Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Abigail Keng | -- | 1253 | 2023-03-22 20:24:41 | | | |
| 2 | Rita Xu | -5 word(s) | 1248 | 2023-03-23 04:09:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Keng, A.; Botezatu, A. Haloanisoles in Wine. Encyclopedia. Available online: https://encyclopedia.pub/entry/42448 (accessed on 07 February 2026).
Keng A, Botezatu A. Haloanisoles in Wine. Encyclopedia. Available at: https://encyclopedia.pub/entry/42448. Accessed February 07, 2026.
Keng, Abigail, Andreea Botezatu. "Haloanisoles in Wine" Encyclopedia, https://encyclopedia.pub/entry/42448 (accessed February 07, 2026).
Keng, A., & Botezatu, A. (2023, March 22). Haloanisoles in Wine. In Encyclopedia. https://encyclopedia.pub/entry/42448
Keng, Abigail and Andreea Botezatu. "Haloanisoles in Wine." Encyclopedia. Web. 22 March, 2023.
Copy Citation
Haloanisoles in wine have devastating effects on the aroma and quality of the wine. 2,4,6-trichloroanisole (TCA) was discovered and coined as “cork taint” in 1982. There are many more haloanisoles that contribute to these musty odors, including 2,4,6-Tribromoanisiole (TBA), 2,3,4,6-tetrachloroanisole (TeCA), and pentachloroanisole (PCA). While TCA, TeCA, and PCA can all be traced back to the cork, TBA’s phenol precursor is ubiquitous in building material as a fire retardant, making it a much larger vector. All haloanisoles have the ability to aerosolize and resettle onto surfaces in the winery, making this a very difficult problem to eliminate.
haloanisole
wine
pentachloroanisole
halophenol
tetrachloroanisole
trichloroanisole
tribromoanisole
cork taint
1. Introduction
In 1982, 2,4,6-trichloroanisole (TCA) was the first haloanisole identified as causing musty odors in wine. Even before its discovery, wine makers suspected that corks had something to do with the odor, thus naming the fault that leads to musty odors in wine “cork taint”. This groundbreaking research also concluded that in some wines the concentration of TCA did not correlate with the level of musty odors the wine had [1]. Researchers now know that there are multiple haloanisoles that can be the cause of musty odors in wine, including 2,4,6-tribromoanisole (TBA), 2,3,4,6-tetrachloroanisole (TeCA), and pentachloroanisole (PCA) [2].
2. What Are Haloanisoles in Wine?
Haloanisoles in wine all cause the same musty and moldy odors and are indistinguishable from each other in a wine matrix [3]. Although detrimental to wine quality, haloanisoles are not toxic; however, their halophenol precursors are “highly toxic” [1]. The main difference between the haloanisoles are their sources and how they come into contact with wine.
2.1. Trichloroanisole
Trichloroanisole was the first haloanisole identified as the causal compound for musty, moldy aromas in wine in 1982 and is the most well-researched haloanisole in wine. Previously, TCA was only studied as an off flavor in chicken eggs and broilers [1]. TCA was originally found in corks that had been bleached with chlorine bleach in order to create a uniform white color for the corks [1]. The chlorine would mix with the natural fungus present in the cork and produce TCA. The TCA would then be released into the wine once it was sealed and left to age in bottle. Buser proposed that the “replacement of chlorine treatment in the processing of cork” would fix this issue. The industry has since eradicated the use of chlorine bleaching of corks, so how can we still have “corked” wines? Another change that cork producers made after becoming aware of TCA is not washing the corks using public water that contains chlorine for human health and safety [4]. Wineries now also know to dechlorinate the water that they use for wine making if they are using public water.
