
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vishal Ram | -- | 11734 | 2023-03-22 14:28:30 | | | |
| 2 | Rita Xu | -4 word(s) | 11730 | 2023-03-23 02:46:02 | | | | |
| 3 | Vishal Ram | -818 word(s) | 10912 | 2023-03-27 14:21:58 | | |
Video Upload Options
A concise yet comprehensive overview of major synthetic fuels currently in production worldwide. The fuels are virtually categorised in an intelligible manner with detailed descriptions of their chemical equations, feedstocks, production processes, applications, and global market leaders.
1. Introduction
-
Renewable energy: One of the most important solutions to climate change is the transition to renewable energy sources, such as wind, solar, and hydropower. Renewable energy is clean, sustainable, and abundant, and it can help to reduce greenhouse gas emissions and mitigate the impacts of climate change. Some of the renewable energy technologies are solar photovoltaic, wind turbine, hydroelectric power plants, geothermal energy, and tidal energy technologies.
-
Energy efficiency: Another important solution to climate change is improving energy efficiency, which refers to the use of less energy to achieve the same level of service. This can be achieved through a variety of measures, such as better insulation, more efficient appliances, and smarter transportation systems.
-
Carbon capture and storage: Carbon capture and storage (CCS) is a technology that captures carbon dioxide emissions from power plants and other sources and stores them underground, preventing them from entering the atmosphere. CCS can help to reduce greenhouse gas emissions and mitigate the impacts of climate change [19].
-
Synthetic fuels: Synthetic fuels, also known as e-fuels or artificial fuels, are produced from renewable or non-renewable sources that resemble the characteristics of fossil-derived fuels. They can be used in place of traditional fossil fuels in a variety of applications, such as transportation, heating, and power generation.
-
Carbon pricing: Carbon pricing is a policy that puts a price on carbon dioxide emissions, through taxes or cap-and-trade systems. This provides a financial incentive for businesses and individuals to reduce their emissions and transition to cleaner technologies [20].
2. Classification of Synthetic Fuels
The future landscape for synthetic fuels is uncertain, as it will depend on the development and deployment of new technologies and the adoption of policies that support their use. However, synthetic fuels will likely play a larger role in the energy mix in the future as a way to reduce greenhouse gas emissions and improve energy security.
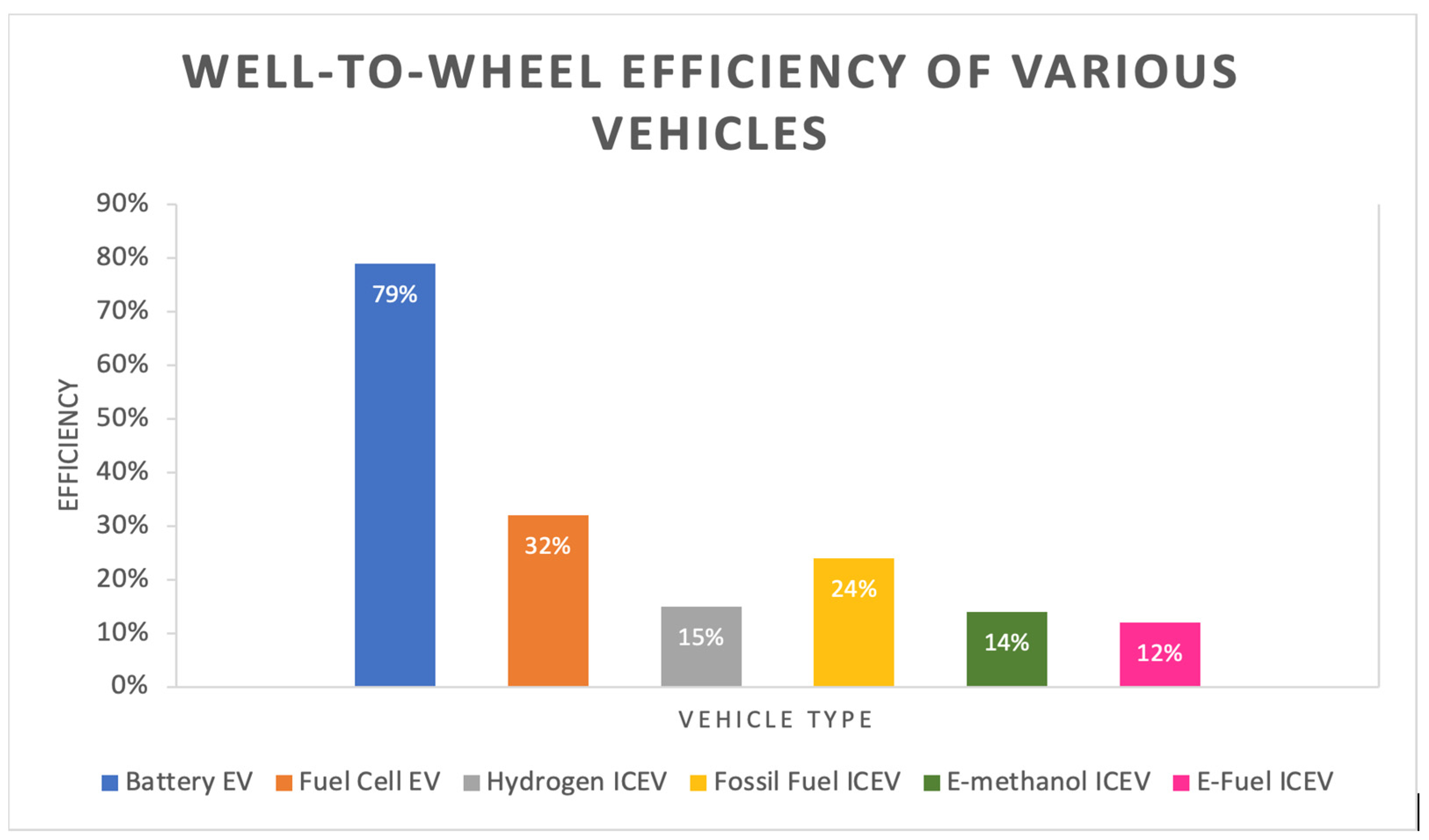
Figure 1. Comparison of well-wheel efficiency between vehicles of various fuels.
The classification of synthetic fuels as broad, mutually exclusive groups is a challenging attempt and is contextual since it depends on various discussed factors such as production process, feedstock, application, sustainability, and chemical composition. For simplicity, this research has adopted the method of classifying synthetic fuels based on their feedstock as it allows the formation of thicker groups of similar fuels. This classification system can aid in a better understanding of the vast number of the major synthetic fuels. The feedstock-based classifications are
-
Biofuels: These are synthetic fuels that are produced from biological materials, such as vegetable oils, animal fats, or waste products. Examples of biofuels include biodiesel, bioethanol, and biogas.
-
Hydrogen fuels: These are synthetic fuels that are produced by reacting hydrogen with other molecules, commonly carbon dioxide. Examples of fuels considered under this category include methanol, DME, and synthetic natural gas.
-
Power-to-liquid (PtL) fuels: These are synthetic fuels that are produced by using electricity from renewable sources to convert water and carbon dioxide into liquid fuel. Examples of PtL fuels include synthetic gasoline, synthetic diesel, and synthetic aviation fuel.
-
Gas-to-liquids (GtL) fuels: These are synthetic fuels that are produced by using natural gas or other gases as feedstocks. Examples of GtL fuels include synthetic diesel, synthetic gasoline, and synthetic aviation fuel.
3. Biofuels
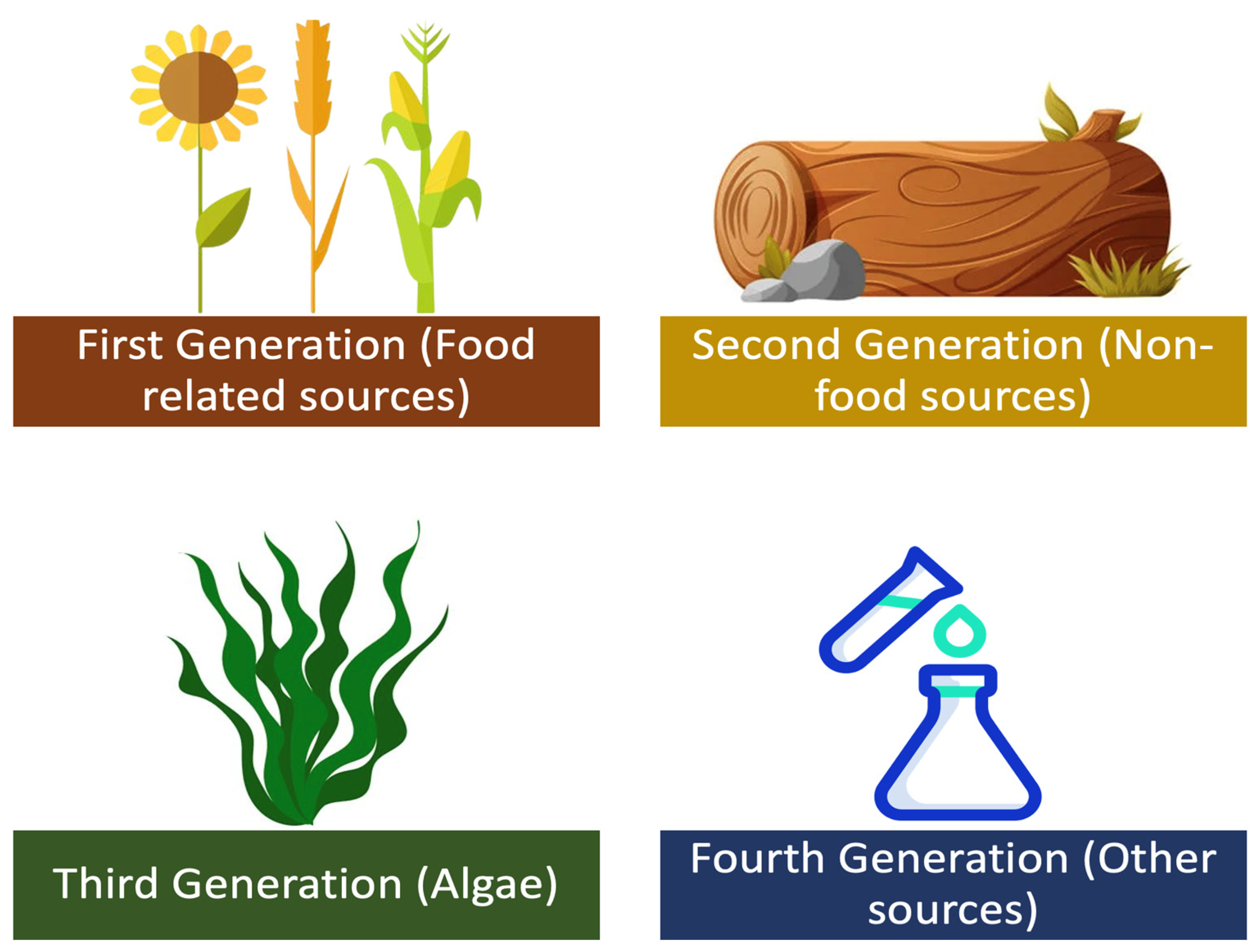
Figure 2. Generations of biofuels.
The advantages of biofuels include biodegradability, non-inflammable nature, low toxicity, safety, high combustion efficiency, abundance, lubricant nature, high cetane number, lower emission, and renewability. Their disadvantages include low calorific value, nitrous oxide emissions, poor shelf life, and their tendency to cause engine wear [51][52]. They can be used in their pure form and can even be blended with fossil fuels owing to their miscibility [53]. The nomenclature for biodiesel blends is denoted as Bxx, where xx represents the percentage of biofuel in the blend. For example, B40 indicates a 40% biofuel and 60% fossil fuel mixture. A blend of up to B20 can be used in existing internal combustion engines without any structural design changes [54].
3.1. Biodiesel
Biodiesel is composed of fatty acid alkyl esters (FAAEs) and is considered a viable low-carbon alternative for fossil-based diesel, particularly in the transportation sector [55][56]. Biodiesel can be produced through four main routes: oil-blends, micro-emulsion, pyrolysis, and transesterification. The transesterification route is the most preferred due to the quality of fuel produced [57]. Transesterification is an eco-friendly chemical process in which the fatty acids in vegetable oil or animal fat are converted into esters [53]. This reaction is typically carried out using a catalyst, such as sodium hydroxide or potassium hydroxide, and an alcohol, such as methanol or ethanol [58][59]. The parameter with the highest impact on the yield is the temperature, followed by reaction time and pressure. The general reaction of transesterification reaction is depicted in Equation (1).
In this reaction, the fatty acids in the vegetable oil or animal fat react with the alcohol to produce biodiesel and glycerol, where glycerol is a by-product of the reaction and is typically removed and used in other applications, such as soap or animal feed [60][61]. The production of biodiesel is a relatively simple and straightforward process, and it can be carried out on a small or large scale, using a variety of different feedstocks. Biodiesel is a versatile fuel that can be used in a wide range of applications, and it offers several advantages over petroleum-based diesel, including improved air quality, reduced greenhouse gas emissions, and improved energy security [62][63][64]. It is important to note that FT-diesel (covered in later sections), produced through biomass feedstock, is also commonly called biodiesel; however, it is not the same as the FAME biodiesel discussed in this section [65]. A significant obstacle to biodiesel’s widespread commercialisation is its high price and poor efficiency [66]. The high cost of feedstock and lack of infrastructure for moving significant amounts of biodiesel from refineries to major population areas further restrict the availability of this fuel.
3.2. Hydrogenated Vegetable Oil (HVO)
HVO is an alternative fuel made from a variety of vegetable oils and fats containing triglycerides and fatty acids. HVO is another name for hydro-processed esters and fatty acids (HEFA) [67]. It is also known as renewable diesel or green diesel. It is generated through hydrogenation and hydrocracking of paraffinic hydrocarbon forms of lipids where its feedstock is composed of vegetable oil, tallow, and animal fat [68]. Rapeseed is found to produce the highest product yield of HVO of all the feedstocks [69]. Although HVO is obtained from similar feedstocks as biodiesel, it is produced through a hydrotreated process, as opposed to biodiesel, which is obtained through transesterification [70]. This difference helps in improving the oxidation stability of HVO. This results in better resilience to bacterial growth in comparison to biodiesel, making it a better solution for longer and standby applications [71]. Its chemical characteristics are comparable to those of fossil diesel, but differ from diesel with lower density and energy content [72]. It also has a high cetane number and is devoid of sulphur, oxygen, and aromatic hydrocarbons [73]. It is now the second largest renewable diesel alternative, and it is blended with fossil diesel combinations, which are now offered at petrol stations [74][75][76]. The production flow diagram of HVO is illustrated in Figure 3. The general reaction for HVO production is depicted in Equation (2).
The production of HVO is a complex and multi-step process, and it requires specialised equipment and expertise [77]. In the process shown in the equation, the fatty acids in the vegetable oil or animal fat are first converted into paraffin, olefins, and naphthene using a series of chemical reactions, which is followed by combining these chemicals to form the HVO fuel [78][79]. The resulting fuel has a number of advantages over biodiesel, including improved stability, improved energy density, and improved handling characteristics. As a result, HVO is increasingly being used as a replacement for petroleum-based diesel in a variety of applications. Although HVO is comparably better than biodiesel and synthetic diesel with respect to cetane number and other fluid properties, it requires a high capital for its production compared to synthetic and biodiesel [80].
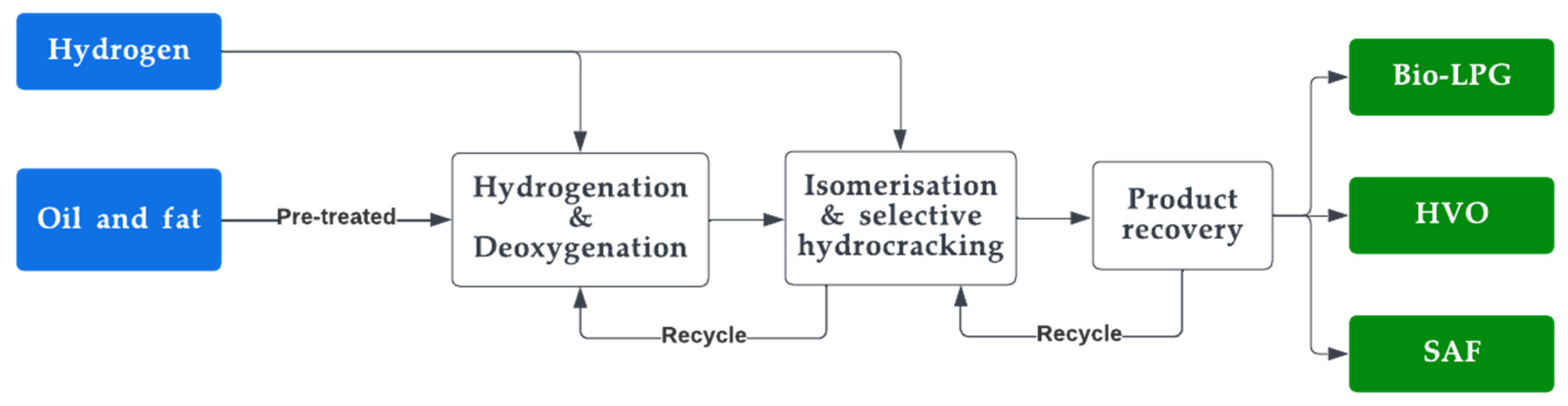
Figure 3. HVO production process.
3.3. Bio-Methanol and Bioethanol
Bioethanol is a type of biofuel that is produced by fermenting biomass, such as corn or sugarcane, with yeast [81]. The resulting liquid can be blended with gasoline to create a fuel that can be used in conventional gasoline engines [82][83]. Mixing bioethanol with unleaded petrol in a ratio of 10% bioethanol and 90% petrol is known as E10, which is commonly offered at filling stations. Another mixture, E85, also exists and contains 85% of ethanol in the mixture. However, this mixture cannot be continuously used on conventional engines as it would eventually result in wear [84][85]. Nevertheless, a specially designed engine for E85 is promising and offers engine operation at lower temperatures [86][87]. Bioethanol has several advantages over gasoline, including reduced greenhouse gas emissions and improved air quality [88]. It is worth noting that, chemically, there is no difference between ethanol and bioethanol. The difference in the name implies that bioethanol is ethanol produced through the biochemical process of fermentation which predominantly uses biological matter as feedstock. The production of bioethanol involves several steps, including harvesting the biomass, grinding it into a fine powder, and mixing it with water and yeast to create a mash. The mash is then fermented, typically using a type of yeast called Saccharomyces cerevisiae [89], which converts the sugars in the biomass into alcohol. The alcohol is then separated from the mash and purified to produce bioethanol. There are various terms coined for the aforementioned processes, namely: saccharification, fermentation, distillation, and ethanol dehydration. The feedstock classification of bio-methanol is the same as that of the general biofuel feedstock classification shown in Figure 2. Currently, most of the biomass produced today is of the first generation, where the most used first-generation feedstocks to produce methanol and ethanol are sugarcane extract and starch [90][91]. Figure 4 illustrates the production of bioethanol and Equation (3) depicts the general reaction of bioethanol production.
