Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LU CHENG | -- | 2516 | 2023-03-21 02:19:28 | | | |
| 2 | Lindsay Dong | + 22 word(s) | 2538 | 2023-03-21 04:16:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cheng, L.; Liu, S.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Mechanisms of Gut Microbiota Intervention in the CNS. Encyclopedia. Available online: https://encyclopedia.pub/entry/42368 (accessed on 13 January 2026).
Cheng L, Liu S, Liu Y, Zhan S, Wu Z, Zhang X. Mechanisms of Gut Microbiota Intervention in the CNS. Encyclopedia. Available at: https://encyclopedia.pub/entry/42368. Accessed January 13, 2026.
Cheng, Lu, Siyu Liu, Yanan Liu, Shengnan Zhan, Zufang Wu, Xin Zhang. "Mechanisms of Gut Microbiota Intervention in the CNS" Encyclopedia, https://encyclopedia.pub/entry/42368 (accessed January 13, 2026).
Cheng, L., Liu, S., Liu, Y., Zhan, S., Wu, Z., & Zhang, X. (2023, March 21). Mechanisms of Gut Microbiota Intervention in the CNS. In Encyclopedia. https://encyclopedia.pub/entry/42368
Cheng, Lu, et al. "Mechanisms of Gut Microbiota Intervention in the CNS." Encyclopedia. Web. 21 March, 2023.
Copy Citation
Cognitive, mood and sleep disorders are common and intractable disorders of the central nervous system, causing great inconvenience to the lives of those affected. The gut–brain axis plays a vital role in studying neurological disorders such as neurodegenerative diseases by acting as a channel for a bidirectional information exchange between the gut microbiota and the nervous system.
intestinal flora
neurodegenerative diseases
gut–brain axis
food
1. Gut Microbiota Modulates Mood through Neural Pathways
Currently, an increasing number of studies are focusing on the relationship between gut flora and psychiatric disorders and their possible mechanisms. As a key regulator of the gut–brain axis, the gut flora has emerged as an important factor in the development of depression [1]. The structure of the gut flora is significantly altered in patients with depression compared to healthy controls [2]. Animal studies have shown that gut flora dysbiosis leads to an abnormal stress response in the host, reduced neurogenesis and increased neuroinflammation. The transplantation of faeces from depressed loyalists into germ-free mice induced behavioural and physiological features of depression, including a lack of pleasure and depressive-like behaviour [3]. Furthermore, altered intestinal flora may cause increased intestinal barrier permeability, the activation of systemic inflammatory and immune responses, and the modulation of the release of the monoamine neurotransmitter S-HT, altering the activity and function of the hypothalamic–pituitary–adrenal (HPA) axis and levels of brain-derived neurotic nutritional factor (BDNF), ultimately leading to depression [4]. Disrupting the gut flora in healthy individuals, for example, increases depression. Rebalancing the intestinal flora (e.g., faecal transplantation, healthy diet, probiotics, prebiotics, exercise or medication), on the other hand, can have an antidepressant effect [5].
An analysis targeting neural pathways that regulate emotions showed three main pathways in the gut–brain axis (GBA) to regulate the interaction between the gastrointestinal system and the brain nervous system: the neurological endocrine pathway, the immune regulatory pathway and the VN pathway. The vagus nerve (VN) is a neural pathway composed of sympathetic fibre bundles which can connect the gastrointestinal system with the nervous system in the GBA. The VN is transmitted to the end of the neurotomy, involving histamines, 5-hydroxytryptamine (5-HT), prostaglandins, and cytokines [6]. The VN pathway is not directly related to the intestinal microbial group but instead determines brain activity by transmitting sensor signals between the intestinal microbial metabolites, dopamine and amino acids [7]. Intestinal microorganisms may inhibit the growth and development of T cells in the intestinal lymph tissue in the immune system, causing intestinal inflammation and affecting neurological diseases through VN such as depression, anxiety, and autism [8]. The intestinal flora can also affect the endocrine system of the brain through the hypothalamus–pituitary–adrenal axis (HPA), stimulate the central nervous system (CNS) and cause anxiety and other emotional disorders. Through measuring the concentration of norepinephrine (NE) and aldosterone (ALD) with different intestinal microorganisms, the conclusion is that the intestinal flora affects the endocrine system through HPA [9].
