment, society, and economy, as well as for use in everyday items like electrical appliances, vehicles, aircraft, biomedical devices, and medications [40]. Because they are uniform in size, shape, composition, surface charge, and optical properties, metal-based NPs have many advantageous medical and industrial applications. However, the high cost and purity of metals during synthesis is a major concern in the biomedical and pharmaceutical sectors [41][42]. Greener approaches to synthesizing biogenic NPs are constantly in demand by researchers to reduce toxic effects and maintain a green environment because of their biocompatible, clean, cost-effective, and eco-friendly properties [43][44].
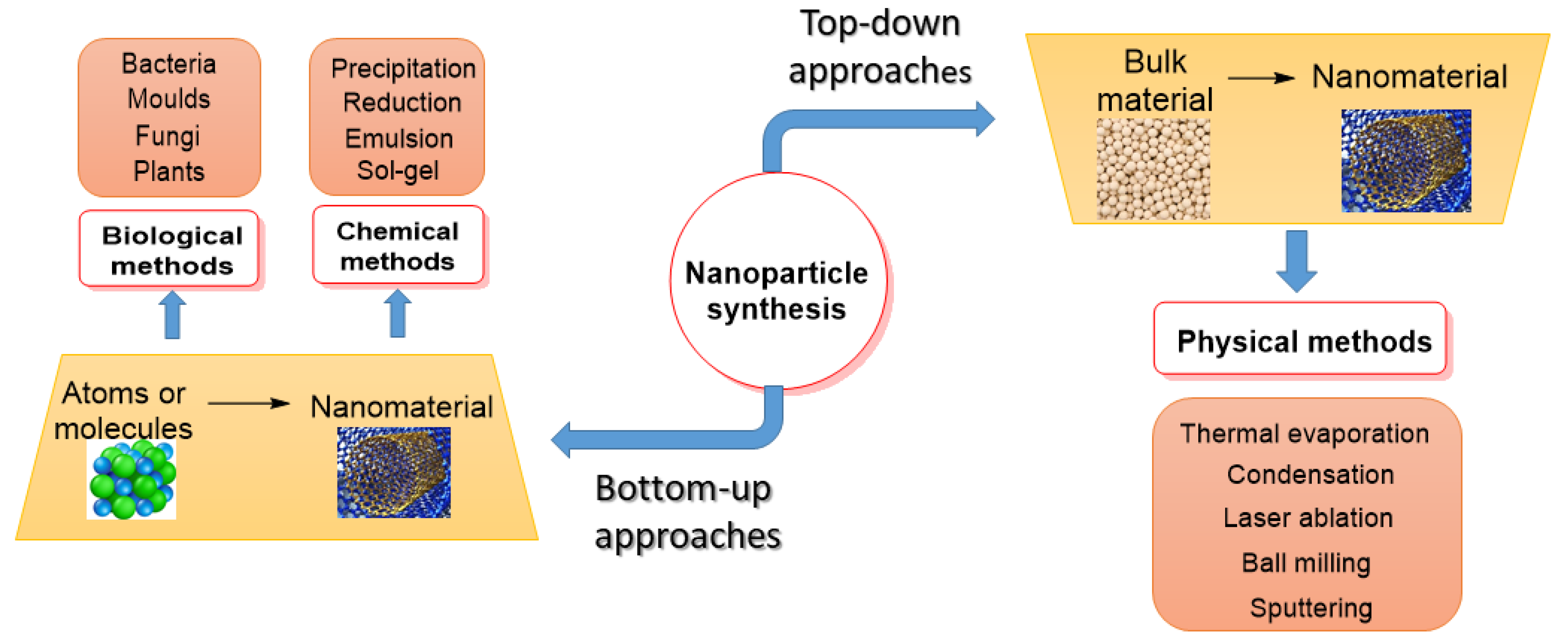
Figure 2. A flow chart showing the synthesis of PDMNPs by applying top-down and bottom-up approaches.
3.2. Plant Mediated Synthesis
Use of fungi, bacteria, plant, and plant-derived products are the best examples and best studied topics currently to synthesize green NPs over conventionally synthesized NPs [45]. However, the production of NPs by microorganisms like bacteria and fungi is a costly and time-consuming process, and since microbes are the main contributor to many diseases, high-grade care is always needed. PDMNPs do not pose these dangers and typically preserve positive health effects. In the suspension of plant extracts and metal salts/oxides, the synthesis of PDMNPs is completed in three steps, viz., activation, growth, and termination [46]. The synthesis begins with the activation of metal salts/oxides by reducing them followed by the growth of smaller nanoparticles into larger NPs, and finally, with the production of desired nanoparticles [47]. The usage of plant extract helps to overcome the poor solubility, decreased stability, and extended duration, which are the main drawbacks of the synthesis of metal NPs (Figure 3). The aqueous extracts of the leaves of numerous plants have received the most attention among plant extracts for the production of metal-chelated NPs [48]. Given the rapid rate of synthesis, short reaction times, and repeatable yields of Ag and Au, these metals are excellent choices for creating plant-based NPs [49]. All plant parts, including the flowers, leaves, stems, fruits, and roots, are abundant sources of bioactive compounds, which include terpenoids, polyphenols, flavonoids, and tannins. Numerous studies have demonstrated that various plant extracts contain polyphenols, flavonoids, tannins, quinol, chlorophyll, and saponin, contributing as reducing, capping, and stabilizing agents to improve the stability of PDMNPs [50].