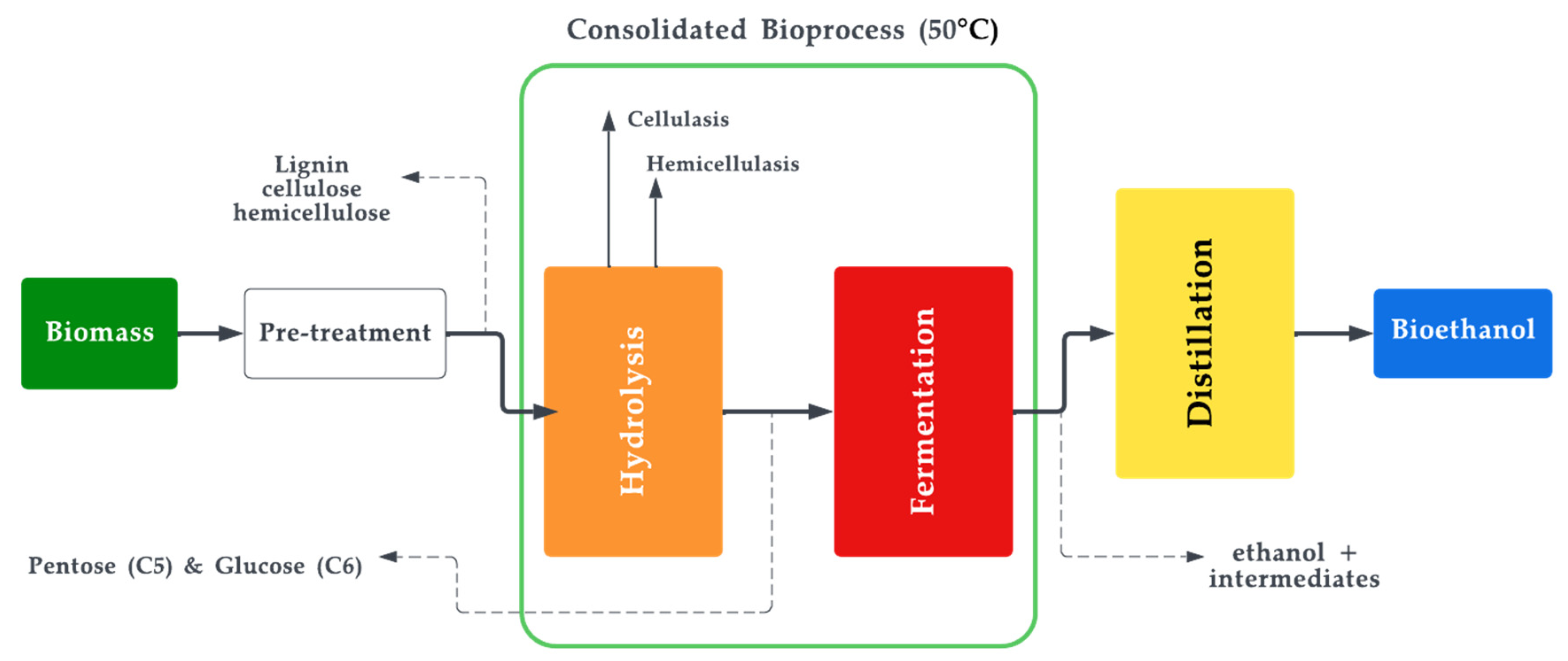
Figure 4. Bioethanol production process.
Production of bio-methanol is similar to that of bioethanol. It is produced through the fermentation of biomass, such as wood or agricultural waste, using microorganisms that are capable of producing methanol [92]. The process is similar to the production of bioethanol, with the main difference being the type of microorganism. The yield of methanol/ethanol is variable and is determined by various factors. Some of the factors include carbon source concentration, fermentation time, temperature, and pH [90]. The general reaction for bio-methanol production through this method is described in Equation (4). It is worth noting that methanol is also produced through the catalytic conversion of syngas commonly around the world [93], which is a mixture of carbon monoxide and hydrogen that is produced from the gasification of biomass. Since the majority of the methods for producing methanol/ethanol include utilizing biomass, solid waste, and carbon capture technologies, the production of methanol and ethanol is sustainable. The reaction for the production of bio-methanol from syngas involves the reverse water-gas shift reaction, depicted in Equation (5), followed by reacting CO with H2, as depicted in Equation (6).
-
Methanol can react with steam at lower temperatures and carries the advantage of a simpler reforming process due to the presence of only one carbon atom [94].
-
Ethanol is non-toxic, as opposed to methanol, which is toxic in nature [94].
-
Ethanol has better combustion performance and lower corrosion when blended with gasoline when compared to methanol [95].
-
Ethanol has higher specific energy than methanol.
-
Methanol is cheaper to produce than ethanol [96].
-
Methanol is preferred for the production of other synthetic fuels, such as biodiesel through the transesterification process, due to its reactivity and higher equilibrium conversion [97].
-
Both ethanol and methanol can be used as fuel in direct alcohol fuel cells, where each fuel displays superiority under different parameters such as catalysts used and temperature [98].
4. Hydrogen Fuels
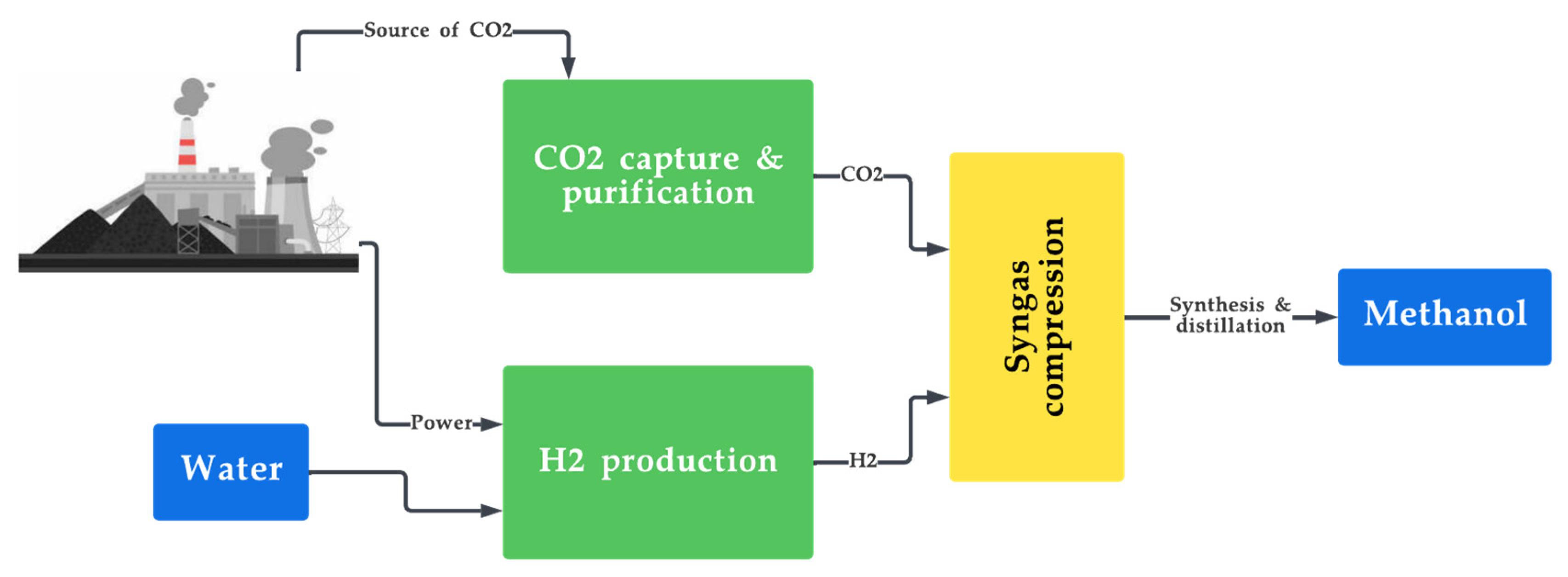
Figure 5. Production of synthetic fuels from CO2 capture and H2 production.
Hydrogen-based synthetic fuels come with their own set of pros and cons. Some of the advantages include the abundant and renewable nature of its feedstock (H2 and CO2) and its ability to be produced locally, eliminating the need for long-distance transportation and thus improving energy security [117][118][119]. Its major disadvantage is its high cost of production as it requires complex and exclusive infrastructure [120][121].
4.1. Hydrogen
Hydrogen energy is a form of clean, renewable energy that can be used for a wide range of applications. It is produced through the process of electrolysis, in which electricity is used to split water into hydrogen and oxygen. There are three main types of hydrogen energy: grey hydrogen, blue hydrogen, and green hydrogen. Grey hydrogen is produced from fossil fuels, such as natural gas, and is the most common type of hydrogen energy. It has high energy density but its production releases carbon dioxide into the atmosphere, contributing to climate change [122]. Green hydrogen, on the other hand, is produced from renewable energy sources such as solar or wind power and is currently being promoted for large-scale adoption for a variety of applications. All three major colour codes of hydrogen are illustrated in Figure 6. In general, hydrogen energy can be used in a number of ways. To name a few:
-
As a fuel for vehicles: It can be used to power cars, buses, and other vehicles. It has a high energy density and can be used to store and transport energy, making it a potential alternative to gasoline and other fossil fuels.
-
For electricity generation: Hydrogen can be burned in a fuel cell to produce electricity, with water as the only by-product. Fuel cells are highly efficient and can be used to power homes, businesses, and other facilities [123].
-
For heating and cooling: Hydrogen can be used as a fuel for heating and cooling systems, providing an alternative to natural gas and other fossil fuels.
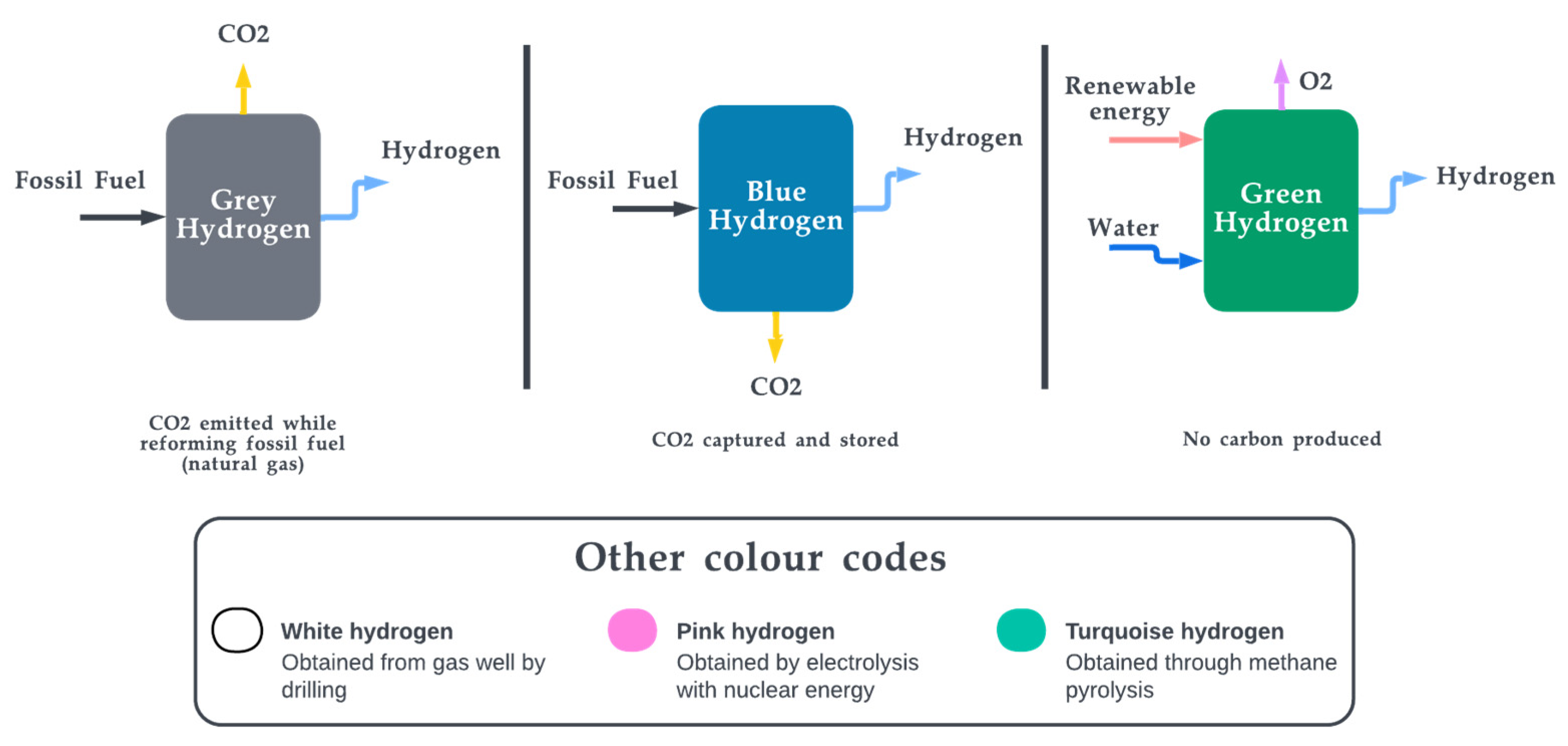
Figure 6. Popular hydrogen colour codes.
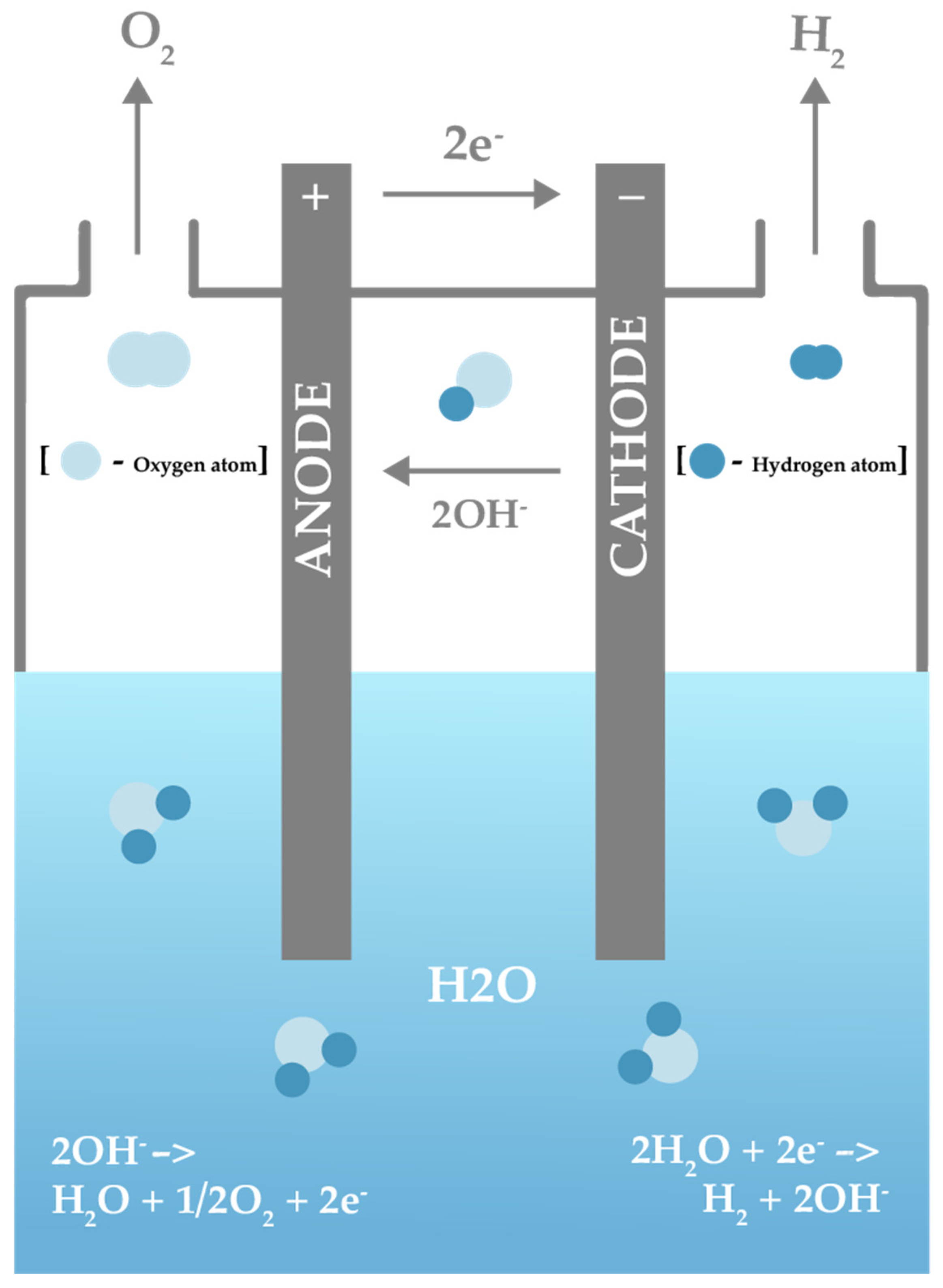
Figure 7. Electrolysis reaction.
Hydrogen energy is being considered to have the potential to play a significant role in the transition to a cleaner and more sustainable energy system on a global scale. As renewable energy sources become more widespread, the production of green hydrogen will increase, helping to reduce the reliance on fossil fuels and decrease greenhouse gas emissions. Currently, worldwide energy organisations are focusing to reduce the costs of producing hydrogen and improving the efficiency of existing systems [131]. An example of one such organisation is IRENA [132]. On the flip side, several academists and professors started highlighting the hydrogen economy to be a scam hosted by fossil fuel industries to delay the current green energy transition [133][134][135][136]. This theory is proposed based on the facts built on the practical efficiencies and costs of incorporating hydrogen systems into the energy transition.