The blood–brain barrier (BBB) and intestinal barriers are key components in brain control and intestinal flora regulation. The exchange of molecules and nutrients between the BBB control the circulation system, and the brain substance plays a role in maintaining the stability of the CNS [10]. The intestinal barrier can protect the intestinal mucosa by secreting short-chain fatty acids (SCFAs) and helps the growth of intestinal epithelial cells, maintaining the stability of the intestinal system. At the same time, intestinal microorganisms will affect the host’s γ-aminobutyric acid (GABA) system. The loss of brain-derived neurotic nutritional factor (BDNF) will affect the CNS, causing depression and hindering neurological development, synapse formation, neuron survival, cell differentiation and other processes, which will cause nervous system injury [11].
Many studies have found that exploring the mechanisms of action between the nervous system and the gut microbiota has focused more on the gut microbiota’s modification of the nervous system [12]. Intestinal microorganisms can affect the colour’s normal metabolic and movement levels. Syminopine is a crucial substance in the entire brain–intestine axis system, and it also prefaces the main body of 5-hydroxytryptamine (5-HT) secretion and metabolism [13]. SCFAs can stimulate the intestinal enterochromaffin cells to produce 5-HT, affecting the brain’s emotions and changing the metabolism of 5-hydroxylidin in the CNS. Therefore, the lack of 5-HT is a crucial feature of the depression pathogenesis mechanism [14].