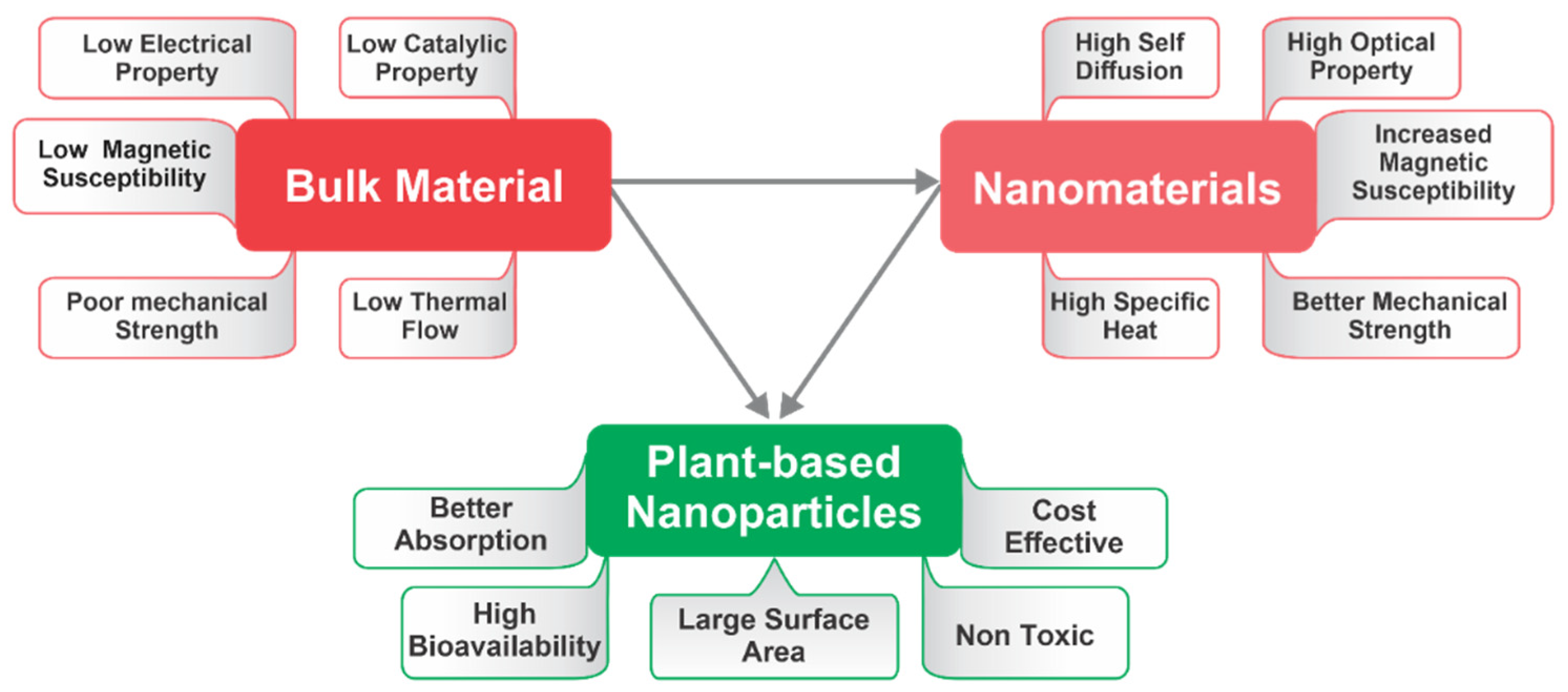
Figure 3. Diagrammatic representation of numerous properties of bulk material, nanomaterials, and plant-based nanoparticles (PDMNPs).