4.2. Syngas
Syngas, also called synthesis gas, is a mixture of carbon monoxide (CO), H2, and CO2 gases that can be used as a fuel or as a feedstock for producing chemicals and other synthetic fuels. Syngas is typically produced through a several-step process with their feedstock being carbon-containing materials, such as coal or biomass [137]. It is produced through the gasification of heavy hydrocarbons and steam reforming or partial oxidation of light hydrocarbons. First, a carbon-containing feedstock is gasified by heating the feedstock in the absence of air but in the presence of partial oxygen, which causes it to break down into its constituent gases. The mixture usually contains H2, CO2, CO, CH4 and nitrogen (N2), where the N2 is inactive and does not participate in any reaction [138]. The preferred composition varies depending on the application, with H2/CO ratios of two or higher for methanol and Fischer–Tropsch synthesis, while a higher ratio is preferred for hydrogen production. DME, higher alcohols, oxo-alcohols, and ethanol are all synthesised from lower H2/CO ratios of around one [139]. When there is a requirement for the ratio to be reduced, the water–gas shift reaction (WGSR) is employed to increase the concentration of H2 and reduce the amount of CO by reforming the mixture with steam and making it more suitable for use as a fuel or for production of other fuels [140]. In contrast, the ratio is increased by converting CO2 content in the mixture into CO through the Reverse-WGSR, also known as catalytic hydrogenation of CO2, whereby the mixture is subjected to high temperature and pressure in the presence of a catalyst, typically a metal such as nickel or cobalt [141][142][143][144]. During the production stages, the composition depends on the reaction conditions and technology employed. The temperature and pressure at which the gasification takes place, as well as the type of feedstock used, can be varied to produce syngas with different compositions and properties. The most important parameters that determine the quality of syngas are the equivalence ratio (ER) and feedstock ash content. The syngas equivalency ratio is a measure of the ratio of the actual fuel–air ratio to the stoichiometric fuel–air ratio necessary for full syngas combustion, where the value of the ratio indicates the quantity of air present to burn the fuel [145][146]. High-quality syngas may be generated at any temperature and pressure combination by adjusting the optimum ER ratio. Figure 8 illustrates the process of producing syngas. The sustainability of syngas production depends on the method and feedstock used to produce it. Syngas produced from fossil fuel sources can be environmentally detrimental. In addition, even when renewable feedstocks are used, the production process requires significant amounts of energy, which often comes from fossil fuels, thus imposing sustainability challenges.
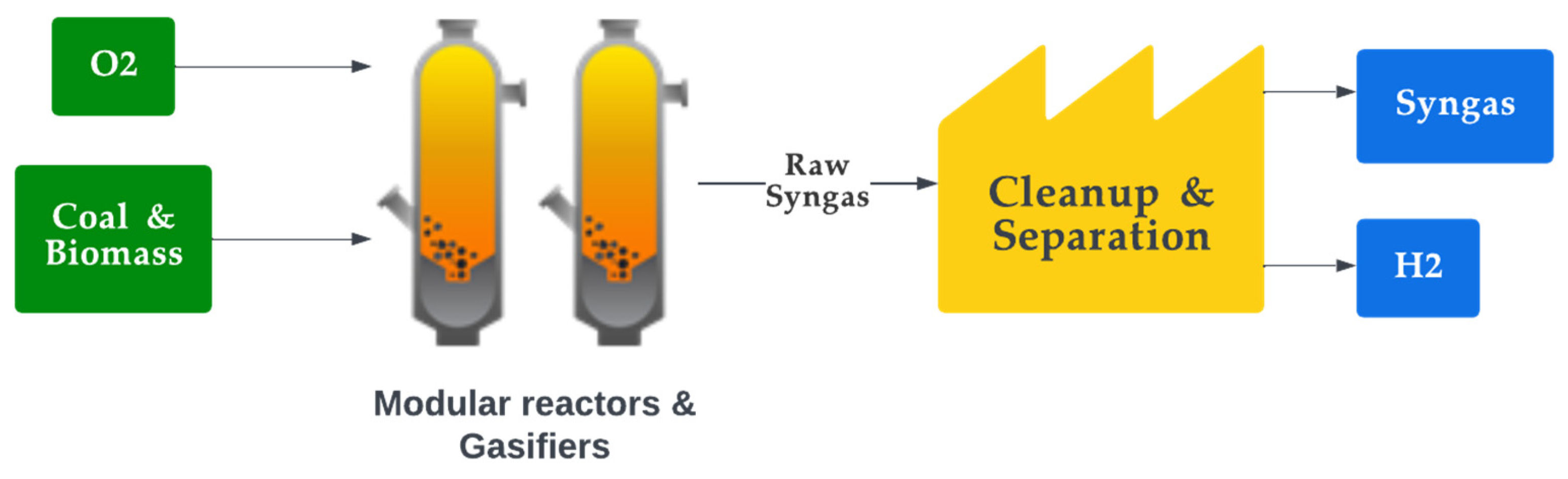
Figure 8. Production of syngas.
Syngas is commonly used for the production of chemicals, such as methanol and synthetic natural gas (SNG), as well as for the production of transportation fuels, such as diesel and jet fuel [147][148]. The technology required for stable production of syngas production from the gasification of biomass continues to be in its infancy, making commercial-scale production a bottleneck [149]. Figure 9 depicts the various uses of the syngas [150].
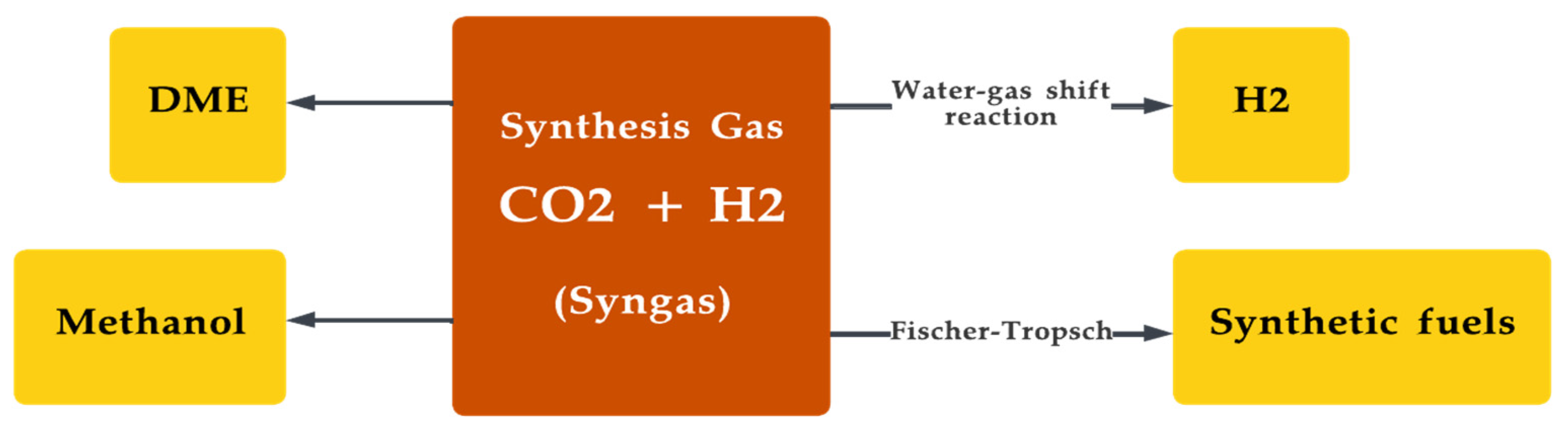
Figure 9. Production of various synthetic fuels from synthesis gas.
4.3. Methanol
-
The fermentation of biomass: In this process, biomass, such as wood or agricultural waste, is fermented to produce methanol. It basically converts methane produced from biomaterial into methanol. This type is also known as bio-methanol and was discussed in detail in the previous sections. This method is typically used on a small scale and it is often used to produce methanol from waste materials or other low-value feedstocks. Figure 10 depicts the feedstocks for all types of methanol.
-
Direct methane to methanol conversion: The industrial process of methane to methanol transformation is carried out through the conventional (indirect) route, which is expensive, owing to its high energy requirements. Direct conversion routes for methane to methanol such as plasma, photocatalytic, supercritical water, and biological routes have been developed to achieve cost-effectiveness and feasibility [93]. However, these technologies are in their early stages and have a time window of 5 to 20 years for industrial feasibility.
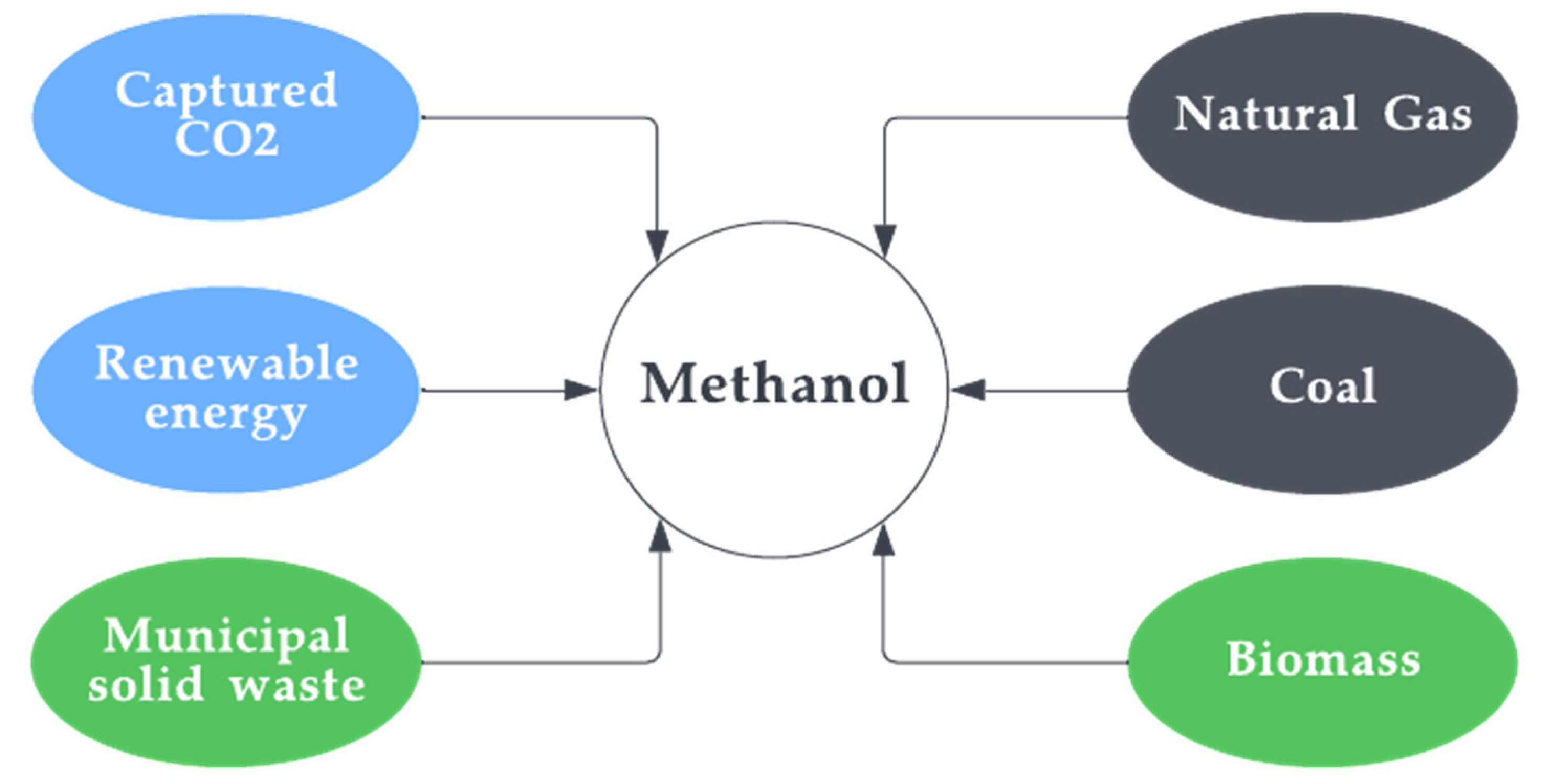
Figure 10. Feedstocks of methanol.
Conventionally, methanol is produced on a large scale from natural gas. This method is considered to be indirect since the natural gas (methane) is first reformed to obtain CO, followed by its hydrogenation in the presence of a catalyst, such as zinc oxide or copper chromite, to produce methanol. The process is typically carried out at high temperatures and pressures, which helps to increase the reaction rate and the yield of methanol. The reaction is exothermic, which means it releases heat, and the heat is typically removed through cooling systems to prevent the reaction from getting out of control. The main advantage of this method for producing methanol is that it can be carried out on a small scale and it can be used to produce methanol from waste gases or other carbon-rich feedstocks. This makes it a potentially economical and sustainable method for producing methanol and a way to reduce greenhouse gas emissions and air pollution. However, there are also some disadvantages to the hydration of carbon monoxide as a method for producing methanol. The process requires specialised equipment and a high-quality catalyst, both of which can add to expenses. Figure 11 illustrates the above-mentioned process pictorially.
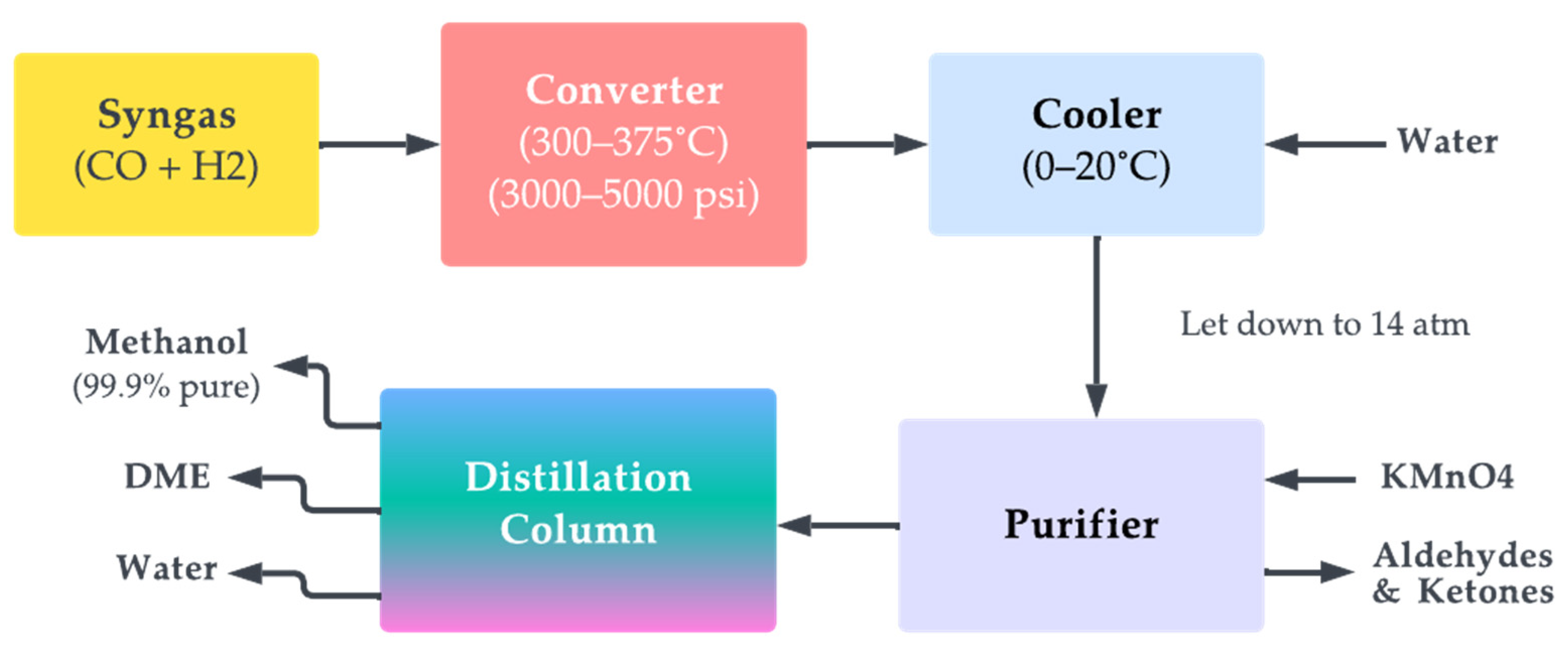
Figure 11. Methanol synthesis process.
4.4. Dimethyl Ethers (DME)
DME is a synthetic alternative to diesel that is intended for use in specifically engineered compression-ignition diesel engines [160]. It has physical characteristics comparable to liquefied petroleum gases and it burns with a visible blue flame and does not generate peroxides in its pure form or in the aerosol formulation. It also produces significantly lower levels of CO2, NOx, SOx, and particulate matter, giving it a significantly lower emissions profile compared to diesel and other fossil fuels [161][162]. It is the simplest form of ether, with a chemical formula of CH3OCH3. Its advantages include clean combustion, high-efficiency compression, the ability to be easily reformed to hydrogen at low temperatures, near non-toxic nature, and cost-effective production and transportation compared to other fuels [163]. The conventional production of DME involves the dehydration of methanol. In this process, methanol is heated in the presence of a strong acid catalyst, such as sulfuric acid, to remove the water molecule and produce DME. As discussed in the previous sections, syngas is first produced from biomass, followed by the simultaneous production and dehydration of methanol as per the methods discussed earlier. The reaction is also called the dimerization of methanol and its general reaction is depicted in Equation (8) [164]. This is the conventional process used on large scale for producing DME as it utilises the same infrastructure used for methanol production [165]. The DME fuel mixture typically has a composition of DME, methanol, and water, where the fuel-grade DME is of its purest form with >99% of DME in the mixture, while technical grade DME has a lower concentration of DME in the mixture [163][166]. As the name suggests, the fuel grade DME is typically used as fuel in the transportation sector, while the technical grade DME is used as an intermediate in the production of other chemicals and hydrocarbon fuels [163][167]. It has promising applications, ranging from being used as a transportation fuel or a solvent in propellants to being used as a direct fuel in fuel cells (DDMEFC) [163][168]. However, its usage in fuel cells is in its early laboratory stages.
Oligomers of DME are also synthetic fuels that are comparable to DME. They are a group of oxymethylene ethers (OMEx), where the term oligomer implies that OMEx is a chain of repeating units of the DME ester [169]. These OMEx have similar properties to DME but have higher molecular mass contributing to significant changes in their physical properties such as higher boiling points or different states at lower temperatures [170]. Mentioning OMEx here is significant since DME has properties similar to that of LPG, while OMEx behaves similarly to LPG but has physical and chemical properties similar to that of diesel [170]. OME of different oligomer lengths is chosen based on the use and need. Coming to the nomenclature, DME is considered OME0, whereas OME1 would imply that one more ester block is added to the initial DME block. For OME2, two extra ester blocks would be added to the chain and so on for other oligomers. Collectively, OMEx is known as polyoxymethylene dimethyl ethers. Figure 12 illustrates the structure of DME and OMEx with an example. OMEx can be prepared from DME through two routes, as illustrated in Figure 13.
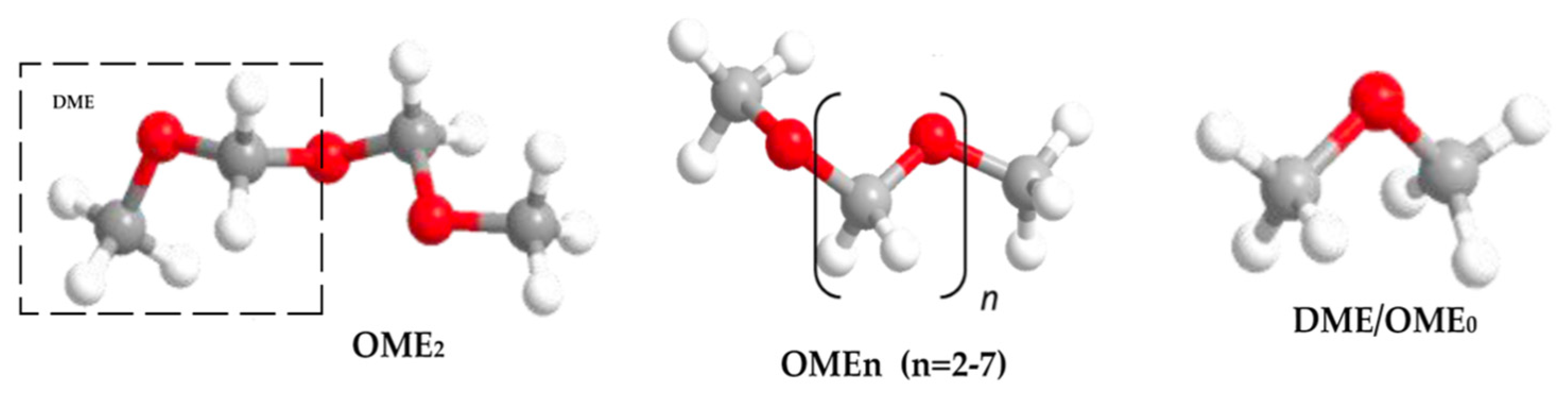
Figure 12. Nomenclature of OMEx.