Emotional disorders such as depression, anxiety and autism will also, in turn, affect the richness and diversity of the intestinal microbial group [15]. For patients with depression, the levels of mobilization and alcohol in the body increase, and the level of thick-wall bacteria decreases. For patients with autism, the level of thick-wall bacteria and deformation bacteria in the body increases, and the level of bacteria decreases [16]. The changes in the intestinal microbial groups of autism patients are also related to the level of neurotoxins generated. Neurotoxins increase the expression of C-FOS in VN sensory ganglia and inhibit the release of neurotransmitters [17].
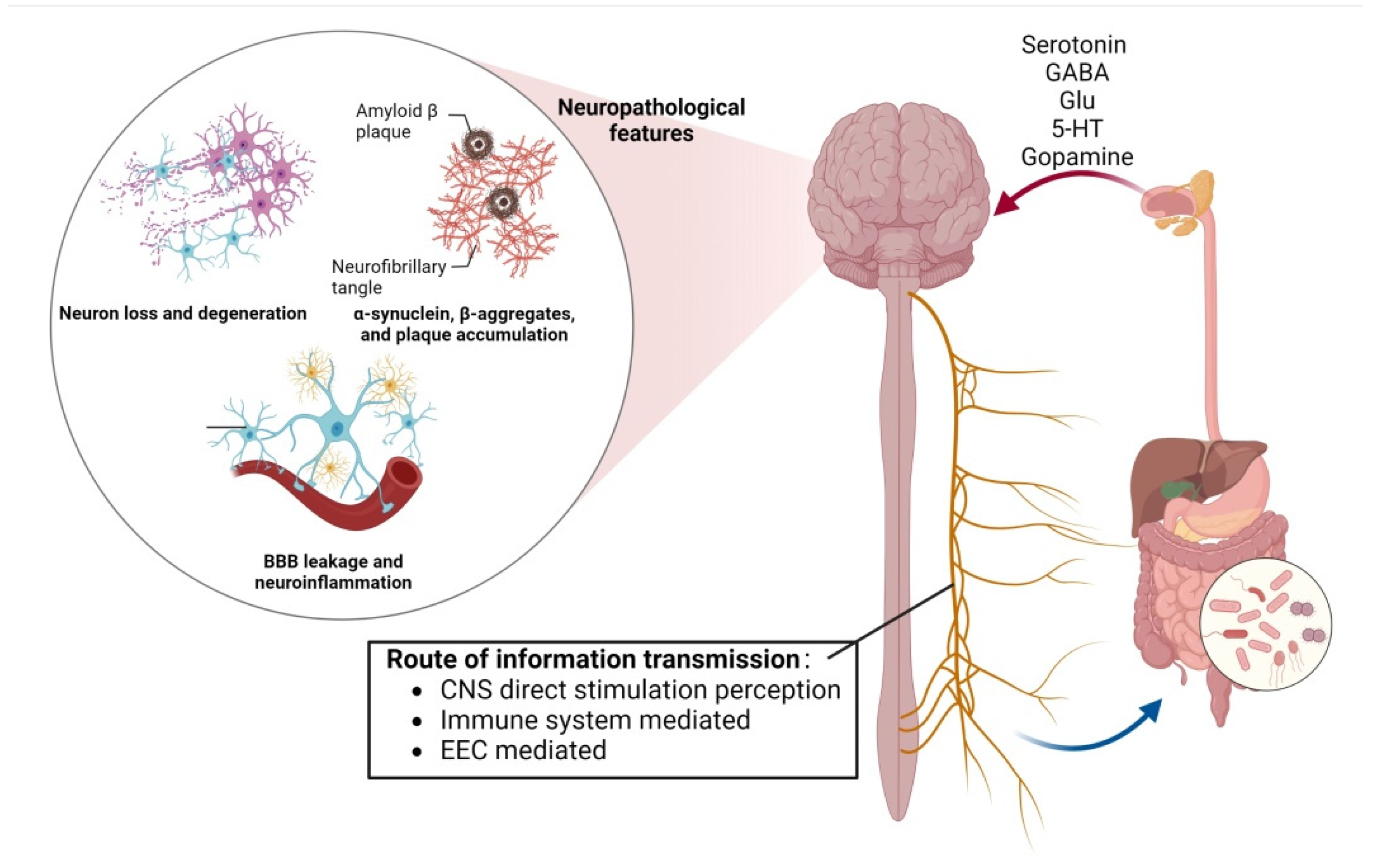
2. Mechanisms of Gut-Flora-Induced Cognitive Impairment
With an increase in age and inflammatory response, a decreased antioxidant capacity, BBB damage, and hippocampus structural changes are related to the decline in cognitive function caused by ageing. Cognitive dysfunction includes dementia, amnesia and delirium. Patients cannot perceive information about time and space, and the back of the brain cannot perceive and analyse data. It is difficult for patients to establish information models. Information models can be divided into perception, memory and thinking [18]. Intestinal flora can affect cognitive functions through the GBA. The intestinal flora affects brain memory, concentration, emotion and other brain functions through the neurotransmitter, VN, neurobiological endocrine, immune regulation, and other channels. It also affects the cognitive function of β-starch-like protein deposition, fat polysaccharide levels, and the development of small glue cells. Disorders of the intestinal flora can cause damage to cognitive function. The intestinal flora and metabolites of patients with cognitive impairment, that is, abnormal metabolites, stimulate external immune inflammation, promote peripheral inflammatory immune cells into the brain-induced inflammation and seriously affect cognitive ability [19].