When PDMNPs are synthesized, the bioactive compounds found in plant extracts provide great stability, reduce the clumping, as well as inhibiting the agglomeration, which promotes good dispersion and more active sites in the suspension [51]. These bioactive compounds have a variety of functional groups, including carboxylic acid (-COOH), aldehyde (-CHO), hydroxyl (-OH), nitrile (-CN), and amines (-NH2), which are engaged in reducing metal ions into free metals (Figure 4) like Ag+ → Ag0, Au+ → Au0, Cu2+ → Cu0, Zn2+ → Zn0, and Pt+ → Pt0 [52]. Bioactive compounds also improve the adsorption ability of NPs by increasing the surface area of PDMNPs through molecular interactions such as electrostatic interaction, dipole–dipole induction, π–π interaction, and hydrogen bonding [53]. According to research studies, functional groups of bioactive compounds are involved in reducing the energy gap in metals, which promotes electron release and makes it easier for free radical species to develop. They may readily pass through the cell membrane of pathogens and other host cells followed by killing the diseased cells and as a result, they exhibit a variety of biological activities such as antioxidant and anticancer [54]. In some studies, polyphenols and flavonoids form a capping layer around the NPs via electrostatic interactions, which increases the PDMNPs’ ability to bind to bacterial cell surfaces [55]. Copper-based PDMNPs have been synthesized by using Cu(NO3)2 salt and plant extracts. In these studies, the bioactive compounds such as anthraquinone glycosides, tannin, and quercetin contained in plant extracts have been found to play key roles in enveloping and stabilizing the PDMNPs by reducing Cu2+ ions into Cu0 form [56][57]. Furthermore, Kumar et al. reported the formation of highly stable Zn nanoparticles when they used a suspension of Citrus paradisi peel extract and zinc oxide. Following these studies, researchers found that the hydroxyl and carboxyl functional groups of flavonoids, carotenoids, and limonoids were involved in reducing the Zn2+ ions into Zn0, a zero valent state [58]. Moreover, Kesharwani et al. demonstrated that alkaloids, amino acids, and sugar compounds reduce Ag+ to Ag0 [59]. Therefore, these investigations supported the significance of bioactive compounds in the synthesis of PDMNPs by demonstrating how they operate as reducing, capping, and stabilizing agents.
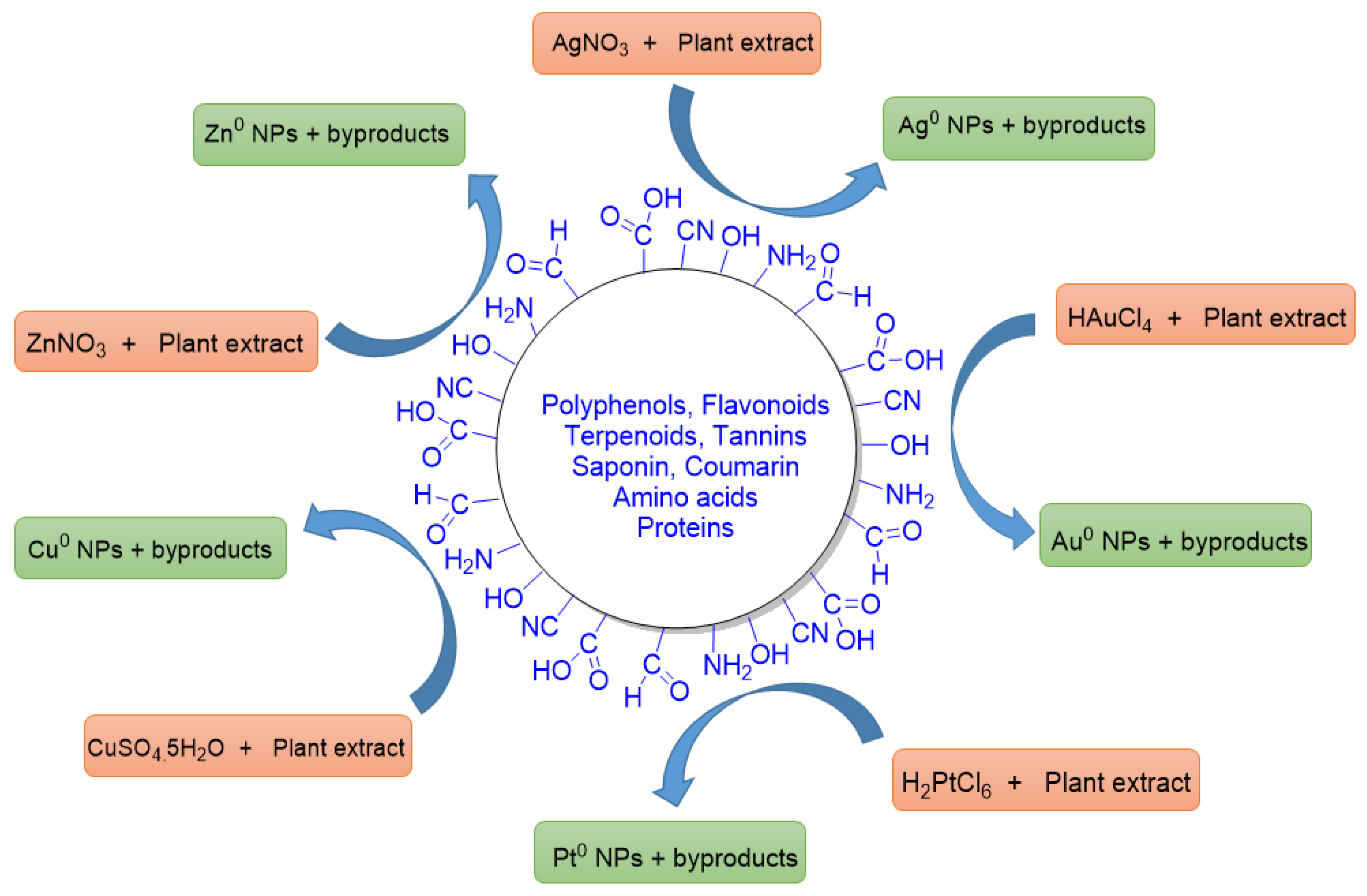
Figure 4. A diagrammatic representation of reducing abilities of functional groups (CHO, COOH, OH, NH2, CN, etc.) of bioactive compounds that are present in the various plant extracts. These functionalities reduce the metal ions into their zero valent states. Some byproducts are also formed in the reactions.