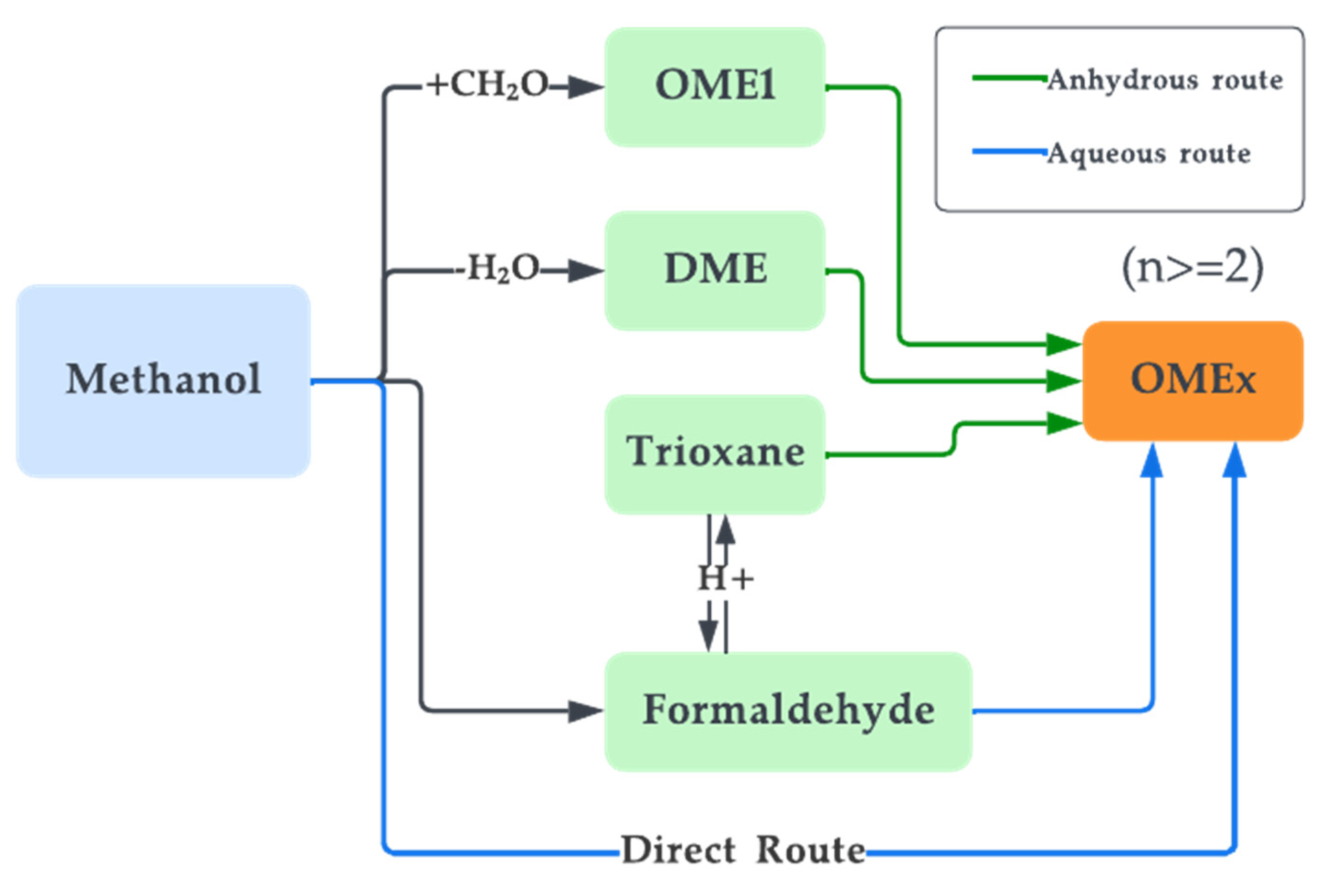
Figure 13. OMEx synthesis routes.
DME can thus be used in a variety of applications such as fuel for heating, transportation, and electricity generation. DME can also be converted into other chemicals and fuels [171]. For example, it can be converted into dimethyl sulphate (DMS), which is used in the production of detergents and other household products. It has a high cetane number and is currently considered for replacing diesel as fuel for trucks and ships. It is thus considered a fuel with significant potential [172]. DME carries similar disadvantages to that of methanol, stopping its large-scale adoption.
5. Power-to-Liquid (PtL)
-
Hydrogen: It is considered a PtL fuel since it can be produced purely from renewable electricity and water as its feedstock. It can be used as a standalone fuel in fuel cell vehicles or blended with natural gas to produce a lower-carbon fuel for use in internal combustion engines.
-
Methanol, Ethanol and DME: The production of methanol, ethanol, and DME are discussed in the earlier sections in detail, which brings ambiguity in mentioning them here again. With the other feedstocks remaining the same, if the hydrogen required for their production is obtained from clean energy, they are considered as PtL fuels. The production methods, however, remain the same. Methanol and ethanol produced as PtL fuels are also called e-methanol and e-ethanol.
-
Synthetic diesel: Synthetic diesel is a PtL fuel that can be used in any diesel engine with little or no modification.
-
PtL-kerosene: This is an e-fuel produced through renewable energy that is under development stages in the aviation industry.
-
Ammonia: Ammonia is a chemical compound that can be produced from renewable electricity and used as fuel in specialised engines or fuel cells.
One of the main benefits of PtL fuels is their ability to store excess renewable electricity for use when it is needed [177][178]. This can help to smooth out fluctuations in the electricity grid and make it easier to integrate renewable energy sources into the energy mix [179]. PtL fuels also have the potential to reduce greenhouse gas emissions, as they do not release additional carbon dioxide during their production or use [180]. PtL fuels are a promising technology that could help to reduce the reliance on fossil fuels and lower greenhouse gas emissions, especially in the transportation sector [181][182]. While further research and development are needed to bring these fuels to market, they have the potential to play a significant role in the transition to a more sustainable energy future.
5.1. Synthetic Diesel
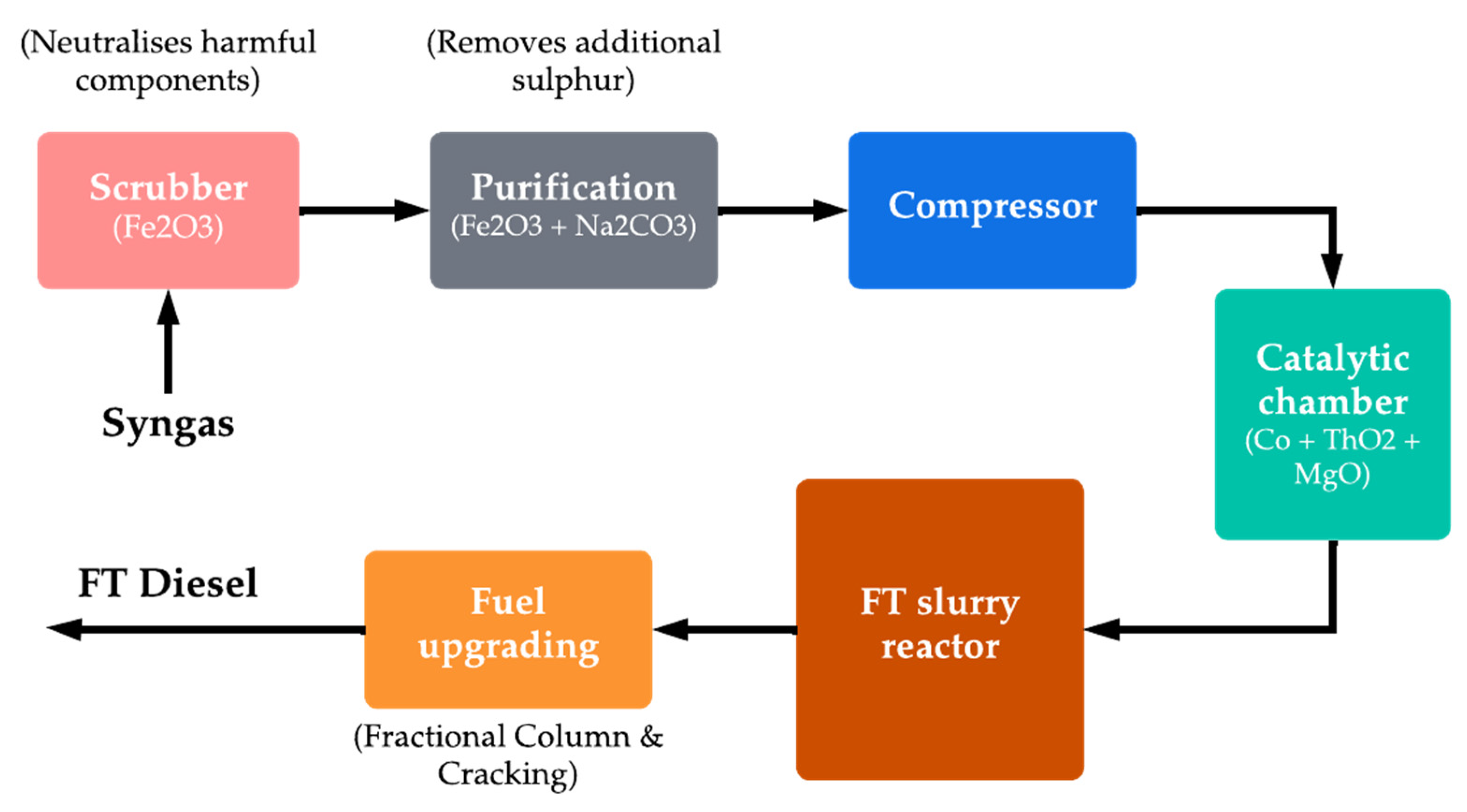
Figure 14. Typical Fischer–Tropsch process for FT diesel production from syngas.
The mentioned process is used in a variety of applications, including the production of synthetic fuels such as synthetic diesel, synthetic gasoline, and jet fuel for aviation, naphtha, and in the production of chemicals and other industrial products [188][189][190]. The method can be employed to produce synthetic fuels at economical costs when compared to the production of synthetic fuels through other methods. However, the process is energy-intensive and can be costly under certain conditions, and the feedstocks used can have significant environmental impacts, depending on their source [190][191]. After obtaining the fuel by Fischer–Tropsch, the excess by-products are processed under the water–gas shift (WGS) reaction [192], which is a chemical reaction in which CO and water are converted into H2 and CO2, which can be once again used as feedstock for the production of other synthetic fuels [193]. Synthetic diesel is considered to be sustainable if the energy required to synthesise the fuel is obtained from renewable sources. As mentioned in earlier sections, the technological limitation of commercial-scale biomass gasification is also the limiting factor for the expansion of FT diesel.
5.2. PtL-Kerosene
PtL-kerosene is another synthetic fuel produced through the Fischer–Tropsch process. The production of PtL-kerosene typically involves the use of electrolysis to produce hydrogen from water, which is then combined with carbon dioxide to form syngas, followed by the Fischer–Tropsch process to produce PtL-kerosene. To improve the quality of its production, a hydrocracker is employed after the FT process to break long hydrocarbon chains and aid the production of hydrocarbons in the middle distillate range, where kerosene resides [194]. PtL-kerosene is considered to be a Sustainable Aviation Fuel (SAF) as its main application is for powering aircraft [176][178][195]. The PtL-kerosene produced is blended with kerosene-based jet fuels such as Jet A and Jet A-1, JP-5, and JP-8. Jet A-1 is the closest form of jet fuel to PtL-kerosene, as it itself is a highly refined form of kerosene [196][197][198]. Figure 15 shows the cycle of PtL-kerosene production and its use.
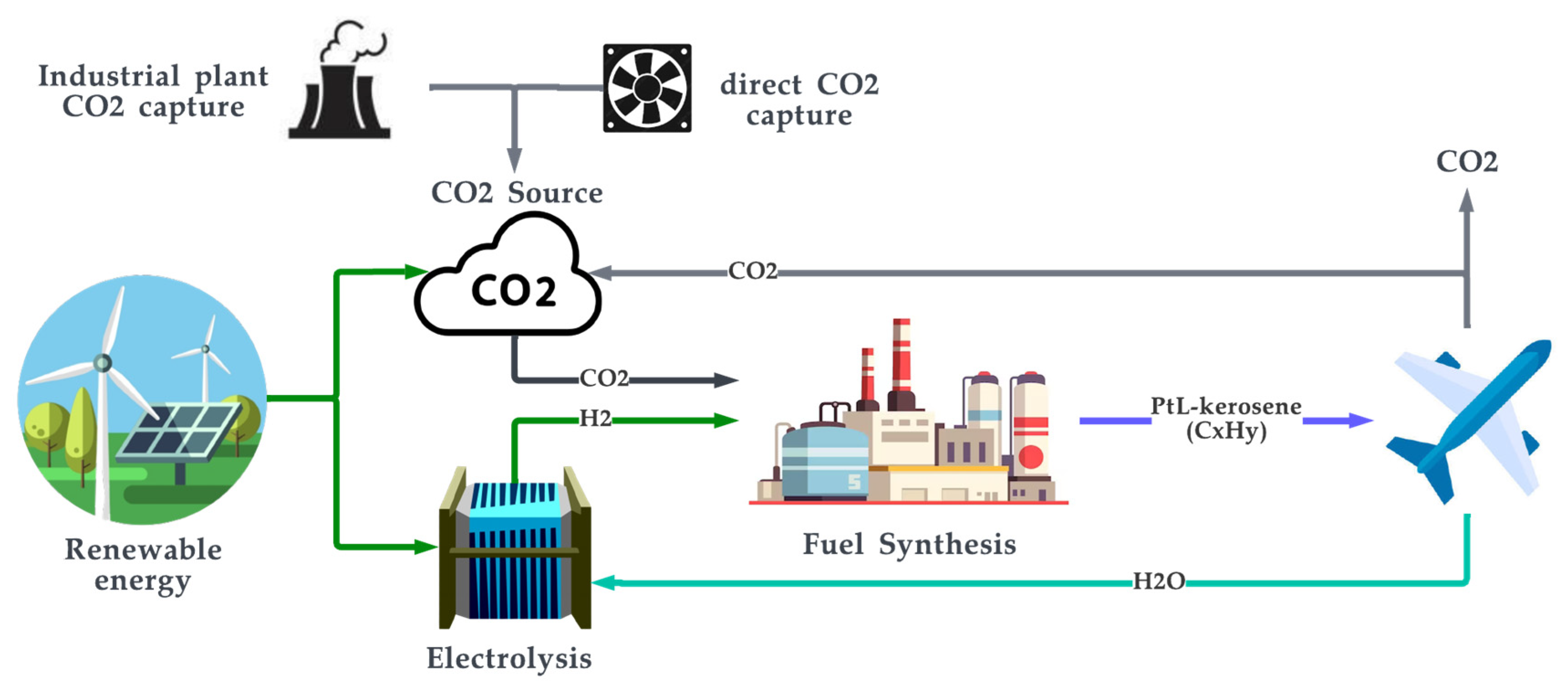
Figure 15. PtL-kerosene production and use cycle.
The production quantity of SAF is negligible compared to conventional aviation fuel production volumes, as the commercial-scale production of this fuel has a few challenges to consider, such as compositional complexity and variability of feedstock, rapid growth in hydrogen generation technologies, and risk in investment of erecting plants considering a history of abandoned flagship projects in the aviation industry [199]. Despite this, its adoption is likely to increase as more companies and countries seek to transition to a cleaner and more sustainable energy system.
5.3. Ammonia
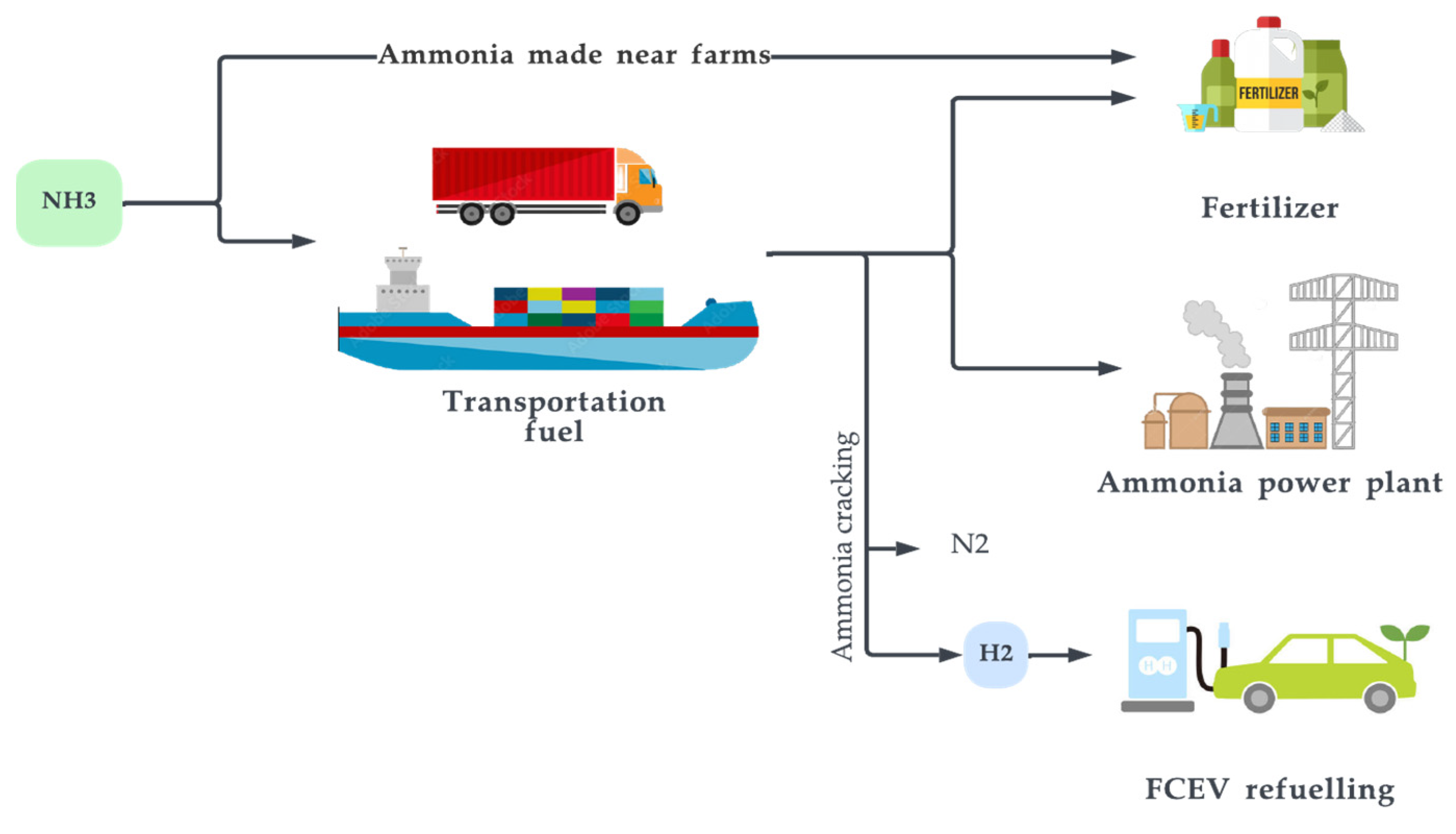
Figure 16. Major uses of ammonia.