The nerve transmission of intestinal flora stimulation (GABA, 5-HT, glutamic acid (GLU), NE, dopamine, etc.) can directly stimulate the CNS to generate signals. Among them, metabolic by-products generated by the flora can also interact with adrenaline to stimulate the hypothalamus to generate BNDF, which further affects cognitive ability. It is worth noting that the materials (such as serum, amino acids and GLU) produced by the intestinal microorganisms can also be used as information transmission media to coordinate communication between the systems in the body [20]. Among them, GLU stimulates the CNS, and GABA exists in many immune cells which suppress the CNS by regulating the immune system [21]. The horizontal influence of GABA on the intestinal flora affects the VN system, and GABA further plays a role in preventing neurological diseases [22]. GLU and GABA are neurotransmitters that stimulate different areas of the CNS, including the cerebral cortex, base nerve section, edge system and hypothalamus [23]. Intestinal microorganisms may affect the GLU/GABA ratio in the intestinal cavity, thereby affecting the transition of the GBA’s mid-intestinal signal. During the ageing process, small glue cells are activated during the development of the CNS. Small glue cells are tissue macrophages of the CNS that are mainly responsible for repairing damaged neural networks [24]. Microglia can increase β-secretase and γ-secretase activity and contribute to β-amyloid accumulation, creating a disruptive feedback loop that leads to neuronal interactions, leading to the remodelling of dendritic spines during synaptogenesis [25]. The occurrence of inflammatory cytokines that lead to increased inflammation may be related to sepsis [26]. Inflammatory cytokines destroy the hippocampal neurons by reducing the dendritic branches of the hippocampus. The hippocampus is an integral part of the brain’s ability to learn, cognition, and memory in the CNS. In ageing groups with cognitive obstacles, the hippocampus will show signs of shrinking and significant atrophy. Therefore, most studies have speculated that the atrophy of the hippocampus is also a mechanism that induces severe cognitive impairment. The synapse structure of the hippocampus CAL region has changed, reducing the speed of neurotransmitting and the transmission and recession of the brain’s storage information capacity, thereby increasing the degree of cognitive obstacles for ageing people. Many studies have built mouse models to compare the degree of intervention of mouse mental obstacles. Compared with the control mice, AD mice in a sterile environment have less amyloid β-protein [27]. Wang et al. checked the permeability of the BBB in living and disease-free primary mice. Their results showed that the permeability of the BBB in the body of sterile mice increased, and the expression of proteins changed [28]. However, the increase in the number of normal intestinal flora in infertile mice will lead to a decrease in the BBB permeability, and the reduction in the BBB permeability causes the loss of nutrients and cannot ensure the balance and stability of the neurons. This can easily lead to the accumulation of amyloid β-protein, thereby aggravating cognitive obstacles. These factors indicate that intestinal microorganisms play a role in the starch and protein changes in mental barriers. Heijtza et al. compared the behavioural activity and neurotransmitters of mice without specific pathogens and mice without bacteria. Early exposed intestinal microorganisms reduced the expression of PSD-95 and synapses in the two types of mouse pattern. The fungal mice demonstrated increasing exercise and reduced anxiety, which is related to the long-term enhancement of the cerebellum [10]. These results show that the microbial regulation process of host physiology affects the neural circuit signal mechanism that affects anxious behaviour. Mahmoudiandehkordi et al. also found a strong interaction between AD and the intestinal system. Concentrations of secondary bile acids, deoxycholic acid and their conjugates are consistently increased in AD. As bile acid levels increase, cognitive impairment in the host becomes more severe [29].
The conclusion that sleep deprivation affects cognitive ability has also been verified in recent years. After 40 hours of sleep deprivation, healthy adult subjects have demonstrated reduced immunity, increased inflammation, and severe impairment to cognitive functions such attention, memory and other cognitive functions. To further prove that the sleep deprivation of intestinal flora affects cognitive function, between sterile mice and ordinary mice, the cognitive ability of the sterile mice was found to be less damaged. Cognitive function was significantly impaired in germ-free mice after faecal transplantation. This series of animal experiments and clinical studies suggest intestinal flora disorders may mediate sleep-deprivation-induced cognitive impairment (Figure 1) [30].

Figure 1. The bidirectional action between the gut–brain axis can be regulated by the blood–brain barrier, neurons and amyloid β-protein, affecting the emotional and cognitive parts of the brain.
3. Intestinal Flora Interacts with Circadian Rhythms through the GBA Bidirectional Circulatory System
The GBA is a two-way communication chain between the four systems (endocrine system, intestinal system, endocrine system and CNS) [31]. The regulatory factor in the system can affect the interaction between each system and, through the GBA’s transmission, regulate the steady state of the host microbial group. Microorganisms produce metabolites in the gastrointestinal system, and SCFAs can cross the BBB to affect the growth of neurons and synapses in the hippocampus and amygdala, interacting with sympathetic nerves and nerve cells [32].