4. Factors Affecting the Synthesis of PDMNPs
There are some challenges during the synthesis of PDMNPs, particularly, non-uniformity in the particle’s shape and size, that need to be overcome in order to maintain monodispersity in the suspension phase. There are also some other factors that cause delays in the production rate, reaction time, and overall yield of synthesized nanoparticles. These factors include the concentration of plant extract, metal salt concentration, incubation time, pH, temperature, types of bioactive compounds, and agitation processes to make the plant extract and suspension of nanoparticles [60].
4.1. Effect of Extract Concentration
The concentration of extracts is one of the most important factors that influence the synthesis of PDMNPs as well as the time required for their formation [61]. In most of the studies, generally, the extract volume ranges from 1 mL to 250 mL have been applied to observe their effects on the synthesis [62]. The effects of extract concentration can be easily observed by changes in color while synthesizing PDMNPs. Moreover, color changes have been observed as the ratio of concentrations of plant extract varies, indicating variations in the size of nanoparticles. When the concentration of plant extract containing significant number of bioactive compounds is high, a sharp peak (small particle size) is observed. A broad peak is observed as the concentration of extract decreases, indicating that the size of the nanoparticle is large [63]. Furthermore, Adeyemia et al. synthesized the bimetallic Ag and Au nanoparticles by using different concentrations (50, 100, and 250 mL) of Dovyalis caffra (Kei apple) fruit extract. This study demonstrated that different concentrations of extracts affect the shape and size of nanoparticles with a low reaction time in 250 mL volume [64]. Other researchers also reported the different extract concentrations regarding the best synthesis of PDMNPs using Ocimum sanctum (Tulsi) leaves extract (0.1, 0.2, 0.3, 0.4, and 0.5 mL), Aegle marmelose leaves extract (10, 20, and 30 mL), and Aloe barbadensis Mill. (Aloe Vera) leaves extract (5, 10, and 15 mL) concentrations [65][66][67].
4.2. Effect of Time
The incubation period of suspension containing plant extract and metal salts/oxides is a vital period that influences the synthesis of PDMNPs in an appropriate time. Smaller-size nanoparticles are produced by using a short incubation time, but larger nanoparticles with a propensity to assemble are often produced by employing a longer incubation time [68]. Numerous investigations have shown that the ideal incubation time, during which all essential reaction steps are properly carried out, is in between 30 min and 2 h. By using Garcinia indica fruit extract, Sangaonkar et al. successfully carried out the experiment of UV spectroscopy to investigate the incubation time from 2.0 to 120 h in a reaction mixture and then found that 24 h was the ideal incubation time for the synthesis of Ag nanoparticles. Similar to this, when fruit extract from Garcinia indica was utilized as a reducing agent, the Au ions are reduced, followed by transformation into nanoparticles in less than two hours [69][70]. In the earlier studies, by using the extracts of Chenopodium leaf and Tansy fruits, Ag and Au nanoparticles started to appear within 10 min of the reaction started and increased up to 2 h by the time the reaction was finished [71][72]. In contrast, other research indicated that the incubation period was slightly longer than the recommended 2 h [73][74]. Additionally, several reports indicated that the ideal incubation duration for the nucleation reactions used to generate metal nanoparticles from plant extracts is 60 min, indicating great stability of the metal nanoparticles [75][76].
4.3. Effect of pH
The parameter of pH cannot be ignored at the time of nanoparticle synthesis because it affects the morphology of nanoparticles in terms of shape and size [77]. The absorbance peaks in UV-Visible absorption spectra throughout their investigation make it simple to see changes caused by pH variations [78]. The formation of big-sized nanoparticles at pH 2.0 and large amounts of smaller-sized nanoparticles at pH 3.0 to 4.0 during the synthesis of gold nanoparticles using Avena sativa plant extract demonstrate the strong pH dependence of nanoparticle size [79]. Another study found that increasing the pH from 3.0 to 7.0 causes nanoparticle size to decrease, whereas increasing the pH from 7 to 11 causes nanoparticle size to rise [80]. Similarly, by using some other plant extracts, studies hypothesized that higher pH (alkaline) values produce small, mostly spherical nanoparticles whereas lower pH (acidic) values produce big, ellipsoidal nanoparticles [81][82][83][84]. According to an experiment performed by Chitra and Annadurai, the size of Ag nanoparticles synthesized using Bacillus brevis cell culture was highly dependent on pH changes, with larger size (60–110 nm) at pH 5 and smaller size (10–40 nm) at pH 9 [85]. Moreover, Suresh et al. reported the synthesis of smaller size nanoparticles derived from low-pH ginger extract, which was attributed to the presence of the highest total phenolic content as compared to neutral and alkaline pH extracts [86]. Overall, pH influences the size and shape of PDMNPs because exposure of polar functional groups such as carbonyl and hydroxyl groups increase the stability as well as binding ability of metal ions during the nucleation and growth phases, which encourages less aggregation of synthesized nanoparticles [87].