Studies conducted in cork tree forests in Portugal indicated the presence of TCA in the bark. Further research showed a higher concentration of TCA at the base of the trees. The researchers hypothesized that the past use of chlorophenol-based biocides, containing either TCP or PCP, led to these compounds being still present, even though the spraying regime was stopped in the 1980’s [4]. In 2005, a study conducted by Herve found a 76% decrease of ‘releasable TCA’ in corks. He defined releasable TCA as the amount of TCA able to move from the cork into a solution such as wine. It is possible that in the past 17 years since the study conducted by Herve, this amount of releasable TCA has decreased even more.
The precursor to TCA is 2,4,6-trichlorophenol or TCP. TCP can be methylated by different fungi to produce TCA as shown in Figure 1. Different fungi showed varying aptitudes to transform the TCP to TCA. Trichoderma and Fusarium were found to be strong methylators with a 25% conversion of TCP to TCA, and Penicillium, A. strictum, C. sitophila, and C. oxysporum were found to be medium methylators, with only a 5–10% transformation of TCP into TCA [1]. A recent study in 2021 found that Aspergillus versicolor and Paecilomyces variotii were found to be strong methylators and are very common in wooden constructions [5].
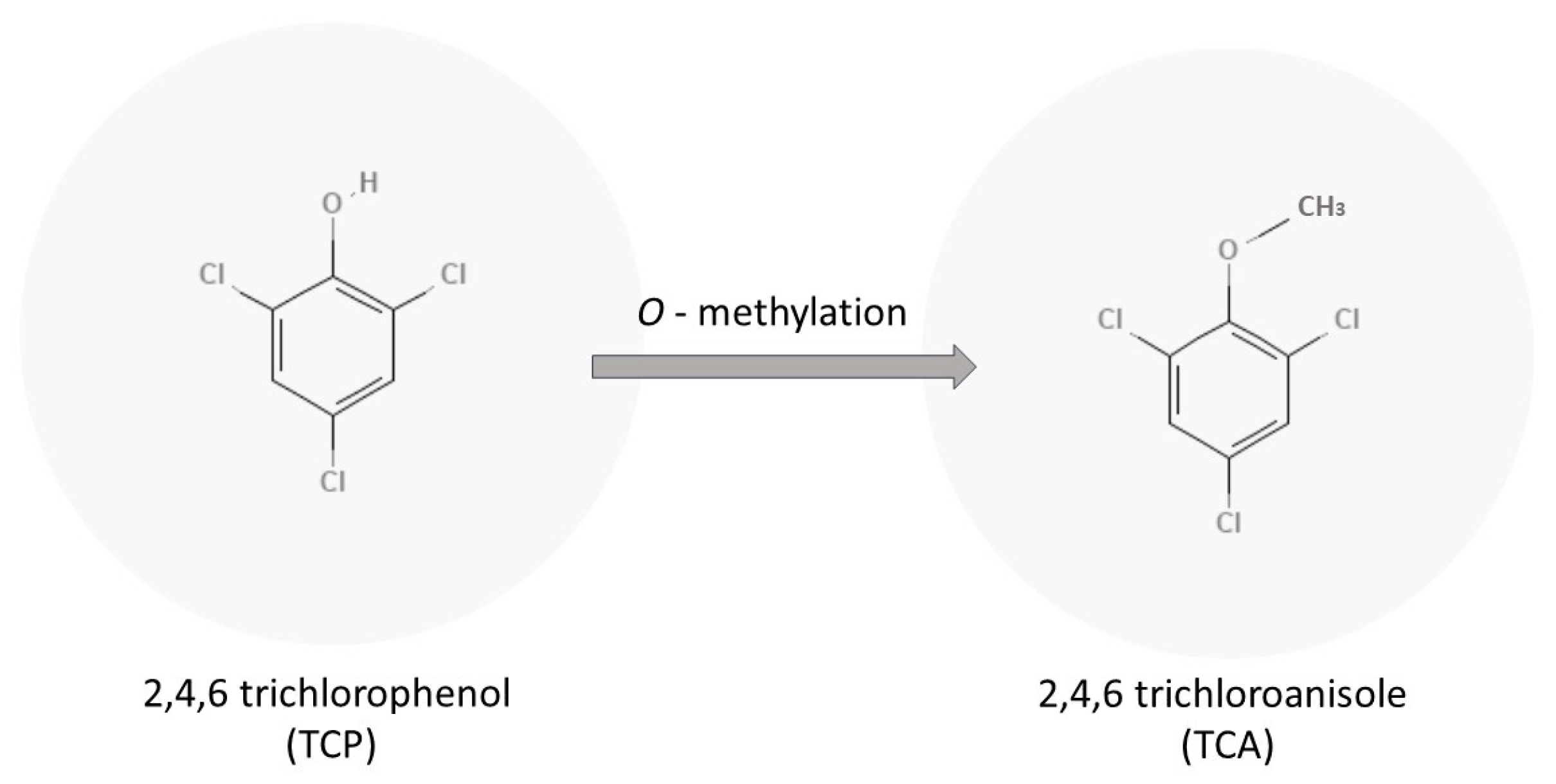
Figure 1. Microbiological formation of TCA by O-methylation of TCP.