Successive sleep plays a crucial role in maintaining physical functions, and lack of sleep is a driving factor in the cause of various diseases. A host with intestinal disease has abnormal levels of reactive oxygen species (ROS), which may cause inflammation by destroying DNA and the immune system [33]. In the absence of sleep, there is a large amount of ROS accumulation in the intestine. ROS are unstable, vital oxidation short-cycle molecules [34]. The valence electron in a large amount of ROS is unpaired and unstable; therefore, it depends on oxygen and has a strong oxidation ability. It is easy to deprive other macromolecules of electrons to meet their needs. ATP is also required for the oxidation of ROS, and it is well-known that ATP increases in the waking state to meet the needs of ROS in large quantities. In contrast, sleep, as a traditional metabolic regulation process, will put pressure on the endoplasmic reticulum, inhibit the massive production of mitochondrial ATP, inhibit the accumulation of ROS and protect the CNS [35]. In fly experiments conducted by Vaccaro et al., sleep-deprived flies died rapidly. When sleep-deprived flies were fed antioxidant compounds or gut-targeted antioxidant enzymes, the survival rate of flies that prevented the accumulation of ROS was significantly higher [36]. Kempf et al. also found that sleep deprivation altered the REDOX state of sleep-regulating neurons in flies, affecting the CNS activity [37]. Conditional phosphate in the brain is caused by sleep, affecting day and night rhythm. If sleep is interrupted, phosphoric acidization will gradually decrease [38].
Biological rhythms exist in all living organisms, including circannual rhythms and circadian rhythms, and are closely related to metabolism and nutrition, affecting the activity of bioactive compounds [39]. Recently, the interaction of circadian rhythms with the gut flora has become key to the study of the metabolic regulation of the organism [40]. The day and night rhythm is a physiological shock process produced by environmental synchronization and endogenous creature clocks [41]. The day and night rhythm consists of two systems (central and peripheral clock systems) which affect the expression of most genes in the body [42]. The central clock system, located in the suprachiasmatic nucleus (SCN), is an oscillator of circadian rhythms. It passes the light to the SCN through retinal stimulation to regulate neuron and blood circulation under dark conditions [43]. The molecular rhythm clock comprises CLOCK and BMAL1 activists and PER and CRY blocking agents. The rhythm of day and night is driven by negative feedback between factors [44]. By improving the composition of the intestinal flora, the rhythm of day and night participates in various physiological reactions (including nutritional absorption, energy regulation, glucose metabolism and immune system regulation) [45]. The intestinal microorganisms themselves also affect rhythm of the host. Despite different environmental factors, the expression of clock genes in sterile mice due to a lack of intestinal microbial groups will also be damaged [46]. Most disease mechanisms are exposed when the central endogenous clock (liver clock) is destroyed. Moreover, liver clocks are not only affected by time but also by eating habits [47]. When the rhythm of day and night is destroyed, the harmful bacteria (such as inflammatory bacteria) in the intestinal microorganisms will increase. In contrast, beneficial bacteria (anti-inflammatory and SCFAs) will be reduced. Long-term rhythmic disorder affects the brain’s normal development, destroys the bodily environment’s steady state, and causes the host to suffer from depression and bipolar mood disorders. It also hurts memory and cognitive functions through the CAMP Map Mapk CREB pathway, which seriously harms health [48]. The interaction between the host’s circadian rhythm and gut flora is bidirectional. The gut flora affects the host’s energy metabolic system by regulating the efficiency of energy extraction from food and the way in which energy is obtained [49]. Oxidation-related substances, such as enzymes, ROS and oxidants, also significantly affect the stability of circadian rhythms [50]. Melatonin has solid oxidative properties that remove ROS and, due to its lipophilic nature, it is readily involved in interactions between the brain and the gut microbiota via the BBB [44]. The loss of the gut microbiota affects the activity of rhythm promoters and terminators, altering gene expression and ultimately reprogramming circadian chromatin [51]. In contrast, circadian arrhythmias also provide negative feedback to the gut flora, increasing the permeability of the gut barrier and leading to an abnormal metabolism and even endotoxemia [52].