4.4. Effect of Temperature
Another crucial factor that must be monitored throughout the synthesis of nanoparticles is temperature. A rise in temperature typically speeds up the reaction, increasing the amount of product produced. For instance, the rate of nanoparticles formation is higher at high temperatures than it is at normal temperature [88]. The greater yield of synthesized nanoparticles is made possible by an increase in metal reduction and homogenous nucleation, both of which are influenced by temperature. It is interesting to note that the temperature range needed for the synthesis of PDMNPs is lower (37–100 °C) than the temperature range needed for synthesis using physical and chemical methods, which is around 350 °C [89]. Because of the increment in surface plasmon resonance and sharpness of peak by increasing temperature, Ag and Au nanoparticles have been synthesized at higher rates at higher temperatures, transitioning from nanorod to platelet-shaped size, as reported in various research [90][91][92][93]. Additionally, some research used spectrophotometers to analyze the strong peaks of absorbance, which directly linked the experiment’s increased temperature to the nanoparticle’s rapid growth [94]. The sharpness of the absorbance relies on the size of the synthesized nanoparticles.
4.5. Effect of Types of Bioactive Compounds
Plant extracts rich in bioactive compounds (polyphenols, flavonoids, tannins, saponin, etc.), which function as reducing and stabilizing agents, influence the reduction of metal ions, the size, shape, processing, and stability of the PDMNPs [95]. These variations are explained by the presence of varying amounts of phytochemicals, which rely on extract preparation techniques, species variances within the same plant, and geographical and seasonal variations [96]. There are many publications on PDMNPs that reported the use of bioactive compounds as reducing, capping, and stabilizing agents, but no clear study is available so far on which types of phytochemicals are responsible for these properties. Most often, the reported bioactive compounds are polyphenols, flavonoids, terpenoids, and alkaloids, which contain oxygen functionalities and could act as reductants of metal ions for the synthesis of PDMNPs [97][98]. Some publications showed that ascorbic acid and glucose could reduce some metals like Au and Ag into their zero valent states, leading to PDMNPs synthesis [99]. Proteins, enzymes, and sugars found in plant extracts also lead to bio reduction of metals as well as agglomeration of synthesized nanoparticles [100][101]. Thus, the various types of bioactive compounds, which are good reducing and capping agents, may enhance the yield of synthesized PDMNPs by inhibiting the aggregation of the nanoparticles.
4.6. Effect of Agitation
Naturally, bioactive compounds are synthesized in cytoplasm or in specific cells and organelles, followed by transportation to other organs. Among them, terpenoids and alkaloids are synthesized in chloroplast whereas the endoplasmic reticulum contains the steroids and sesquiterpenoids. Plant vacuoles contain water-soluble compounds like polyphenols, flavonoids, saponins, and tannins. Lipid soluble compounds such as monoterpenoids and fats are transported through resin ducts and lactiferous glands from their origin sites to storage locations [102]. Most of the compounds are synthesized in the leaves and aerial parts and stored in other parts like roots, fruits, and stems. Thus, a metabolic profile based on bioactive compounds that varies from species to species of plants depends upon season, time, and stage of development [103]. It also depends on the location and types of compounds by which extraction should be done so that we can obtain bioactive compounds in good yield [104]. Agitation during extraction is a very important aspect that should be done because most of the bioactive compounds are enclosed within the cells or vacuoles. Usually, proper agitation causes the breakdown of the cell walls or vacuole rupturing, followed by enhancing the squeezing out of the compounds [105]. Hot aqueous extraction, among them, is the best way for easy preparation of PDMNPs. In this type of extraction, polar compounds are widely extracted, the majority of which are polyphenols and flavonoids, along with their glycosides. Further, when compounds are extracted for an extended time of period, their yield improves [106][107][108]. In addition, pressurized agitation of plant material is very important; applying pressure increases extraction yield [109].
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112.
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954.
- Gatoo, M.A.; Naseem, S.; Arfat, M.Y.; Dar, A.M.; Qasim, K.; Zubair, S. Physicochemical Properties of Nanomaterials: Implication in Associated Toxic Manifestations. Biomed Res. Int. 2014, 2014, 498420.
- Mansoori, G.; Fauzi, S.T. Nanotechnology—An Introduction for the Standards Community; ASTM International: West Conshohocken, PN, USA, 2005; Volume 2, pp. 1–22.