2.2. Tribromoanisole
There are many sources in the winery for tribromoanisole and its precursor tribromophenol. Fireproofing agents can contain tribromoanisole or tribromophenol (TBP); these agents can become aerosolized, contaminate wood or other products treated with brome fumigates, and can spread to all parts of the winery [2][6].
Another source of bromine-based fire retardants is spray foam insulation. By itself, spray foam insulation is flammable; however, when bromine flame retardants are added they “enhance the flame retardancy of the foams, helping to inhibit ignition or slow down the process of combustion” [7]. According to the FDA, spray foam insulation can be installed and left unexposed—not covered by drywall, for example. Older bromine-based fire retardants such as Hexabromocyclododecane (HBCD) are being phased out; however, newer ones are being implemented.
Methyl Bromide is a chemical fumigant that is used to treat shipping containers, wood products, and other materials in order to protect them from invasive pests. It is also a substance that is being phased out of use by the Environmental Protection Agency (EPA) due to its damaging effects on the atmosphere [8].
Recently, a study showed that there are still measurable concentrations of fumigants in both treated and untreated containers. These concentrations are higher in containers that are treated with methyl bromide and in containers that contain metal or glass items, as well as containers from China [9].
Methyl bromide is still in use for wood treatment in order to adhere to the International Standards for Phytosanitary Measures. These measures are necessary to prevent the introduction of harmful species that could potentially disrupt our ecosystems [10].
TBP can become TBA by O-methylation of fungus, such as Paecelomyces variotii, which has also been found to convert TCP into TCA [11].
A study performed in 2004 tested three wineries for TBA contamination. The authors sampled a variety of places, including the atmosphere, wines stored in stainless steel vs. barrel, walls of the buildings, and barrels of different ages. All wineries had TBA present, ranging in concentration from 2–2185 ng/L [12].
TBA can become aerosolized and resettle onto many things in the winery and lead to drastic quality loss in wines.
2.3. Tetrachloroanisole & Pentachloroanisole
Tetrachloroanisole (TeCA) and pentachloroanisole (PCA) are unique haloanisoles due to the co-use of their phenol precursors—2,4,6-tetrachlorophenol (TeCP) and pentachlorophenol (PCP)—in various applications. PCP was once the most widely used biocide in the United States, but its use has since been heavily restricted by the EPA. Despite this, PCP is still used to preserve utility poles and railroad ties. TeCP is often found in combination with PCP, as it is used as a component of PCP based biocides. This means that when one of the compounds is detected, it is likely that the other is present as well [13]. Due to its previous use as a biocide in the United States, residual PCP and TeCP can be found in oak forests used to create many products found in the winery. PCP and TeCP are easily methylated by fungi found in the air that includes, but are not limited to, Trichderma virgatum, Aspergillus sydowi, Scopulatriopsis brevicaulis, and penicillium spp. [14]. Both the phenol and the anisole can become airborne and resettle in and on soil, water, trees, etc., thus making it extremely difficult to be completely eliminated in natural settings. Wood is commonly used in wineries, from the building structure to wood pallets, barrels, and more. Though it may be a useful material, it is important to consider the potential for contamination from TeCA and PCA, as well as their phenol precursors.