References
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41.
- Chen, J.; Zheng, P.; Liu, Y.; Zhong, X.; Wang, H.; Guo, Y.; Xie, P. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 647–655.
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796.
- Liu, Y.; Wang, H.; Gui, S.; Zeng, B.; Pu, J.; Zheng, P.; Zeng, L.; Luo, Y.; Wu, Y.; Zhou, C.; et al. Proteomics analysis of the gut-brain axis in a gut microbiota-dysbiosis model of depression. Transl. Psychiatry 2021, 11, 568.
- Hori, T.; Matsuda, K.; Oishi, K. Probiotics: A dietary factor to modulate the gut microbiome, host immune system, and gut-brain interaction. Microorganisms 2020, 8, 1401.
- Keita, A.V.; Soderholm, J.D. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol. Motil. 2010, 22, 718–733.
- Sun, Y.; Ho, C.T.; Zhang, Y.; Hong, M.; Zhang, X. Plant polysaccharides utilized by gut microbiota: New players in ameliorating cognitive impairment. J. Tradit. Complement. Med. 2022, 13, 128–134.
- Fetissov, S.O.; Dechelotte, P. The new link between gut-brain axis and neuropsychiatric disorders. Curr. Opin. Clin. Nutr. Metab. 2011, 14, 477–482.
- Huo, R.; Zeng, B.; Zeng, L.; Cheng, K.; Li, B.; Luo, Y.; Wang, H.; Zhou, C.; Fang, L.; Li, W.; et al. Microbiota Modulate Anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front. Cell. Infect. Microbiol. 2017, 7, 489.
- Heijtza, R.D.; Wang, S.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of bdnf synthesis and release: Implications in cns function. J. Neurosci. 2009, 29, 12764–12767.
- Liu, Y.; Wu, Z.; Cheng, L.; Zhang, X.; Yang, H. The role of the intestinal microbiota in the pathogenesis of host depression and mechanism of tps relieving depression. Food Funct. 2021, 12, 7651–7663.
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723.
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464.
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194.
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602.
- Lyte, M.; Li, W.; Opitz, N.; Gaykema, R.P.A.; Goehler, L.E. Induction of anxiety-like behavior in mice during the initial stages of infection with the agent of murine colonic hyperplasia citrobacter rodentium. Physiol. Behav. 2006, 89, 350–357.
- Luo, A.; Xie, Z.; Wang, Y.; Wang, X.; Li, S.; Yan, J.; Zhan, G.; Zhou, Z.; Zhao, Y.; Li, S. Type 2 diabetes mellitus-associated cognitive dysfunction: Advances in potential mechanisms and therapies. Neurosci. Biobehav. Rev. 2022, 137, 104642.
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099.
- Bermudez-Humaran, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Alejandro Morales, J.; Bitzer-Quintero, O.K. From probiotics to psychobiotics: Live beneficial bacteria which act on the brain-gut axis. Nutrients 2019, 11, 890.
- Julio-Pieper, M.; Hyland, N.P.; Bravo, J.A.; Dinan, T.G.; Cryan, J.F. A novel role for the metabotropic glutamate receptor-7: Modulation of faecal water content and colonic electrolyte transport in the mouse. Br. J. Pharmacol. 2010, 160, 367–375.
- Zhang, Z.; Yu, Q.; Zhang, X.; Wang, X.; Su, Y.; He, W.; Li, J.; Wan, H.; Jing, X. Electroacupuncture regulates inflammatory cytokines by activating the vagus nerve to enhance antitumor immunity in mice with breast tumors. Life Sci. 2022, 291, 119539.