- Liu, Y.; Mai, S.; Li, N.; Yiu, C.K.; Mao, J.; Pashley, D.H.; Tay, F.R. Differences between top-down and bottom-up approaches in mineralizing thick, partially demineralized collagen scaffolds. Acta Biomater. 2011, 7, 1742–1751.
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576.
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Adv. Mater. Res. 2013, 832, 350–355.
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. The Potentiality of Plant-Derived Nanovesicles in Human Health-A Comparison with Human Exosomes and Artificial Nanoparticles. Int. J. Mol. Sci. 2022, 23, 4919.
- Roy, A. Plant Derived Silver Nanoparticles and their Therapeutic Applications. Curr. Pharm. Biotechnol. 2021, 22, 1834–1847.
- Vippola, M.; Valkonen, M.; Sarlin, E.; Honkanen, M.; Huttunen, H. Insight to Nanoparticle Size Analysis—Novel and Convenient Image Analysis Method versus Conventional Techniques. Nanoscale Res. Lett. 2016, 11, 169.
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743.
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95.
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res. 2018, 122, 877–902.
- Alzoghaibi, M.A. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J. Gastroenterol. 2013, 19, 6540–6547.
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975.
- Rasheed, A.; Li, H.; Tahir, M.M.; Mahmood, A.; Nawaz, M.; Shah, A.N.; Aslam, M.T.; Negm, S.; Moustafa, M.; Hassan, M.U.; et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front. Plant Sci. 2022, 13, 976179.
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120.
- AshaRani, P.V.; Low, K.M.G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009, 24, 279–290.
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.J.R. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B. 2008, 112, 13608–13619.
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871.
- Nancharaiah, Y.V.; Mohan, S.V.; Lens, P.N.L. Biological and bioelectrochemical recovery of critical and scarce metals. Trends Biotechnol. 2016, 34, 137–155.
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug. Deliv. Sci. Technol. 2019, 53, 101174.
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; Abdalla, M.S.; Bechelany, M. Review on Natural, Incidental, Bioinspired, and Engineered Nanomaterials: History, Definitions, Classifications, Synthesis, Properties, Market, Toxicities, Risks, and Regulations. Nanomaterials 2022, 12, 177.
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074.
- Shehzad, K.; Xu, Y.; Gaoc, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588.
- Vollath, D. Nanomaterials: An Introduction to Synthesis, Properties and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; ISBN 978-3-527-33379-0.
- Paras; Yadav, K.; Kumar, P.; Teja, D.R.; Chakraborty, S.; Chakraborty, M.; Mohapatra, S.S.; Sahoo, A.; Chou, M.M.C.; Liang, C.-T.; et al. A Review on Low-Dimensional Nanomaterials: Nanofabrication, Characterization and Applications. Nanomaterials 2023, 13, 160.
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677.
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Ibrahim, M.N.M. Recent Advances in Metal Decorated Nanomaterials and Their Various Biological Applications: A Review. Front. Chem. 2020, 8, 341.
- Yaqoob, S.B.; Adnan, R.; Khan, R.M.R.; Rashid, R. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376.
- Chavali, M.S.; Nikolova, M.P. Metal oxide nanoparticles and their applications in nanotechnology. SN Appl. Sci. 2019, 1, 607.
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174.
- Nguyen, T.K.; Thanh, N.M.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630.
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226.
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245.
- Thakkar, K.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanomed.-Nanotechnol. 2010, 6, 257–262.
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037.
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368.
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84.
- Viswanath, B.; Kim, S. Influence of nanotoxicity on human health and environment: The alternative strategies. Rev. Environ. Contam. Toxicol. 2015, 240, 77.
- Rastogi, A.; Tripathi, D.K.; Yadav, S.; Chauhan, D.K.; Živčák, M.; Ghorbanpour, M.; El-Sheery, N.I.; Bresticet, M. Application of silicon nanoparticles in agriculture. 3 Biotech 2019, 9, 90.
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K.; Saion, E. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett. 2013, 8, 1–10.
- Ullah, A.; Lim, S.I. Plant extract-based synthesis of metallic nanomaterials, their applications, and safety concerns. Biotechnol. Bioeng. 2022, 119, 2273–2304.
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 799.
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565.
- Park, Y.; Hong, Y.N.; Weyers, A.; Kim, Y.S.; Linhardt, R.J. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78.
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844.
- Das, S.K.; Thatoi, H. Biotechnological Utilization of Mangrove Resources, 1st ed.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–370.
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627.
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, F. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940.
- Xulu, J.H.; Ndongwe, T.; Ezealisiji, K.M.; Tembu, V.J.; Mncwangi, N.P.; Witika, B.A.; Siwe-Noundou, X. The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics. Pharmaceutics 2022, 14, 2437.
- Gawande, M.B.; Goswami, A.; Felpi, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811.
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836.
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526.
- Benassai, E.; Bubba, M.D.; Ancillotti, C.; Colzi, I.; Gonnelli, C.; Calisi, N.; Salvatici, M.C.; Casalone, E.; Ristori, S. Green and cost-effective synthesis of copper nanoparticles by extracts of non-edible and waste plant materials from Vaccinium species: Characterization and antimicrobial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111453.