6. Gas-to-Liquid (GtL)
-
GtL Diesel: Gas-to-liquid (GtL) diesel and power-to-liquid (PtL) diesel are both essentially the same synthetic diesel fuel produced through different processes. One key difference between GtL and PtL diesel is the source of the starting material. GtL diesel is produced through the conversion of natural gas or other hydrocarbon gases into a liquid form, while it is considered PtL when the energy required is obtained from renewable sources. As a result, PtL diesel is considered to be a more environmentally friendly option compared to GtL diesel, as it does not release additional carbon dioxide into the atmosphere during its production. GtL diesel is commonly used in transportation and other applications and is often considered to be a cleaner burning fuel compared to traditional diesel, as it contains fewer impurities and produces fewer emissions.
-
Jet fuel: Jet fuel is another type of GtL fuel that is commonly used in the aviation industry. GtL jet fuel is often considered to be a more sustainable alternative to traditional jet fuel, as it has a lower carbon intensity and produces fewer emissions. This is the same as the SAF discussed in the earlier sections and the differentiation between them is once again analogous to that of synthetic diesel in the earlier point.
-
Naphtha: Naphtha is a type of GtL fuel that is used as a feedstock for the production of chemicals and plastics. It can be produced from other hydrocarbon gases and is sometimes used as a blend stock for gasoline.
-
LPG: LPG, or liquefied petroleum gas, is a type of GtL fuel that is commonly used for heating purposes. It is a mixture of propane and butane and is often considered to be a cleaner-burning alternative to traditional fossil fuels.
6.1. Naphtha
Naphtha is a type of liquid hydrocarbon that is produced as a by-product while refining crude oil in the petrochemical industry [214]. It is a colourless or light-yellow liquid with a gasoline-like odour and is a complex mixture of hydrocarbons that can be used as a feedstock for the production of various chemicals and fuels. Naphtha is considered a GtL fuel since it can be produced through the FT process discussed in earlier sections [215]. However, on an industrial scale, it typically is produced through a refining process known as distillation [216]. First, crude oil or coal tar is heated and distilled to separate it into various components based on their boiling points [217]. The resulting mixture is then further processed through a series of distillation columns, where the naphtha is separated from other components such as gasoline and kerosene. It is typically produced from the middle distillates of crude oil, which are the components that have a boiling point between approximately 200–300 °C. The naphtha is then treated with hydrogen and passed over a catalyst, such as a metal or metal oxide, to remove impurities and improve its quality. The purified naphtha is then cooled and stored for further use or transportation to other facilities for further processing. Naphtha is typically composed of hydrocarbons with carbon numbers in the range of about 5 to 12 [218]. It is made up of n-paraffins and olefins; the paraffin yield quality favours high temperature and low pressure while the olefin composition varies on the reaction pathway, where a greater olefin composition denotes a higher octane number and, consequently, better fuel quality [219][220]. During the refining process of petroleum, crude oil is heated and distilled to separate it into various hydrocarbon components based on their boiling points. In addition, bio-naphtha is a type of naphtha that is produced from biomass feedstocks rather than fossil fuels such as petroleum or coal. Similar to the typical FT process, bio-naphtha can be produced through a biomass-to-liquid Fischer–Tropsch (BTL FT) process [221]. Figure 17 depicts the cycle of naphtha’s applications.
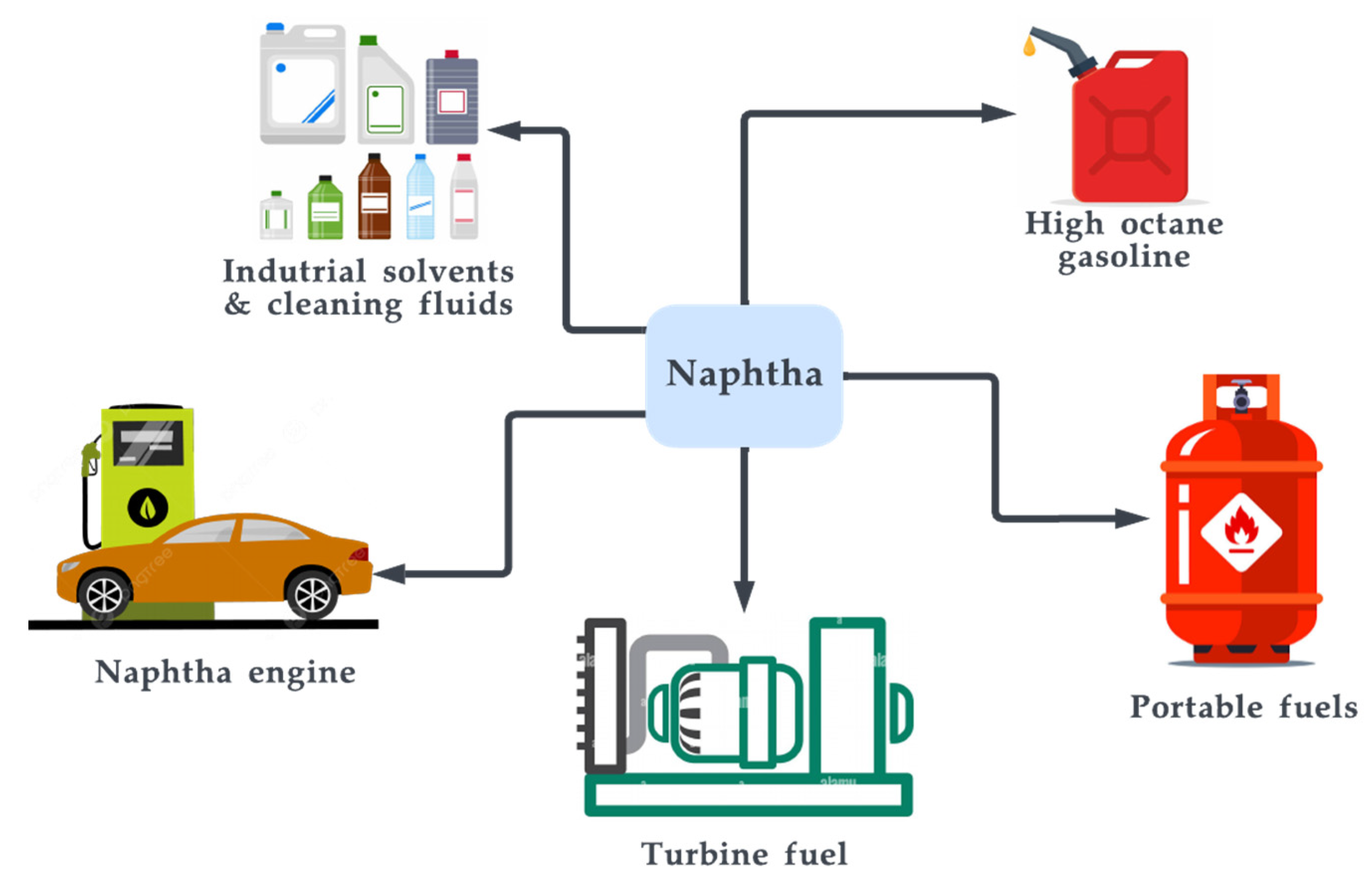
Figure 17. Applications of naphtha.
Naphtha has a wide range of uses in the chemical and fuel industries. It is commonly used as a feedstock for the production of gasoline, as well as for the production of other chemicals such as petrochemicals, solvents, plastics, and resins [222]. Naphtha is also used as fuel for portable stoves and other heating and cooking applications. One of the key properties of naphtha is its high energy density, which makes it an attractive fuel for transportation applications [223][224]. On the other hand, it is important to note that the production and use of naphtha, like other fossil fuels, can have environmental impacts. However, the environmental impacts of naphtha can be mitigated through the use of advanced technologies and practices. It is also important to be aware that naphtha can have potentially harmful effects on human health if contacted directly.
6.2. Liquefied Petroleum Gas (LPG)
LPG is a mixture of propane and butane and is produced through the refining of petroleum or the extraction of natural gas [225]. It is a GtL fuel that is produced through several steps involving the conversion of natural gas or other gases into liquid form. First, natural gas or other gases are cleaned and purified to remove impurities such as water and sulphur. The purified gases are then converted into synthesis gas, or syngas, through a process called steam methane reforming. This process involves the reaction of the gas with steam and a catalyst to produce hydrogen and carbon monoxide. The syngas is then passed over a catalyst, such as a metal or metal oxide, to convert it into a range of hydrocarbons, including LPG [226]. LPG is also produced through fractional distillation as explained in the previous section, with it being in the top tier as shown in Figure 18. The parameters that favour a high yield of LPG include lower temperature, optimum steam-to-feed ratio and more trays in the furnace [227].
LPG has a wide range of applications, including as a fuel for cooking and heating in residential and commercial settings, where it is a popular choice due to its clean-burning nature, which results in fewer emissions compared to other fossil fuels [228]. It is also used as fuel for vehicles, including cars, buses, and trucks. It is also used as feedstock for the production of chemicals and plastics and as a solvent for a variety of products. It is commonly used in the production of propylene, which is used in the manufacture of plastics, resins, and other products [229]. An uncommon usage of LPG is its usage as a fuel for recreational vehicles, such as boats and recreational vehicles (RVs). It is also a relatively inexpensive fuel, making it an attractive option for many consumers [230]. It is abundant in many parts of the world but is often difficult to transport due to its gaseous nature [231]. Its transportation difficulty, higher engine burning temperatures, higher consumption, NOx emission, non-sustainable nature, and high cost of equipment are its major drawbacks [232]. Although LPG is not sustainable, it has a significantly lower impact on the environment as opposed to other fossil fuels.
7. Current challenges and the Future Landscape of Synthetic Fuels
-
Cost: Transitioning to low-carbon or zero-emissions technologies can be expensive. Many countries rely heavily on fossil fuels for energy, and transitioning away from these sources will require significant investments in new infrastructure.
-
Technological challenges: There may be technical challenges involved in transitioning to low-carbon or zero-emissions technologies, such as the need to develop new technologies or modify existing ones.
-
Political challenges: There may be political challenges to transitioning to net zero emissions, as it may require significant policy changes and the implementation of new regulations. This can be difficult to achieve, particularly if there is resistance to change from certain groups or sectors of society.
-
Behavioural change: Achieving net zero emissions will also require individuals and businesses to change their behaviour, such as using low-carbon transportation options and adopting energy-efficient practices. Changing behaviours can be difficult, particularly if people are resistant to change.
-
International cooperation: Achieving net zero emissions will require cooperation between countries, as global emissions need to be reduced. This can be challenging, as different countries have different priorities and may not agree on the best course of action.
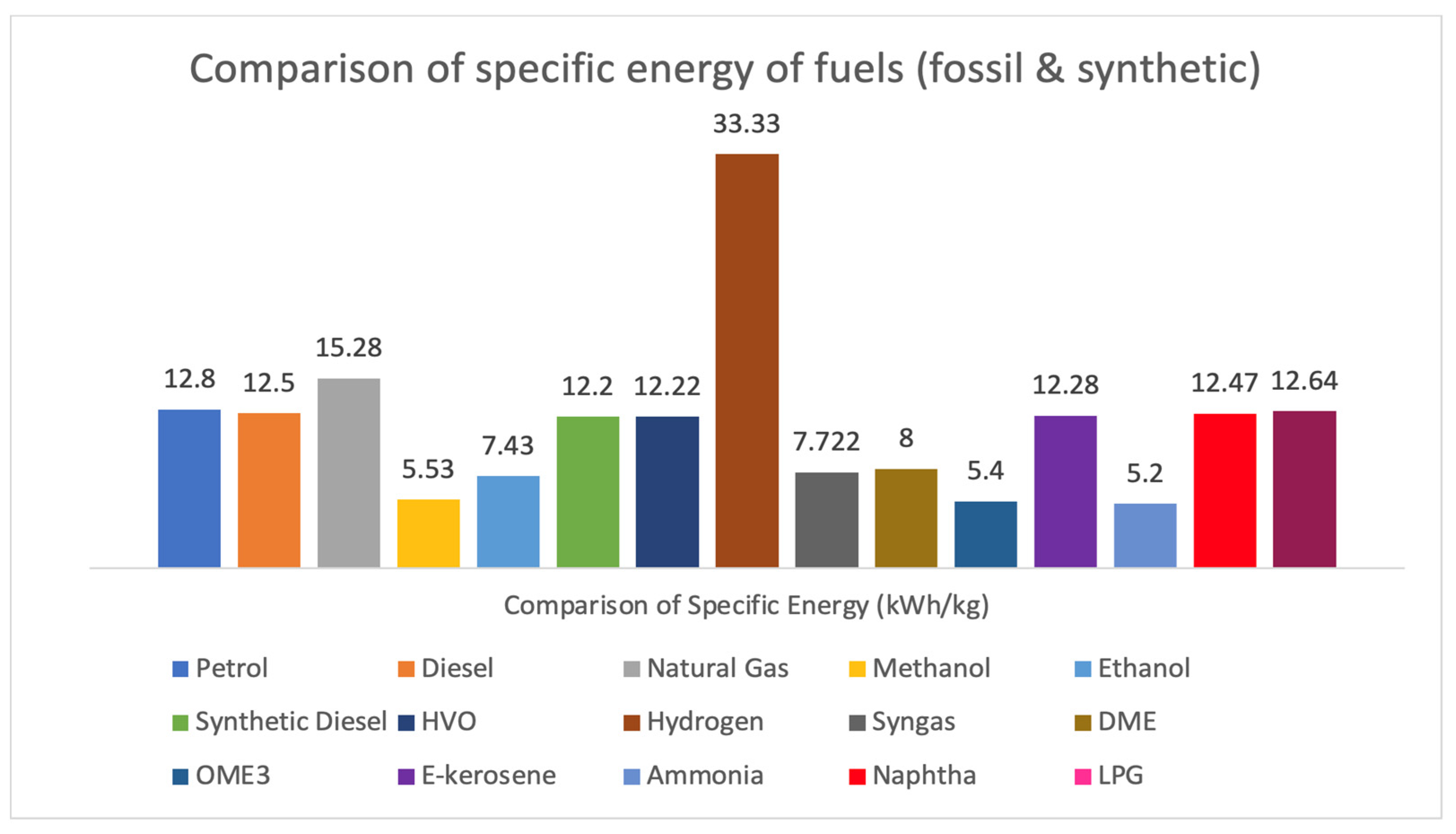
Figure 18. Fuel comparison chart.
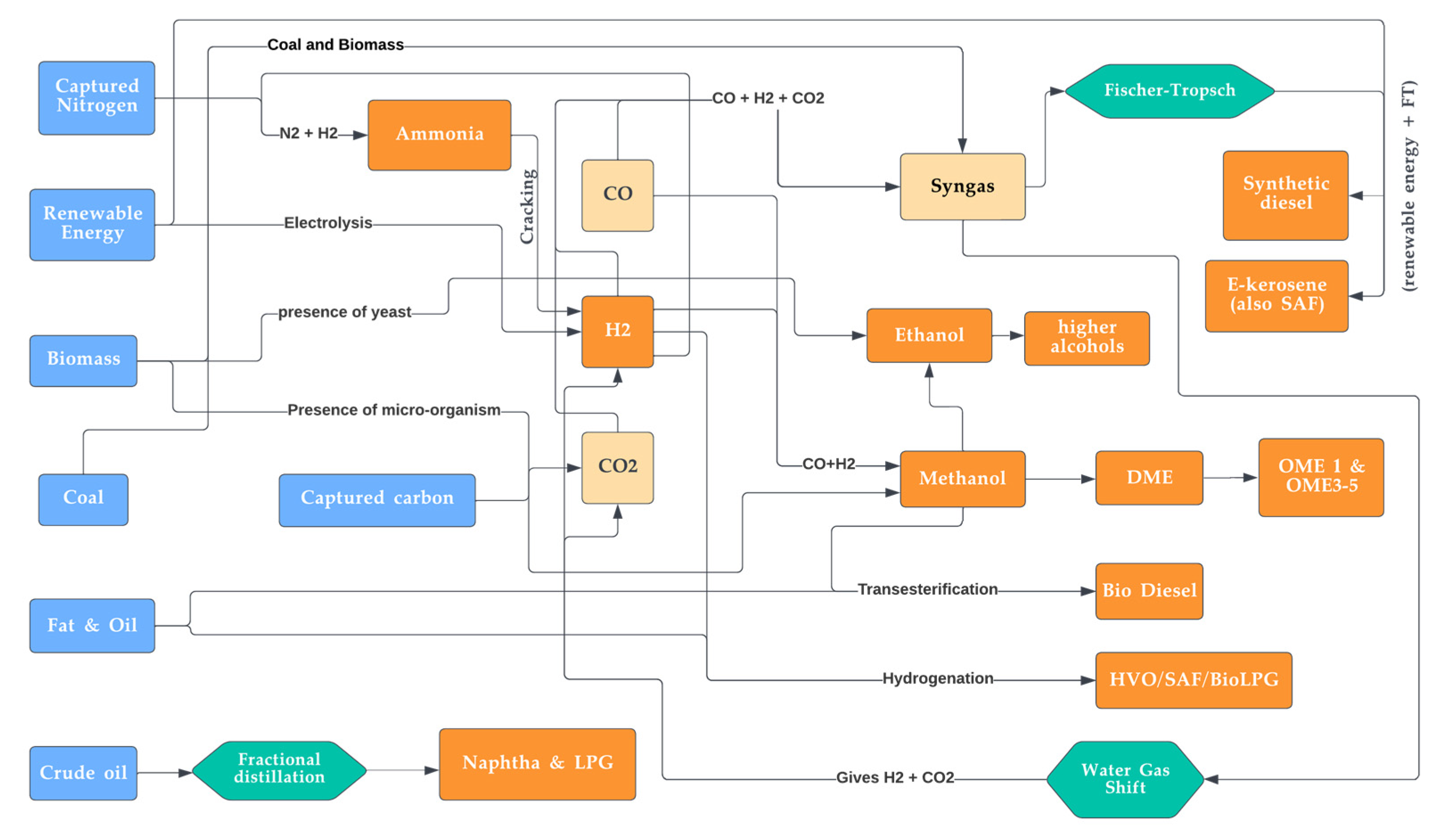
Figure 19. Overview of combined routes for the synthesis of various major synthetic fuels.