- Kondoh, T.; Mallick, H.N.; Torii, K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am. J. Clin. Nutr. 2009, 90, 832S–837S.
- Lenz, K.M.; Nelson, L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018, 9, 698.
- Edmonson, C.A.; Ziats, M.N.; Rennert, O.M. A non-inflammatory role for microglia in autism spectrum disorders. Front. Neurol. 2016, 7, 9.
- Richwine, A.F.; Godbout, J.P.; Berg, B.M.; Chen, J.; Escobar, J.; Millard, D.K.; Johnson, R.W. Improved psychomotor performance in aged mice fed diet high in antioxidants is associated with reduced ex vivo brain interleukin-6 production. Brain. Behav. Immun. 2005, 19, 512–520.
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of abeta amyloid pathology in appps1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 46856.
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of sesamin alleviates stress-induced behavioral and psychological disorders via reshaping the gut microbiota structure. J. Agric. Food Chem. 2019, 67, 12441–12451.
- Mahmoudiandehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Ahmad, S.; Jia, W.; Xia, G.; Louie, G.; Kueider, A.; Moseley, M.A.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-an emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 604.
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292.
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62.
- Zhao, K.; Yao, M.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y.; Wang, H. Flavonoids and intestinal microbes interact to alleviate depression. J. Sci. Food Agric. 2022, 102, 1311–1318.
- Li, R.; Xiao, J.; Cao, Y.; Huang, Q.; Ho, C.T.; Lu, M. Capsaicin attenuates oleic acid-induced lipid accumulation via the regulation of circadian clock genes in HepG2 cells. J. Agric. Food Chem. 2022, 70, 794–803.
- Li, W.; Young, J.F.; Sun, J. NADPH Oxidase-generated reactive oxygen species in mature follicles are essential for drosophila ovulation. Proc. Natl. Acad. Sci. USA 2018, 115, 7765–7770.
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327.
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell 2020, 181, 1307–1328.e15.
- Kempf, A.; Song, S.M.; Talbot, C.B.; Miesenbock, G. A potassium channel beta-subunit couples mitochondrial electron transport to sleep. Nature 2019, 568, 230–234.
- Bruening, F.; Noya, S.B.; Bange, T.; Koutsouli, S.; Rudolph, J.D.; Tyagarajan, S.; Cox, J.; Mann, M.; Brown, S.A.; Robles, M.S. Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 2019, 366, eaav3617.
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and polyphenols: Roles and diseases. Nutrients 2019, 11, 2602.
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92.
- Engin, A. Circadian Rhythms in Diet-Induced Obesity. In Obesity and Lipotoxicity; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 19–52.
- Welsh, D.; Logothetis, D.; Meister, M.; Reppert, S. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 1995, 14, 697–706.
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549.
- Liu, F.; Zhang, X.; Zhao, B.; Tan, X.; Wang, L.; Liu, X. Role of food phytochemicals in the modulation of circadian clocks. J. Agric. Food Chem. 2019, 67, 8735–8739.
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529.
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadimpalli, A.; Hubert, N.; et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015, 17, 681–689.
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. 2014, 20, 1006–1017.
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian disorganization alters intestinal microbiota. PLoS ONE 2014, 9, e97500.
- Deaver, J.A.; Eum, S.Y.; Toborek, M. Circadian disruption changes gut microbiome taxa and functional gene composition. Front. Microbiol. 2018, 9, 737.
- Khapre, R.V.; Kondratova, A.A.; Susova, O.; Kondratov, R.V. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 2011, 10, 4162–4169.
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalova, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota diumal rhythmicity programs host transcriptome oscillations. Cell 2016, 167, 1495–1510.e12.
- Summa, K.C.; Voigt, R.M.; Forsyth, C.B.; Shaikh, M.; Cavanaugh, K.; Tang, Y.; Vitaterna, M.H.; Song, S.; Turek, F.W.; Keshavarzian, A. Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS ONE 2013, 8, e67102.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Revisions:
2 times
(View History)
Update Date:
21 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