References
- Buser, H.R.; Zanier, C.; Tanner, H. Identification of 2,4,6-Trichloroanisole as a Potent Compound Causing Cork Taint in Wine. J. Agric. Food Chem. 1982, 30, 359–362.
- Alvarez-Rodríguez, M.L.; Lopez-Ocana, L.; Miguel Lopez-Coronado, J.; Rodríguez, E.; Jesus Martınez, M.; Larriba, G.; Coque, J.J.R. Cork Taint of Wines: Role of the Filamentous Fungi Isolated from Cork in the Formation of 2,4,6-Trichloroanisole by O Methylation of 2,4,6-Trichlorophenol. Appl. Environ. Microbiol. 2002, 68, 5860–5869.
- Cravero, M.C. Musty and Moldy Taint in Wines: A Review. Beverages 2020, 6, 41.
- Simpson, R.F.; Sefton, M.A. Origin and Fate of 2,4,6-Trichloroanisole in Cork Bark and Wine Corks. Aust. J. Grape Wine Res. 2007, 13, 106–116.
- Ekberg, O. Literature Reveiw of Fungi in Buildings and Their Ability to Methylate Chlorophenols into Malodorous Chloroanisoles. J. Phys. Conf. Ser. 2021, 2069, 012207.
- Callejón, R.M.; Ubeda, C.; Ríos-Reina, R.; Morales, M.L.; Troncoso, A.M. Recent Developments in the Analysis of Musty Odour Compounds in Water and Wine: A Review | Elsevier Enhanced Reader. J. Chromatogr. A 2015, 1428, 72–85.
- BSEF. Electronics and Electrical Equipment—Flame Retardants—Preventing Firesand Protecting People. Available online: http://flameretardantsguide.com/flame-retardants-applications/electronics-and-electrical-equipment/ (accessed on 20 December 2022).
- US EPA. Methyl Bromide. Available online: https://www.epa.gov/ods-phaseout/methyl-bromide (accessed on 20 December 2022).
- Hinz, R.; Mannetje, A.; Glass, B.; McLean, D.; Douwes, J. Airborne Fumigants and Residual Chemicals in Shipping Containers Arriving in New Zealand. Ann. Work Expo. Health 2022, 66, 481–494.
- Sela, S.; Schroeder, T.; Mamoru, M.; Ormsby, M. Explanatory document for International Standards for Phytosanitary Measures 15 (Regulation of Wood Packaging Material in International Trade); International Plant Protection Convention: Rome, Italy, 2017.
- Chatonnet, P.; Fleury, A.; Boutou, S. Identification of a New Source of Contamination of Quercus Sp. Oak Wood by 2,4,6-Trichloroanisole and Its Impact on the Contamination of Barrel-Aged Wines. J. Agric. Food Chem. 2010, 58, 10528–10538.
- Chatonnet, P.; Bonnet, S.; Boutou, S.; Labadie, M.-D. Identification and Responsibility of 2,4,6-Tribromoanisole in Musty, Corked Odors in Wine. J. Agric. Food Chem. 2004, 52, 1255–1262.
- PubChem 2,3,4,6-Tetrachlorophenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6028 (accessed on 19 December 2022).
- Ruckdeschel, G.; Renner, G. Effects of Pentachlorophenol and Some of Its Known and Possible Metabolites on Fungi. Appl. Environ. Microbiol. 1986, 51, 1370–1372.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.6K
Revisions:
2 times
(View History)
Update Date:
23 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