- Naghdi, S.; Sajjadi, M.; Nasrollahzadeh, M.; Rhee, K.Y.; Sajadi, S.M.; Jaleh, B. Cuscuta refexa leaf extract mediated green synthesis of the Cu nanoparticles on graphene oxide/manganese dioxide nanocomposite and its cata lytic activity toward reduction of nitroarenes and organic dyes. J. Taiwan Inst. Chem. Eng. 2018, 86, 158–173.
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green approach for fabrication and applications of zinc oxide nanoparticles. Bioinorg. Chem. Appl. 2014, 2014, 523869.
- Kesharwani, J.; Yoon, K.Y.; Hwang, J.; Rai, M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: Hypothetical mechanism involved in synthesis. J. Bionanoscience 2009, 3, 39–44.
- Patra, J.K.; Baek, K.-H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 417305.
- Pyrzynska, K.; Sentkowska, A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2022, 12, 467–480.
- Gopinath, K.; Arumugam, G.S. Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. J. Nanostruc. Chem. 2013, 3, 68.
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Ul-Islam, R. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 2021, 11, 2625–2660.
- Adeyemia, J.O.; Elemikec, E.E.; Onwudiwea, D.C.; Singh, M. Bio-inspired synthesis and cytotoxic evaluation of silver-gold bimetallic nanoparticles using Kei-Apple (Dovyalis caffra) fruits. Inorg. Chem. Comm. 2019, 109, 107569.
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 15867.
- Christopher, J.S.G.; Saswati, B.; Ezilrani, P.S. Optimization of Parameters for Biosynthesis of Silver Nanoparticles Using Leaf Extract of Aegle marmelose. Braz. Arch. Biol. Technol. 2015, 58, 702–710.
- Moosa, A.A.; Ridha, A.M.; Al-Kaser, M. Process Parameters for Green Synthesis of Silver Nanoparticles using Leaves Extract of Aloe Vera Plant. Int. J. Multidiscip. Curr. Res. 2015, 3, 966–976.
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28.
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloids Surf. B 2018, 164, 210–217.
- Krishnaprabha, M.; Pattabi, M. Synthesis of gold nanoparticles using garcinia indica fruit rind extract. Int. J. Nanosci. 2016, 15, 1660015.
- Dwivedi, A.D.; Gopal, K. Biosynthesis of gold and silver nanoparticles using Chenopodium album leaf extract. Colloids Surf. A 2010, 369, 27–33.
- Dubey, S.P.; Lahtinen, M.; Sillanpaa, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071.
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal based synthesis of silver nanoparticles-an effect of temperature on the size of particles. Colloids Surf. B 2009, 74, 123–126.
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009, 44, 939–943.
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J. Saudi Chem. Soc. 2011, 15, 113–120.
- Ghoreishi, S.M.; Behpour, M.; Khayatakashani, M. Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary applications in electrochemistry. Phys. E 2011, 44, 97–104.
- Gardea-Torresdey, J.L.; Tiemann, K.J.; Gamez, G.; Dokken, K.; Cano-Aguilera, I.; Furenlid, L.R.; Renner, M.W. Reduction and accumulation of gold(III) by Medicago sativa alfalfa biomass: X-ray absorption spectroscopy, pH and temperature dependence. Environ. Sci. Technol. 2000, 34, 4392–4396.
- Begum, R.; Farooqi, Z.H.; Naseem, K.; Ali, F.; Batool, M.; Xiao, J.; Irfan, A. Applications of UV/Vis Spectroscopy in Characterization and Catalytic Activity of Noble Metal Nanoparticles Fabricated in Responsive Polymer Microgels: A Review. Crit. Rev. Anal. Chem. 2018, 48, 506–516.
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanopartilce formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanopart. Res. 2004, 6, 377–382.
- Singh, A.K.; Srivastava, O.N. One-step Green synthesis of gold nanoparticles using black cardamom and effect of pH on its synthesis. Nanoscale Res. Lett. 2015, 10, 353.
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B 2009, 73, 332–338.
- Vincent, J.; Lau, K.S.; Evyan, Y.C.-Y.; Chin, S.X.; Sillanpää, M.; Chia, C.H. Biogenic Synthesis of Copper-Based Nanomaterials Using Plant Extracts and Their Applications: Current and Future Directions. Nanomaterials 2022, 12, 3312.
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The Effect of pH on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444.
- Bernabé-Antonio, A.; Martínez-Ceja, A.; Romero-Estrada, A.; Sánchez-Carranza, J.N.; Columba-Palomares, M.C.; Rodríguez-López, V.; Meza-Contreras, J.C.; Silva-Guzmán, J.A.; Gutiérrez-Hernández, J.M. Green Synthesis of Silver Nanoparticles Using Randia aculeata L. Cell Culture Extracts, Characterization, and Evaluation of Antibacterial and Antiproliferative Activity. Nanomaterials 2022, 12, 4184.