References
- Ready, E.; Collings, P. “All the Problems in the Community Are Multifaceted and Related to Each Other”: Inuit Concerns in an Era of Climate Change. Am. J. Hum. Biol. 2021, 33, e23516.
- de Guttry, C.; Süsser, D.; Döring, M. Situating Climate Change: Psychological Distances as Tool to Understand the Multifaceted Dimensions of Climate Change Meanings. Geoforum 2019, 104, 92–100.
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Šegvić Klarić, M.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment Changes, Aflatoxins, and Health Issues, a Review. Int. J. Environ. Res. Public Health 2020, 17, 7850.
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Jorgenson, A.K.; Fiske, S.; Hubacek, K.; Li, J.; McGovern, T.; Rick, T.; Schor, J.B.; Solecki, W.; York, R.; Zycherman, A. Social Science Perspectives on Drivers of and Responses to Global Climate Change. WIREs Clim. Chang. 2019, 10, e554.
- Chen, T.; Bao, A.; Jiapaer, G.; Guo, H.; Zheng, G.; Jiang, L.; Chang, C.; Tuerhanjiang, L. Disentangling the Relative Impacts of Climate Change and Human Activities on Arid and Semiarid Grasslands in Central Asia during 1982–2015. Sci. Total Environ. 2019, 653, 1311–1325.
- Sanson, A.V.; Burke, S.E.L. Climate Change and Children: An Issue of Intergenerational Justice. In Children and Peace: From Research to Action; Balvin, N., Christie, D.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 343–362. ISBN 978-3-030-22176-8.
- Mah, A.Y.J.; Chapman, D.A.; Markowitz, E.M.; Lickel, B. Coping with Climate Change: Three Insights for Research, Intervention, and Communication to Promote Adaptive Coping to Climate Change. J. Anxiety Disord. 2020, 75, 102282.
- Froese, R.; Schilling, J. The Nexus of Climate Change, Land Use, and Conflicts. Curr. Clim. Chang. Rep. 2019, 5, 24–35.
- Marx, W.; Haunschild, R.; Bornmann, L. Heat Waves: A Hot Topic in Climate Change Research. Theor. Appl. Climatol. 2021, 146, 781–800.
- Stillman, J.H. Heat Waves, the New Normal: Summertime Temperature Extremes Will Impact Animals, Ecosystems, and Human Communities. Physiology 2019, 34, 86–100.
- Perera, A.T.D.; Nik, V.M.; Chen, D.; Scartezzini, J.-L.; Hong, T. Quantifying the Impacts of Climate Change and Extreme Climate Events on Energy Systems. Nat. Energy 2020, 5, 150–159.
- Cianconi, P.; Betrò, S.; Janiri, L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front. Psychiatry 2020, 11, 74.
- FitzGerald, D.M.; Hughes, Z. Marsh Processes and Their Response to Climate Change and Sea-Level Rise. Annu. Rev. Earth Planet. Sci. 2019, 47, 481–517.
- Konapala, G.; Mishra, A.K.; Wada, Y.; Mann, M.E. Climate Change Will Affect Global Water Availability through Compounding Changes in Seasonal Precipitation and Evaporation. Nat. Commun. 2020, 11, 3044.
- Ballew, M.T.; Leiserowitz, A.; Roser-Renouf, C.; Rosenthal, S.A.; Kotcher, J.E.; Marlon, J.R.; Lyon, E.; Goldberg, M.H.; Maibach, E.W. Climate Change in the American Mind: Data, Tools, and Trends. Environ. Sci. Policy Sustain. Dev. 2019, 61, 4–18.
- Treen, K.M.D.I.; Williams, H.T.P.; O’Neill, S.J. Online Misinformation about Climate Change. WIREs Clim. Chang. 2020, 11, e665.
- Schäfer, M.S. Online Communication on Climate Change and Climate Politics: A Literature Review. WIREs Clim. Chang. 2012, 3, 527–543.
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Arenas, L.F.V.; Navarrete, B. Carbon Capture and Utilization Technologies: A Literature Review and Recent Advances. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 1403–1433.
- Maestre-Andrés, S.; Drews, S.; van den Bergh, J. Perceived Fairness and Public Acceptability of Carbon Pricing: A Review of the Literature. Clim. Policy 2019, 19, 1186–1204.
- Abdin, Z.; Mérida, W. Hybrid Energy Systems for Off-Grid Power Supply and Hydrogen Production Based on Renewable Energy: A Techno-Economic Analysis. Energy Convers. Manag. 2019, 196, 1068–1079.
- Moioli, E.; Schildhauer, T. Negative CO2 Emissions from Flexible Biofuel Synthesis: Concepts, Potentials, Technologies. Renew. Sustain. Energy Rev. 2022, 158, 112120.
- Toborek-Mazur, J.; Partacz, K.; Surówka, M. Energy Security as a Premise for Mergers and Acquisitions on the Example of the Multi-Energy Concern PKN Orlen in the Face of the Challenges of the 2020s. Energies 2022, 15, 5112.
- Koyamparambath, A.; Santillán-Saldivar, J.; McLellan, B.; Sonnemann, G. Supply Risk Evolution of Raw Materials for Batteries and Fossil Fuels for Selected OECD Countries (2000–2018). Resour. Policy 2022, 75, 102465.
- Białecki, T. Mathematical Model of the Combustion Process for Turbojet Engine Based on Fuel Properties. Int. J. Energy Environ. Eng. 2022, 13, 1309–1316.
- Ngũgĩ, J.M.; Richter, S.; Braun-Unkhoff, M.; Naumann, C.; Riedel, U. A Study on Fundamental Combustion Properties of Oxymethylene Ether-1, the Primary Reference Fuel 90, and Their Blend: Experiments and Modeling. Combust. Flame 2022, 243, 111996.
- Okoro, V.; Azimov, U.; Munoz, J. Recent Advances in Production of Bioenergy Carrying Molecules, Microbial Fuels, and Fuel Design—A Review. Fuel 2022, 316, 123330.
- Pratschner, S.; Hammerschmid, M.; Müller, F.J.; Müller, S.; Winter, F. Simulation of a Pilot Scale Power-to-Liquid Plant Producing Synthetic Fuel and Wax by Combining Fischer–Tropsch Synthesis and SOEC. Energies 2022, 15, 4134.
- Cabrera, E.; de Sousa, J.M.M. Use of Sustainable Fuels in Aviation—A Review. Energies 2022, 15, 2440.
- Galimova, T.; Ram, M.; Bogdanov, D.; Fasihi, M.; Khalili, S.; Gulagi, A.; Karjunen, H.; Mensah, T.N.O.; Breyer, C. Global Demand Analysis for Carbon Dioxide as Raw Material from Key Industrial Sources and Direct Air Capture to Produce Renewable Electricity-Based Fuels and Chemicals. J. Clean. Prod. 2022, 373, 133920.
- Winter, C.; Schröter, B.; Fidaschek, S. The German Cement Industry as a CO2 Source for Other Industries. Fuels 2022, 3, 342–352.
- Kannan, R.; Panos, E.; Hirschberg, S.; Kober, T. A Net-Zero Swiss Energy System by 2050: Technological and Policy Options for the Transition of the Transportation Sector. Futures Foresight Sci. 2022, 4, e126.
- Du, X.; Peng, Y.; Albero, J.; Li, D.; Hu, C.; García, H. Synthetic Fuels from Biomass: Photocatalytic Hydrodecarboxylation of Octanoic Acid by Ni Nanoparticles Deposited on TiO2. ChemSusChem 2022, 15, e202102107.
- Li, Q.; Cherian, J.; Shabbir, M.S.; Sial, M.S.; Li, J.; Mester, I.; Badulescu, A. Exploring the Relationship between Renewable Energy Sources and Economic Growth. The Case of SAARC Countries. Energies 2021, 14, 520.
- Moriarty, P.; Honnery, D. Feasibility of a 100% Global Renewable Energy System. Energies 2020, 13, 5543.
- Taghizadeh-Hesary, F.; Yoshino, N. Sustainable Solutions for Green Financing and Investment in Renewable Energy Projects. Energies 2020, 13, 788.
- Groissböck, M.; Gusmão, A. Impact of Renewable Resource Quality on Security of Supply with High Shares of Renewable Energies. Appl. Energy 2020, 277, 115567.
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent Advances in Solid Oxide Cell Technology for Electrolysis. Science 2020, 370, eaba6118.
- Bogdanov, D.; Ram, M.; Aghahosseini, A.; Gulagi, A.; Oyewo, A.S.; Child, M.; Caldera, U.; Sadovskaia, K.; Farfan, J.; De Souza Noel Simas Barbosa, L.; et al. Low-Cost Renewable Electricity as the Key Driver of the Global Energy Transition towards Sustainability. Energy 2021, 227, 120467.
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-Liquid via Synthesis of Methanol{,} DME or Fischer–Tropsch-Fuels: A Review. Energy Environ. Sci. 2020, 13, 3207–3252.
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A Review of Synthetic Fuels for Passenger Vehicles. Energy Rep. 2019, 5, 555–569.
- Rajak, U.; Verma, T.N. Effect of Emission from Ethylic Biodiesel of Edible and Non-Edible Vegetable Oil, Animal Fats, Waste Oil and Alcohol in CI Engine. Energy Convers. Manag. 2018, 166, 704–718.
- Hájek, M.; Vávra, A.; de Paz Carmona, H.; Kocík, J. The Catalysed Transformation of Vegetable Oils or Animal Fats to Biofuels and Bio-Lubricants: A Review. Catalysts 2021, 11, 1118.
- Padhi, S.; Singh, R.K. Non-Edible Oils as the Potential Source for the Production of Biodiesel in India: A Review. J. Chem. Pharm. Res. 2011, 3, 39–49.
- Shadidi, B.; Najafi, G.; Zolfigol, M.A. A Review of the Existing Potentials in Biodiesel Production in Iran. Sustainability 2022, 14, 3284.
- Abdulkareem-Alsultan, G.; Asikin-Mijan, N.; Lee, H.V.; Rashid, U.; Islam, A.; Taufiq-Yap, Y.H. A Review on Thermal Conversion of Plant Oil (Edible and Inedible) into Green Fuel Using Carbon-Based Nanocatalyst. Catalysts 2019, 9, 350.
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.-H.; Nguyen, T.H.P. Sustainability of the Four Generations of Biofuels—A Review. Int. J. Energy Res. 2020, 44, 9266–9282.
- Hirani, A.H.; Javed, N.; Asif, M.; Basu, S.K.; Kumar, A. A Review on First- and Second-Generation Biofuel Productions. In Biofuels: Greenhouse Gas Mitigation and Global Warming: Next Generation Biofuels and Role of Biotechnology; Kumar, A., Ogita, S., Yau, Y.-Y., Eds.; Springer: New Delhi, India, 2018; pp. 141–154. ISBN 978-81-322-3763-1.
- Chowdhury, H.; Loganathan, B. Third-Generation Biofuels from Microalgae: A Review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44.
- Moravvej, Z.; Makarem, M.A.; Rahimpour, M.R. Chapter 20—The Fourth Generation of Biofuel. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 557–597. ISBN 978-0-12-815162-4.
- Arshad, M.; Zia, M.A.; Shah, F.A.; Ahmad, M. An Overview of Biofuel. In Perspectives on Water Usage for Biofuels Production: Aquatic Contamination and Climate Change; Arshad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–37. ISBN 978-3-319-66408-8.
- Demirbas, A. Progress and Recent Trends in Biofuels. Prog. Energy Combust. Sci. 2007, 33, 1–18.
- Niculescu, R.; Clenci, A.; Iorga-Siman, V. Review on the Use of Diesel–Biodiesel–Alcohol Blends in Compression Ignition Engines. Energies 2019, 12, 1194.
- Mehregan, M.; Moghiman, M. Experimental Investigation of the Distinct Effects of Nanoparticles Addition and Urea-SCR after-Treatment System on NOx Emissions in a Blended-Biodiesel Fueled Internal Combustion Engine. Fuel 2020, 262, 116609.
- Okwundu, O.S.; El-Shazly, A.H.; Elkady, M. Comparative Effect of Reaction Time on Biodiesel Production from Low Free Fatty Acid Beef Tallow: A Definition of Product Yield. SN Appl. Sci. 2019, 1, 140.
- Gotovuša, M.; Pucko, I.; Racar, M.; Faraguna, F. Biodiesel Produced from Propanol and Longer Chain Alcohols & mdash; Synthesis and Properties. Energies 2022, 15, 4996.
- Gebremariam, S.N.; Marchetti, J.M. Biodiesel Production Technologies: Review. AIMS Energy 2017, 5, 425–457.
- Baskar, G.; Aiswarya, R. Trends in Catalytic Production of Biodiesel from Various Feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504.
- Khan, E.; Ozaltin, K.; Spagnuolo, D.; Bernal-Ballen, A.; Piskunov, M.V.; Di Martino, A. Biodiesel from Rapeseed and Sunflower Oil: Effect of the Transesterification Conditions and Oxidation Stability. Energies 2023, 16, 657.
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609.
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen Production from Catalytic Steam Reforming of Biodiesel Byproduct Glycerol: Issues and Challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960.
- Gebremariam, S.N.; Marchetti, J.M. Economics of Biodiesel Production: Review. Energy Convers. Manag. 2018, 168, 74–84.
- Dewangan, A.; Yadav, A.K.; Mallick, A. Current Scenario of Biodiesel Development in India: Prospects and Challenges. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 2494–2501.
- Mishra, V.K.; Goswami, R. A Review of Production, Properties and Advantages of Biodiesel. Biofuels 2018, 9, 273–289.
- Kumar, S.; Salam, P.A.; Shrestha, P.; Ackom, E.K. An Assessment of Thailand’s Biofuel Development. Sustainability 2013, 5, 1577–1597.
- Meira, M.; Quintella, C.M.; Ribeiro, E.M.O.; Silva, H.R.G.; Guimarães, A.K. Overview of the Challenges in the Production of Biodiesel. Biomass Convers. Biorefinery 2015, 5, 321–329.
- Alves, J.C.L. Vibrational Spectroscopy for the Quantification of Hydrotreated Vegetable Oil (HVO) Advanced Biofuels in Petroleum-Derived Fuel Blends: A Minireview. Anal. Lett. 2022, 55, 933–950.
- Vignesh, P.; Pradeep Kumar, A.R.; Shankar Ganesh, N.; Jayaseelan, V.; Sudhakar, K. A Review of Conventional and Renewable Biodiesel Production. Chin. J. Chem. Eng. 2021, 40, 1–17.
- Hor, C.J.; Tan, Y.H.; Mubarak, N.M.; Tan, I.S.; Ibrahim, M.L.; Yek, P.N.Y.; Karri, R.R.; Khalid, M. Techno-Economic Assessment of Hydrotreated Vegetable Oil as a Renewable Fuel from Waste Sludge Palm Oil. Environ. Res. 2023, 220, 115169.
- Yeoh, M.L.; Goh, C.S. Hydrotreated Vegetable Oil Production from Palm Oil Mill Effluents: Status, Opportunities and Challenges. Biofuels Bioprod. Biorefining 2022, 16, 1153–1158.
- Wichmann, J.; Lauersen, K.J.; Biondi, N.; Christensen, M.; Guerra, T.; Hellgardt, K.; Kühner, S.; Kuronen, M.; Lindberg, P.; Rösch, C.; et al. Engineering Biocatalytic Solar Fuel Production: The PHOTOFUEL Consortium. Trends Biotechnol. 2021, 39, 323–327.
- Dimitriadis, A.; Natsios, I.; Dimaratos, A.; Katsaounis, D.; Samaras, Z.; Bezergianni, S.; Lehto, K. Evaluation of a Hydrotreated Vegetable Oil (HVO) and Effects on Emissions of a Passenger Car Diesel Engine. Front. Mech. Eng. 2018, 4, 7.
- d’Ambrosio, S.; Mancarella, A.; Manelli, A. Utilization of Hydrotreated Vegetable Oil (HVO) in a Euro 6 Dual-Loop EGR Diesel Engine: Behavior as a Drop-In Fuel and Potentialities along Calibration Parameter Sweeps. Energies 2022, 15, 7202.
- Nordelöf, A.; Romare, M.; Tivander, J. Life Cycle Assessment of City Buses Powered by Electricity, Hydrogenated Vegetable Oil or Diesel. Transp. Res. Part D Transp. Environ. 2019, 75, 211–222.
- Wikberg, E.; Heikkilä, S.; Sirviö, K.; Välisuo, P.; Niemi, S.; Niemi, A. Calibration Method for the Determination of the FAME and HVO Contents in Fossil Diesel Blends Using NIR Spectroscopy. Fuels 2021, 2, 179–193.
- Rayapureddy, S.M.; Matijošius, J.; Rimkus, A.; Caban, J.; Słowik, T. Comparative Study of Combustion, Performance and Emission Characteristics of Hydrotreated Vegetable Oil–Biobutanol Fuel Blends and Diesel Fuel on a CI Engine. Sustainability 2022, 14, 7324.
- Soam, S.; Hillman, K. Factors Influencing the Environmental Sustainability and Growth of Hydrotreated Vegetable Oil (HVO) in Sweden. Bioresour. Technol. Rep. 2019, 7, 100244.
- Wisniewski, A.; Wiggers, V.R.; Simionatto, E.L.; Meier, H.F.; Barros, A.A.C.; Madureira, L.A.S. Biofuels from Waste Fish Oil Pyrolysis: Chemical Composition. Fuel 2010, 89, 563–568.
- Li, Y.; Xu, H.; Cracknell, R.; Head, R.; Shuai, S. An Experimental Investigation into Combustion Characteristics of HVO Compared with TME and ULSD at Varied Blend Ratios. Fuel 2019, 255, 115757.
- Torres-Ortega, C.E.; Gong, J.; You, F.; Rong, B.-G. Optimal Synthesis of Integrated Process for Co-Production of Biodiesel and Hydrotreated Vegetable Oil (HVO) Diesel from Hybrid Oil Feedstocks. In 27th European Symposium on Computer Aided Process Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 673–678.
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61.
- Lapuerta, M.; Armas, O.; García-Contreras, R. Stability of Diesel–Bioethanol Blends for Use in Diesel Engines. Fuel 2007, 86, 1351–1357.
- Park, S.H.; Cha, J.; Kim, H.J.; Lee, C.S. Effect of Early Injection Strategy on Spray Atomization and Emission Reduction Characteristics in Bioethanol Blended Diesel Fueled Engine. Energy 2012, 39, 375–387.
- Tutak, W. Bioethanol E85 as a Fuel for Dual Fuel Diesel Engine. Energy Convers. Manag. 2014, 86, 39–48.
- Chen, K.-H.; Chao, Y.-C. Characterization of Performance of Short Stroke Engines with Valve Timing for Blended Bioethanol Internal Combustion. Energies 2019, 12, 759.
- Puškár, M. Advanced System Determined for Utilisation of Sustainable Biofuels in High-Performance Sport Applications. Sustainability 2022, 14, 6713.
- Iodice, P.; Cardone, M. Ethanol/Gasoline Blends as Alternative Fuel in Last Generation Spark-Ignition Engines: A Review on CO and HC Engine Out Emissions. Energies 2021, 14, 4034.
- Bautista-Herrera, A.; Ortiz-Arango, F.; Álvarez-García, J. Profitability Using Second-Generation Bioethanol in Gasoline Produced in Mexico. Energies 2021, 14, 2294.
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Srisupa, S.; Chaiyaso, T. Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces Cerevisiae TC-5. J. Fungi 2021, 7, 547.
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268.
- Baez-Gonzalez, A.D.; Kiniry, J.R.; Meki, M.N.; Williams, J.; Alvarez-Cilva, M.; Ramos-Gonzalez, J.L.; Magallanes-Estala, A.; Zapata-Buenfil, G. Crop Parameters for Modeling Sugarcane under Rainfed Conditions in Mexico. Sustainability 2017, 9, 1337.
- Salehi Reza Chaiprapat Sumate Conversion of Biogas from Anaerobic Digestion to Single Cell Protein and Bio-Methanol: Mechanism, Microorganisms and Key Factors—A Review. Environ. Eng. Res. 2022, 27, 210100–210109.
- Srivastava, R.K.; Sarangi, P.K.; Bhatia, L.; Singh, A.K.; Shadangi, K.P. Conversion of Methane to Methanol: Technologies and Future Challenges. Biomass Convers. Biorefinery 2022, 12, 1851–1875.
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts-A Review. Int. J. Hydrog. Energy 2020, 45, 18090–18113.
- Li, S.; Wen, Z.; Hou, J.; Xi, S.; Fang, P.; Guo, X.; Li, Y.; Wang, Z.; Li, S. Effects of Ethanol and Methanol on the Combustion Characteristics of Gasoline with the Revised Variation Disturbance Method. ACS Omega 2022, 7, 17797–17810.
- Marchuk, A.; Likhanov, V.A.; Lopatin, O.P. Alternative Energy: Methanol, Ethanol and Alcohol Esters of Rapeseed Oil as Eco-Friendly Biofuel. Theor. Appl. Ecol. 2019, 2019, 80–86.
- Erchamo, Y.S.; Mamo, T.T.; Workneh, G.A.; Mekonnen, Y.S. Improved Biodiesel Production from Waste Cooking Oil with Mixed Methanol–Ethanol Using Enhanced Eggshell-Derived CaO Nano-Catalyst. Sci. Rep. 2021, 11, 6708.
- Zhou, W.J.; Zhou, B.; Li, W.Z.; Zhou, Z.H.; Song, S.Q.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Goula, M.; Tsiakaras, P. Performance Comparison of Low-Temperature Direct Alcohol Fuel Cells with Different Anode Catalysts. J. Power Sources 2004, 126, 16–22.
- Ram, V.; Infantraj; Salkuti, S.R. Modelling and Simulation of a Hydrogen-Based Hybrid Energy Storage System with a Switching Algorithm. World Electr. Veh. J. 2022, 13, 188.
- Sadik-Zada, E.R. Political Economy of Green Hydrogen Rollout: A Global Perspective. Sustainability 2021, 13, 13464.
- Dahmen, N.; Sauer, J. Evaluation of Techno-Economic Studies on the Bioliq® Process for Synthetic Fuels Production from Biomass. Processes 2021, 9, 684.
- García, A.C.; Moral-Vico, J.; Abo Markeb, A.; Sánchez, A. Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials 2022, 12, 999.
- Giuliano, A.; Catizzone, E.; Freda, C. Process Simulation and Environmental Aspects of Dimethyl Ether Production from Digestate-Derived Syngas. Int. J. Environ. Res. Public Health 2021, 18, 807.
- Sonthalia, A.; Kumar, N.; Tomar, M.; Edwin Geo, V.; Thiyagarajan, S.; Pugazhendhi, A. Moving Ahead from Hydrogen to Methanol Economy: Scope and Challenges. Clean Technol. Environ. Policy 2021, 25, 551–575.
- Fang, M.; Yi, N.; Di, W.; Wang, T.; Wang, Q. Emission and Control of Flue Gas Pollutants in CO2 Chemical Absorption System—A Review. Int. J. Greenh. Gas Control 2020, 93, 102904.
- Krūmiņš, J.; Kļaviņš, M.; Ozola-Davidāne, R.; Ansone-Bērtiņa, L. The Prospects of Clay Minerals from the Baltic States for Industrial-Scale Carbon Capture: A Review. Minerals 2022, 12, 349.
- Elhenawy, S.E.M.; Khraisheh, M.; AlMomani, F.; Walker, G. Metal-Organic Frameworks as a Platform for CO2 Capture and Chemical Processes: Adsorption, Membrane Separation, Catalytic-Conversion, and Electrochemical Reduction of CO2. Catalysts 2020, 10, 1293.
- Markewitz, P.; Zhao, L.; Ryssel, M.; Moumin, G.; Wang, Y.; Sattler, C.; Robinius, M.; Stolten, D. Carbon Capture for CO2 Emission Reduction in the Cement Industry in Germany. Energies 2019, 12, 2432.
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-Combustion Carbon Capture. Renew. Sustain. Energy Rev. 2021, 138, 110490.
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kuś, T. Methods and Techniques for CO2 Capture: Review of Potential Solutions and Applications in Modern Energy Technologies. Energies 2022, 15, 887.
- Regufe, M.J.; Pereira, A.; Ferreira, A.F.P.; Ribeiro, A.M.; Rodrigues, A.E. Current Developments of Carbon Capture Storage and/or Utilization–Looking for Net-Zero Emissions Defined in the Paris Agreement. Energies 2021, 14, 2406.
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-Combustion Carbon Capture by Membrane Separation, Review. Sep. Purif. Technol. 2020, 238, 116448.
- Lau, H.C.; Ramakrishna, S.; Zhang, K.; Radhamani, A.V. The Role of Carbon Capture and Storage in the Energy Transition. Energy Fuels 2021, 35, 7364–7386.
- Mantilla, S.; Santos, D.M.F. Green and Blue Hydrogen Production: An Overview in Colombia. Energies 2022, 15, 8862.
- Zagashvili, Y.; Kuzmin, A.; Buslaev, G.; Morenov, V. Small-Scaled Production of Blue Hydrogen with Reduced Carbon Footprint. Energies 2021, 14, 5194.
- Ishaq, H.; Dincer, I.; Crawford, C. A Review on Hydrogen Production and Utilization: Challenges and Opportunities. Int. J. Hydrog. Energy 2022, 47, 26238–26264.
- Patterson, B.D.; Mo, F.; Borgschulte, A.; Hillestad, M.; Joos, F.; Kristiansen, T.; Sunde, S.; van Bokhoven, J.A. Renewable CO2 Recycling and Synthetic Fuel Production in a Marine Environment. Proc. Natl. Acad. Sci. USA 2019, 116, 12212–12219.
- Bassano, C.; Deiana, P.; Vilardi, G.; Verdone, N. Modeling and Economic Evaluation of Carbon Capture and Storage Technologies Integrated into Synthetic Natural Gas and Power-to-Gas Plants. Appl. Energy 2020, 263, 114590.
- Ghiat, I.; Al-Ansari, T. A Review of Carbon Capture and Utilisation as a CO2 Abatement Opportunity within the EWF Nexus. J. CO2 Util. 2021, 45, 101432.
- Greene, D.L.; Ogden, J.M.; Lin, Z. Challenges in the Designing, Planning and Deployment of Hydrogen Refueling Infrastructure for Fuel Cell Electric Vehicles. eTransportation 2020, 6, 100086.
- Kurtz, J.; Sprik, S.; Bradley, T.H. Review of Transportation Hydrogen Infrastructure Performance and Reliability. Int. J. Hydrog. Energy 2019, 44, 12010–12023.
- Yusaf, T.; Fernandes, L.; Abu Talib, A.R.; Altarazi, Y.S.M.; Alrefae, W.; Kadirgama, K.; Ramasamy, D.; Jayasuriya, A.; Brown, G.; Mamat, R.; et al. Sustainable Aviation—Hydrogen Is the Future. Sustainability 2022, 14, 548.
- Hosseini, S.E.; Wahid, M.A. Hydrogen from Solar Energy, a Clean Energy Carrier from a Sustainable Source of Energy. Int. J. Energy Res. 2020, 44, 4110–4131.
- De Crisci, A.G.; Moniri, A.; Xu, Y. Hydrogen from Hydrogen Sulfide: Towards a More Sustainable Hydrogen Economy. Int. J. Hydrog. Energy 2019, 44, 1299–1327.
- Nicita, A.; Maggio, G.; Andaloro, A.P.F.; Squadrito, G. Green Hydrogen as Feedstock: Financial Analysis of a Photovoltaic-Powered Electrolysis Plant. Int. J. Hydrog. Energy 2020, 45, 11395–11408.
- Lei, Q.; Wang, B.; Wang, P.; Liu, S. Hydrogen Generation with Acid/Alkaline Amphoteric Water Electrolysis. J. Energy Chem. 2019, 38, 162–169.
- Mus, J.; Vanhoutte, B.; Schotte, S.; Fevery, S.; Latré, S.K.; Kleemann, M.; Buysschaert, F. Design and Characterisation of an Alkaline Electrolyser. In Proceedings of the 2022 11th International Conference on Renewable Energy Research and Application (ICRERA), Istanbul, Turkey, 18–21 September 2022; pp. 253–259.
- Ali, S.; Sørensen, K.; Nielsen, M.P. Modeling a Novel Combined Solid Oxide Electrolysis Cell (SOEC)—Biomass Gasification Renewable Methanol Production System. Renew. Energy 2020, 154, 1025–1034.
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-Based Electrolysis for Hydrogen Production: A Review. Membranes 2021, 11, 810.
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen Storage Technologies for Stationary and Mobile Applications: Review, Analysis and Perspectives. Renew. Sustain. Energy Rev. 2021, 149, 111311.
- Wang, S.; Sun, L.; Iqbal, S. Green Financing Role on Renewable Energy Dependence and Energy Transition in E7 Economies. Renew. Energy 2022, 200, 1561–1572.
- IRENA. Green Hydrogen Cost Reduction; IRENA: Masdar City, United Arab Emirates, 2020; ISBN 9789292602956.
- Patt, A. Hydrogen for Ground Transportation and Heating Is a Bad Idea. Available online: https://ethz.ch/en/news-and-events/eth-news/news/2021/11/hydrogen-for-ground-transportation-and-heating-is-a-bad-idea.html (accessed on 28 January 2023).
- Alex Grant, P.M. Hydrogen Is Big Oil’s Last Grand Scam. Available online: https://www.jadecove.com/research/hydrogenscam (accessed on 28 January 2023).
- Porritt, J. Hyping Hydrogen: The Fossil Fuel Industry’s Final Scam. Available online: http://www.jonathonporritt.com/hyping-hydrogen-the-fossil-fuel-industrys-final-scam/ (accessed on 28 January 2023).
- Zubrin, R. The Hydrogen Hoax. Available online: https://www.thenewatlantis.com/publications/the-hydrogen-hoax (accessed on 28 January 2023).
- Wang, M.; Wan, Y.; Guo, Q.; Bai, Y.; Yu, G.; Liu, Y.; Zhang, H.; Zhang, S.; Wei, J. Brief Review on Petroleum Coke and Biomass/Coal Co-Gasification: Syngas Production, Reactivity Characteristics, and Synergy Behavior. Fuel 2021, 304, 121517.
- Gopan, G.; Hauchhum, L.; Pattanayak, S.; Kalita, P.; Krishnan, R. Prediction of Species Concentration in Syngas Produced through Gasification of Different Bamboo Biomasses: A Numerical Approach. Int. J. Energy Environ. Eng. 2022, 13, 1383–1394.
- Medrano-García, J.D.; Ruiz-Femenia, R.; Caballero, J.A. Multi-Objective Optimization of a Carbon Dioxide Utilization Superstructure for the Synthesis of Formic and Acetic Acid. In 28th European Symposium on Computer Aided Process Engineering; Friedl, A., Klemeš, J.J., Radl, S., Varbanov, P.S., Wallek, T., Eds.; Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 43, pp. 1419–1424.
- Chen, W.-H.; Chen, C.-Y. Water Gas Shift Reaction for Hydrogen Production and Carbon Dioxide Capture: A Review. Appl. Energy 2020, 258, 114078.
- Siudyga, T.; Kapkowski, M.; Bartczak, P.; Zubko, M.; Szade, J.; Balin, K.; Antoniotti, S.; Polanski, J. Ultra-Low Temperature Carbon (Di)Oxide Hydrogenation Catalyzed by Hybrid Ruthenium–Nickel Nanocatalysts: Towards Sustainable Methane Production. Green Chem. 2020, 22, 5143–5150.
- Liu, P.; Yang, Y.; Wang, Q. Mechanism Insights into Direct Conversion of Syngas into C2 Oxygenates via Key Intermediate C2O2 over Ni-Supported Graphene. Carbon N. Y. 2021, 175, 322–333.
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A Review of the Catalytic Hydrogenation of Carbon Dioxide into Value-Added Hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598.
- Bahmanpour, A.M.; Héroguel, F.; Kılıç, M.; Baranowski, C.J.; Schouwink, P.; Röthlisberger, U.; Luterbacher, J.S.; Kröcher, O. Essential Role of Oxygen Vacancies of Cu-Al and Co-Al Spinel Oxides in Their Catalytic Activity for the Reverse Water Gas Shift Reaction. Appl. Catal. B Environ. 2020, 266, 118669.
- El-Nagar, R.A.; Ghanem, A.A. Syngas Production, Properties, and Its Importance. In Sustainable Alternative Syngas Fuel; Ghenai, C., Inayat, A., Eds.; IntechOpen: Rijeka, Croatia, 2019.
- Karimipour, S.; Gerspacher, R.; Gupta, R.; Spiteri, R.J. Study of Factors Affecting Syngas Quality and Their Interactions in Fluidized Bed Gasification of Lignite Coal. Fuel 2013, 103, 308–320.
- Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Catalytic Production of Jet Fuels from Biomass. Molecules 2020, 25, 802.
- Borgogna, A.; Salladini, A.; Spadacini, L.; Pitrelli, A.; Annesini, M.C.; Iaquaniello, G. Methanol Production from Refuse Derived Fuel: Influence of Feedstock Composition on Process Yield through Gasification Analysis. J. Clean. Prod. 2019, 235, 1080–1089.
- Parkhey, P. Biomethanol: Possibilities towards a Bio-Based Economy. Biomass Convers. Biorefinery 2022, 12, 1877–1887.
- Kesieme, U.; Pazouki, K.; Murphy, A.; Chrysanthou, A. Biofuel as an Alternative Shipping Fuel: Technological, Environmental and Economic Assessment. Sustain. Energy Fuels 2019, 3, 899–909.
- Dabas, N.; Dubey, V.; Chhabra, M.; Dwivedi, G. Performance Analysis of an IC Engine Using Methanol, Ethanol, and Its Blend with Gasoline and Diesel as a Fuel. In Proceedings of the Advances in Fluid and Thermal Engineering; Saha, P., Subbarao, P.M.V., Sikarwar, B.S., Eds.; Springer: Singapore, 2019; pp. 223–232.
- Bowker, M. Methanol Synthesis from CO2 Hydrogenation. ChemCatChem 2019, 11, 4238–4246.
- Wang, Y.; Bu, G.; Geng, X.; Zhu, Z.; Cui, P.; Liao, Z. Design Optimization and Operating Pressure Effects in the Separation of Acetonitrile/Methanol/Water Mixture by Ternary Extractive Distillation. J. Clean. Prod. 2019, 218, 212–224.
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol Fuel Production, Utilization, and Techno-Economy: A Review. Environ. Chem. Lett. 2022, 20, 3525–3554.
- Zhang, Z.; Wen, M.; Cui, Y.; Ming, Z.; Wang, T.; Zhang, C.; Ampah, J.D.; Jin, C.; Huang, H.; Liu, H. Effects of Methanol Application on Carbon Emissions and Pollutant Emissions Using a Passenger Vehicle. Processes 2022, 10, 525.
- Choe, C.; Byun, M.; Lee, H.; Lim, H. Techno-Economic and Environmental Assessments for Sustainable Bio-Methanol Production as Landfill Gas Valorization. Waste Manag. 2022, 150, 90–97.
- AlNouss, A.; Shahbaz, M.; Mckay, G.; Al-Ansari, T. Bio-Methanol Production from Palm Wastes Steam Gasification with Application of CaO for CO2 Capture: Techno-Economic-Environmental Analysis. J. Clean. Prod. 2022, 341, 130849.
- Tian, Z.; Wang, Y.; Zhen, X.; Liu, Z. The Effect of Methanol Production and Application in Internal Combustion Engines on Emissions in the Context of Carbon Neutrality: A Review. Fuel 2022, 320, 123902.
- Ryabchuk, P.; Stier, K.; Junge, K.; Checinski, M.P.; Beller, M. Molecularly Defined Manganese Catalyst for Low-Temperature Hydrogenation of Carbon Monoxide to Methanol. J. Am. Chem. Soc. 2019, 141, 16923–16929.
- Calis, H.P.; Lüke, W.; Drescher, I.; Schütze, A. Synthetic Diesel Fuels. In Handbook of Fuels; John Wiley & Sons, Ltd.: New York, NY, USA, 2021; pp. 161–200. ISBN 9783527813490.
- Fabiś, P.; Flekiewicz, B. Influence of LPG and DME Composition on Spark Ignition Engine Performance. Energies 2021, 14, 5583.
- Mako\’s, Patrycja; Slupek, Edyta; Sobczak, Joanna; Zabrocki, Dawid; Hupka, Jan; Rogala, Andrzej Dimethyl Ether (DME) as Potential Environmental Friendly Fuel. E3S Web Conf. 2019, 116, 48.
- Fleisch, T.H.; Basu, A.; Sills, R.A. Introduction and Advancement of a New Clean Global Fuel: The Status of DME Developments in China and Beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107.
- Bell, D.A.; Towler, B.F.; Fan, M. Chapter 12—Methanol and Derivatives. In Coal Gasification and Its Applications; Bell, D.A., Towler, B.F., Fan, M., Eds.; William Andrew Publishing: Boston, MA, USA, 2011; pp. 353–371. ISBN 978-0-8155-2049-8.
- Gądek, M.; Kubica, R.; Jędrysik, E. Production of Methanol and Dimethyl Ether from Biomass Derived Syngas—A Comparison of the Different Synthesis Pathways by Means of Flowsheet Simulation. In 23rd European Symposium on Computer Aided Process Engineering; Kraslawski, A., Turunen, I., Eds.; Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2013; Volume 32, pp. 55–60.