- Chitra, K.; Annadurai, G. Antibacterial Activity of pH-Dependent Biosynthesized Silver Nanoparticles against Clinical Pathogen. BioMed Res. Int. 2014, 2014, 725165.
- Suresh, A.P.; Kalarikkal, S.P.; Pullareddy, B.; Sundaram, G.M. Low pH-Based Method to Increase the Yield of Plant-Derived Nanoparticles from Fresh Ginger Rhizomes. ACS Omega 2021, 6, 17635–17641.
- Khan, S.A.; Shahid, S.; Sajid, M.R.; Noreen, F.; Kanwal, S. Biogenic synthesis of CuO Nanoparticles and their biomedical applications: A current review. Int. J. Adv. Res. 2017, 5, 925–946.
- Pereira, L.; Mehboob, F.; Stams, A.J.M.; Mota, M.M.; Rijnaarts, H.H.M.; Alves, M.M. Metallic nanoparticles: Microbial synthesis and unique properties for biotechnological applications, bioavailability and biotransformation. Crit. Rev. Biotechnol. 2013, 35, 114–128.
- Rai, A.; Singh, A.; Ahmad, A.; Sastry, M. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir 2006, 22, 736–741.
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140.
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 2007, 23, 2691–2699.
- Andreescu, D.; Eastman, C.; Balantrapu, K.; Goia, D.V.A. Simple route for manufacturing highly dispersed silver nanoparticles. J. Mater. Res. 2007, 22, 2488–2496.
- Sathishkumar, M.; Krishnamurthy, S.; Yun, Y.S. Immobilization of silver nanoparticles synthesized using the Curcuma longa tuber powder extract on cotton cloth for bactericidal activity. Biores. Technol. 2010, 101, 7958–7965.
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-Chemical Condition Optimization during Biosynthesis lead to development of Improved and Catalytically Efficient Gold Nano Particles. Sci. Rep. 2016, 6, 27575.
- Kobylinska, N.; Klymchuk, D.; Khaynakova, O.; Duplij, V.; Matvieieva, N. Morphology-Controlled Green Synthesis of Magnetic Nanoparticles Using Extracts of ‘Hairy’ Roots: Environmental Application and Toxicity Evaluation. Nanomaterials 2022, 12, 4231.
- Mittal, J.; Batra, A.; Singh, A.; Sharma, M.M. Phytofabrication of nanoparticles through plant as nanofactories. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043002.
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia-A review. Phytochemistry 2002, 59, 117–140.
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502.
- Prasad, R. Synthesis of Silver Nanoparticles in Photosynthetic Plants. J. Nanopart. 2014, 2014, 963961.
- Saxena, A.; Tripathi, R.M.; Zafar, F.; Singh, P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Mater. Lett. 2012, 67, 91–94.
- Aziz, N.; Fatma, T.; Varma, A.; Prasad, R. Biogenic Synthesis of Silver Nanoparticles Using Scenedesmus abundans and Evaluation of Their Antibacterial Activity. J. Nanopart. 2014, 2014, 689419.
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of Synthetic Plant Extracts on the Production of Silver-Derived Nanoparticles. Plants 2021, 10, 1671.
- Kutchan, T.M. A role for intra- and intercellular translocation in natural product biosynthesis. Curr. Opin. Plant Biol. 2005, 8, 292–300.
- Hussain, K.; Saquib, M.; Khan, M.F. Techniques for Extraction, Isolation, and Standardization of Bio-Active Compounds from Medicinal Plants. Natural Bio-Active Compounds: Volume 2: Chemistry, Pharmacology and Health Care Practices; Swamy, M.K., Akhtar, M.S., Eds.; Springer: Singapore, 2019; pp. 179–200. ISBN 9789811372056.
- Teffane, M.; Boudries, H.; Bey, M.B.; Kadi, A.; Boukhalfa, F. Effect of Solvent Type, Extraction Temperature, Agitation Speed and Microwave Power on Phenolic Compound Extraction and Antioxidant Activity of Apricot Kernels (Prunus armeniaca L.). Curr. Bioact. Compd. 2022, 18, e260821191386.
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28.
- Hernández-Pinero, J.L.; Terrón-Rebolledo, M.; Foroughbakhch, R.; Moreno-Limón, S.; Melendrez, M.F.; Solís-Pomar, F.; Pérez-Tijerina, E. Effect of heating rate and plant species on the size and uniformity of silver nanoparticles synthesized using aromatic plant extracts. Appl. Nanosci. 2016, 6, 1183–1190.
- Deen, G.R.; Hannan, F.A.; Henari, F.; Akhtar, S. Effects of Different Parts of the Okra Plant (Abelmoschus esculentus) on the Phytosynthesis of Silver Nanoparticles: Evaluation of Synthesis Conditions, Nonlinear Optical and Antibacterial Properties. Nanomaterials 2022, 12, 4174.
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844.
More
Information
Subjects:
Chemistry, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
23 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