- Sorenson, S.C.; Mikkelsen, S.-E. Performance and Emissions of a 0.273 Liter Direct Injection Diesel Engine Fuelled with Neat Dimethyl Ether. SAE Trans. 1995, 104, 80–90.
- Vu, T.T.N.; Desgagnés, A.; Iliuta, M.C. Efficient Approaches to Overcome Challenges in Material Development for Conventional and Intensified CO2 Catalytic Hydrogenation to CO, Methanol, and DME. Appl. Catal. A Gen. 2021, 617, 118119.
- Basri, S.; Kamarudin, S.K. Chapter 8—Direct Dimethyl Ether Fuel Cells (DDMEFCs). In Direct Liquid Fuel Cells; Akay, R.G., Yurtcan, A.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 177–189. ISBN 978-0-12-818624-4.
- Breitkreuz, C.F.; Hevert, N.; Schmitz, N.; Burger, J.; Hasse, H. Synthesis of Methylal and Poly(Oxymethylene) Dimethyl Ethers from Dimethyl Ether and Trioxane. Ind. Eng. Chem. Res. 2022, 61, 7810–7822.
- Styring, P.; Dowson, G.R.M.; Tozer, I.O. Synthetic Fuels Based on Dimethyl Ether as a Future Non-Fossil Fuel for Road Transport From Sustainable Feedstocks. Front. Energy Res. 2021, 9, 663331.
- Bakhtyari, A.; Bardool, R.; Rahimpour, M.R.; Iulianelli, A. Dehydration of Bio-Alcohols in an Enhanced Membrane-Assisted Reactor: A Rigorous Sensitivity Analysis and Multi-Objective Optimization. Renew. Energy 2021, 177, 519–543.
- Gahtori, J.; Shrivastaw, V.K.; Bordoloi, A. Advances in Direct Thermocatalytic CO2 Conversion to Chemicals and Hydrocarbons. In Heterogeneous Nanocatalysis for Energy and Environmental Sustainability; John Wiley & Sons, Ltd: New York, NY, USA, 2022; pp. 155–193. ISBN 9781119772057.
- Ebner, K.; Moser, L.; Koops, L.; Batteiger, V. Power-to-Liquids: Shedding Light on Levers and Uncertainties in the Process Chain. EUCASS. 2022. Available online: https://www.eucass.eu/doi/EUCASS2022-7340.pdf (accessed on 5 February 2023).
- Isaacs, S.A.; Staples, M.D.; Allroggen, F.; Mallapragada, D.S.; Falter, C.P.; Barrett, S.R.H. Environmental and Economic Performance of Hybrid Power-to-Liquid and Biomass-to-Liquid Fuel Production in the United States. Environ. Sci. Technol. 2021, 55, 8247–8257.
- Lonis, F.; Tola, V.; Cau, G. Assessment of Integrated Energy Systems for the Production and Use of Renewable Methanol by Water Electrolysis and CO2 Hydrogenation. Fuel 2021, 285, 119160.
- Drünert, S.; Neuling, U.; Zitscher, T.; Kaltschmitt, M. Power-to-Liquid Fuels for Aviation—Processes, Resources and Supply Potential under German Conditions. Appl. Energy 2020, 277, 115578.
- Zhang, C.; Gao, R.; Jun, K.-W.; Kim, S.K.; Hwang, S.-M.; Park, H.-G.; Guan, G. Direct Conversion of Carbon Dioxide to Liquid Fuels and Synthetic Natural Gas Using Renewable Power: Techno-Economic Analysis. J. CO2 Util. 2019, 34, 293–302.
- Micheli, M.; Moore, D.; Bach, V.; Finkbeiner, M. Life-Cycle Assessment of Power-to-Liquid Kerosene Produced from Renewable Electricity and CO2 from Direct Air Capture in Germany. Sustainability 2022, 14, 10658.
- Lonis, F.; Tola, V.; Cau, G. Renewable Methanol Production and Use through Reversible Solid Oxide Cells and Recycled CO2 Hydrogenation. Fuel 2019, 246, 500–515.
- Dray, L.; Schäfer, A.W.; Grobler, C.; Falter, C.; Allroggen, F.; Stettler, M.E.J.; Barrett, S.R.H. Cost and Emissions Pathways towards Net-Zero Climate Impacts in Aviation. Nat. Clim. Chang. 2022, 12, 956–962.
- Gnann, T.; Speth, D.; Krail, M.; Wietschel, M.; Oberle, S. Pathways to Carbon-Free Transport in Germany until 2050. World Electr. Veh. J. 2022, 13, 136.
- Speth, D.; Funke, S.Á. Comparing Options to Electrify Heavy-Duty Vehicles: Findings of German Pilot Projects. World Electr. Veh. J. 2021, 12, 67.
- Meyers, D.; Willis, K. Sorting Through the Many Total-Energy-Cycle Pathways Possible with Early Plug-In Hybrids. World Electr. Veh. J. 2008, 2, 66–88.
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140.
- Chun, D.H.; Rhim, G.B.; Youn, M.H.; Deviana, D.; Lee, J.E.; Park, J.C.; Jeong, H. Brief Review of Precipitated Iron-Based Catalysts for Low-Temperature Fischer–Tropsch Synthesis. Top. Catal. 2020, 63, 793–809.
- dos Santos, R.G.; Alencar, A.C. Biomass-Derived Syngas Production via Gasification Process and Its Catalytic Conversion into Fuels by Fischer Tropsch Synthesis: A Review. Int. J. Hydrog. Energy 2020, 45, 18114–18132.
- Konarova, M.; Aslam, W.; Perkins, G. Chapter 3—Fischer-Tropsch Synthesis to Hydrocarbon Biofuels: Present Status and Challenges Involved. In Hydrocarbon Biorefinery; Maity, S.K., Gayen, K., Bhowmick, T.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 77–96. ISBN 978-0-12-823306-1.
- Gupta, P.K.; Kumar, V.; Maity, S. Renewable Fuels from Different Carbonaceous Feedstocks: A Sustainable Route through Fischer–Tropsch Synthesis. J. Chem. Technol. Biotechnol. 2021, 96, 853–868.
- Sun, J.; Yang, G.; Peng, X.; Kang, J.; Wu, J.; Liu, G.; Tsubaki, N. Beyond Cars: Fischer-Tropsch Synthesis for Non-Automotive Applications. ChemCatChem 2019, 11, 1412–1424.
- Jones, M.P.; Krexner, T.; Bismarck, A. Repurposing Fischer-Tropsch and Natural Gas as Bridging Technologies for the Energy Revolution. Energy Convers. Manag. 2022, 267, 115882.
- Tucker, C.L.; Bordoloi, A.; van Steen, E. Novel Single Pass Biogas-to-Diesel Process Using a Fischer-Tropsch Catalyst Designed for High Conversion. Sustain. Energy Fuels 2021, 5, 5717–5732.
- Marchese, M.; Giglio, E.; Santarelli, M.; Lanzini, A. Energy Performance of Power-to-Liquid Applications Integrating Biogas Upgrading, Reverse Water Gas Shift, Solid Oxide Electrolysis and Fischer-Tropsch Technologies. Energy Convers. Manag. X 2020, 6, 100041.
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass: A State-of-the-Art Review of Process Parameters, Reaction Mechanisms and Catalysis. Sustain. Energy Fuels 2019, 3, 578–598.
- Stadler, T.J.; Bertin-Mente, B.; Dittmeyer, R.; Brübach, L.T.; Böltken, T.; Pfeifer, P. Influence of CO2-Rich Syngas on the Selectivity to C10–C14 in a Coupled Fischer-Tropsch/Hydrocracking Process. Chem. Ing. Tech. 2022, 94, 289–298.
- Meurer, A.; Kern, J. Fischer–Tropsch Synthesis as the Key for Decentralized Sustainable Kerosene Production. Energies 2021, 14, 1836.
- Müller-Langer, F.; Dögnitz, N.; Marquardt, C.; Zschocke, A.; Schripp, T.; Oehmichen, K.; Majer, S.; Bullerdiek, N.; Halling, A.-M.; Posselt, D.; et al. Multiblend JET A-1 in Practice: Results of an R & D Project on Synthetic Paraffinic Kerosenes. Chem. Eng. Technol. 2020, 43, 1514–1521.
- Richter, S.; Kukkadapu, G.; Westbrook, C.K.; Braun-Unkhoff, M.; Naumann, C.; Köhler, M.; Riedel, U. A Combined Experimental and Modeling Study of Combustion Properties of an Isoparaffinic Alcohol-to-Jet Fuel. Combust. Flame 2022, 240, 111994.
- Kalligeros, S. Evolution of Fuels with the Advancement of Powertrains. In Advances in Engine and Powertrain Research and Technology: Design Simulation Testing Manufacturing; Parikyan, T., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 207–235. ISBN 978-3-030-91869-9.
- Mawhood, R.; Gazis, E.; de Jong, S.; Hoefnagels, R.; Slade, R. Production Pathways for Renewable Jet Fuel: A Review of Commercialization Status and Future Prospects. Biofuels Bioprod. Biorefining 2016, 10, 462–484.
- Danasa, A.S.; Soesilo, T.E.B.; Martono, D.N.; Sodri, A.; Hadi, A.S.; Chandrasa, G.T. The Ammonia Release Hazard and Risk Assessment: A Case Study of Urea Fertilizer Industry in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 399, 12087.
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062.
- Zincir, B. A Short Review of Ammonia as an Alternative Marine Fuel for Decarbonised Maritime Transportation. In Proceedings of the International Conference on Energy, Environment and Storage of Energy (ICEESEN2020), Kayseri, Turkey, 19–21 November 2020.
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205.
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; de Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on Ammonia as a Potential Fuel: From Synthesis to Economics. Energy Fuels 2021, 35, 6964–7029.
- Morlanés, N.; Katikaneni, S.P.; Paglieri, S.N.; Harale, A.; Solami, B.; Sarathy, S.M.; Gascon, J. A Technological Roadmap to the Ammonia Energy Economy: Current State and Missing Technologies. Chem. Eng. J. 2021, 408, 127310.
- Yousefi Rizi, H.A.; Shin, D. Green Hydrogen Production Technologies from Ammonia Cracking. Energies 2022, 15, 8246.
- Kim, H.; Koo, K.Y.; Joung, T.-H. A Study on the Necessity of Integrated Evaluation of Alternative Marine Fuels. J. Int. Marit. Safety, Environ. Aff. Shipp. 2020, 4, 26–31.
- Berner, U.; Faber, E. Maturity Related Mixing Model for Methane, Ethane and Propane, Based on Carbon Isotopes. Org. Geochem. 1988, 13, 67–72.
- Tan, Y.; Pan, Z.; Feng, X.-T.; Zhang, D.; Connell, L.D.; Li, S. Laboratory Characterisation of Fracture Compressibility for Coal and Shale Gas Reservoir Rocks: A Review. Int. J. Coal Geol. 2019, 204, 1–17.
- Debruyn, J.; Hilborn, D. Anaerobic Digestion Basics. Small 2007, 2007, 1–6.
- Braber, K. Anaerobic Digestion of Municipal Solid Waste: A Modern Waste Disposal Option on the Verge of Breakthrough. Biomass Bioenergy 1995, 9, 365–376.
- Ozgun, H. Anaerobic Digestion Model No. 1 (ADM1) for Mathematical Modeling of Full-Scale Sludge Digester Performance in a Municipal Wastewater Treatment Plant. Biodegradation 2019, 30, 27–36.
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253.
- Elsayed, H.A.; Menoufy, M.F.; Shaban, S.A.; Ahmed, H.S.; Heakal, B.H. Optimization of the Reaction Parameters of Heavy Naphtha Reforming Process Using Pt-Re/Al2O3 Catalyst System. Egypt. J. Pet. 2017, 26, 885–893.
- Alsudani, F.T.; Saeed, A.N.; Ali, N.S.; Majdi, H.S.; Salih, H.G.; Albayati, T.M.; Saady, N.M.C.; Shakor, Z.M. Fisher–Tropsch Synthesis for Conversion of Methane into Liquid Hydrocarbons through Gas-to-Liquids (GTL) Process: A Review. Methane 2023, 2, 24–43.
- Vijayan, S.V.; Mohanta, H.K.; Pani, A.K. Support Vector Regression Modeling in Recursive Just-in-Time Learning Framework for Adaptive Soft Sensing of Naphtha Boiling Point in Crude Distillation Unit. Pet. Sci. 2021, 18, 1230–1239.
- Sweeney, W.J.; McArdle, E.H. Highly Aromatic Petroleum Solvent Naphthas. Ind. Eng. Chem. 1941, 33, 787–791.
- Stijepovic, M.Z.; Vojvodic-Ostojic, A.; Milenkovic, I.; Linke, P. Development of a Kinetic Model for Catalytic Reforming of Naphtha and Parameter Estimation Using Industrial Plant Data. Energy Fuels 2009, 23, 979–983.
- Liu, D.; Zhi, Y.; Bai, Y.; Zhao, L.; Gao, J.; Xu, C. Thermodynamic Analysis of Reaction Pathways and Equilibrium Yields for Catalytic Pyrolysis of Naphtha. Front. Chem. Sci. Eng. 2022, 16, 1700–1712.
- Pine, L.A.; Maher, P.J.; Wachter, W.A. Prediction of Cracking Catalyst Behavior by a Zeolite Unit Cell Size Model. J. Catal. 1984, 85, 466–476.
- Jahangiri, H.; Lappas, A.A.; Ouadi, M.; Heracleous, E. Chapter 14—Production of Biofuels via Fischer-Tropsch Synthesis: Biomass-to-Liquids. In Handbook of Biofuels Production, 3rd ed.; Luque, R., Lin, C.S.K., Wilson, K., Du, C., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2023; pp. 449–509. ISBN 978-0-323-91193-1.
- Matsumoto, R.; Tabata, T. Impact and Challenges of Reducing Petroleum Consumption for Decarbonization. Appl. Sci. 2022, 12, 3738.
- Pei, Y.; Zhang, Y.; Kumar, P.; Traver, M.; Cleary, D.; Ameen, M.; Som, S.; Probst, D.; Burton, T.; Pomraning, E.; et al. CFD-Guided Heavy Duty Mixing-Controlled Combustion System Optimization with a Gasoline-Like Fuel. SAE Int. J. Commer. Veh. 2017, 10, 532–546.
- Ali, O.M.; Alomar, O.R.; Ali, O.M.; Alwan, N.T.; Yaqoob, S.J.; Nayyar, A.; Askar, S.; Abouhawwash, M. Operating of Gasoline Engine Using Naphtha and Octane Boosters from Waste as Fuel Additives. Sustainability 2021, 13, 13019.
- Alsaad, M.A.; Hammad, M.A. The Application of Propane/Butane Mixture for Domestic Refrigerators. Appl. Therm. Eng. 1998, 18, 911–918.
- Al-Qadri, A.A.; Nasser, G.A.; Galadima, A.; Muraza, O. A Review on the Conversion of Synthetic Gas to LPG over Hybrid Nanostructure Zeolites Catalysts. ChemistrySelect 2022, 7, e202200042.
- Kandasamy, V.; Idris, I.; Abdullah, A.; Shamsudin, I.K.; Law, L.C.; Othman, M.R. Optimizing Atmospheric Distillation Unit for Maximum Light Petroleum Gas Yield and Comparative Case Studies. AIP Conf. Proc. 2019, 2124, 20050.
- Caracci, E.; Canale, L.; Buonanno, G.; Stabile, L. Sub-Micron Particle Number Emission from Residential Heating Systems: A Comparison between Conventional and Condensing Boilers Fueled by Natural Gas and Liquid Petroleum Gas, and Pellet Stoves. Sci. Total Environ. 2022, 827, 154288.
- Zhang, W.; Fan, S.; Zhang, J.; Ma, Q.; Wang, K.; Zhao, T.-S. Transformation of LPG on HZSM-5 Catalyst: Effects of Tuned Pores and Acidity on Product Distribution. Fuel 2019, 254, 115615.
- Małek, A.; Caban, J.; Sarkan, B. Research on Low-Emission Vehicle Powered by LPG Using Innovative Hardware and Software. Arch. Automot. Eng.-Arch. Motoryz. 2020, 89, 19–36.
- Dugenci, I.; Arican, O.H.; Kara, G.; Unal, A.U. Operational Process in Lpg and Lng Gas Ships in Maritime Transportation Logistics. In Handbook of Research on the Applications of International Transportation and Logistics for World Trade; Ceyhun, G.Ç., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 310–329. ISBN 9781799813972.
- Naumenko, S.; Bragnik, V.; Sandu, I. Features of Forensic Research on Wheeled Vehicles with LPG Equipment. Theory Pract. Forensic Sci. Crim. 2022, 26, 95–107.
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-Based Synthetic Fuels: A Techno-Economic Comparison of Alcohol, Ether and Hydrocarbon Production. Int. J. Hydrog. Energy 2020, 45, 5395–5414.
- Putrasari, Y.; Ocktaeck, L. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2021, 7, 32–37.
- Ghenai, C. Combustion of Syngas Fuel in Gas Turbine Can Combustor. Adv. Mech. Eng. 2010, 2, 342357.
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102.
- IEA. 2021. Net Zero by 2050, IEA, Paris. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 5 February 2023).
- IEA. Energy Technology Perspectives 2020—Special Report on Clean Energy Innovation; OECD Publishing: Paris, France, 2020.
- Energy Transitions Commission® Mission Possible: Reaching Net-Zero Carbon Emissions from Harder-to-Abate Sectors. Available online: https://www.energy-transitions.org/publications/mission-possible/ (accessed on 5 February 2023).
- IEA. The Future of Hydrogen: Seizing Today’s Opportunities; OECD: Paris, France, 2019.
- Naumenko, S.; Bragnik, V.; Sandu, I. Features of Forensic Research on Wheeled Vehicles with LPG Equipment. Theory Pract. Forensic Sci. Crim. 2022, 26, 95–107.
- Schemme, S.; Breuer, J.L.; Köller, M.; Meschede, S.; Walman, F.; Samsun, R.C.; Peters, R.; Stolten, D. H2-Based Synthetic Fuels: A Techno-Economic Comparison of Alcohol, Ether and Hydrocarbon Production. Int. J. Hydrog. Energy 2020, 45, 5395–5414.
- Putrasari, Y.; Ocktaeck, L. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2021, 7, 32–37.
- Ghenai, C. Combustion of Syngas Fuel in Gas Turbine Can Combustor. Adv. Mech. Eng. 2010, 2, 342357.
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for Power. Prog. Energy Combust. Sci. 2018, 69, 63–102.
- IEA. 2021. Net Zero by 2050, IEA, Paris. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 5 February 2023).
- IEA. Energy Technology Perspectives 2020—Special Report on Clean Energy Innovation; OECD Publishing: Paris, France, 2020.
- Energy Transitions Commission® Mission Possible: Reaching Net-Zero Carbon Emissions from Harder-to-Abate Sectors. Available online: https://www.energy-transitions.org/publications/mission-possible/ (accessed on 5 February 2023).
- IEA. The Future of Hydrogen: Seizing Today’s Opportunities; OECD: Paris, France, 2019.





