
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | giandomenico roviello | -- | 2161 | 2023-03-20 15:40:45 | | | |
| 2 | Lindsay Dong | Meta information modification | 2161 | 2023-03-21 01:56:07 | | |
Video Upload Options
The management of patients with metastatic colorectal cancer (mCRC) has the continuum of care as the treatment paradigm. To date, trifluridine/tipiracil, a biochemically modulated fluoropyrimidine, and regorafenib, a multi-kinase inhibitor, remain the main options for the majority of patients who progressed to standard doublet- or triplet-based chemotherapies, although a tailored approach could be indicated in certain circumstances. Being highly selective for vascular endothelial growth factor receptor (VEGFR)-1, -2 and -3, fruquintinib demonstrated a strong anti-tumor activity in preclinical models and received approval from China’s National Medical Products Administration (NMPA) in 2018 for the treatment of patients with chemo-refractory mCRC. The approval was based on the results of the phase III FRESCO trial. Then, in order to overcome geographic differences in clinical practice, the FRESCO-2 trial was conducted in the US, Europe, Japan, and Australia. In a heavily pretreated patient population, the study met its primary endpoint, demonstrating an advantage of fruquintinib over a placebo in overall survival (OS).
1. Introduction
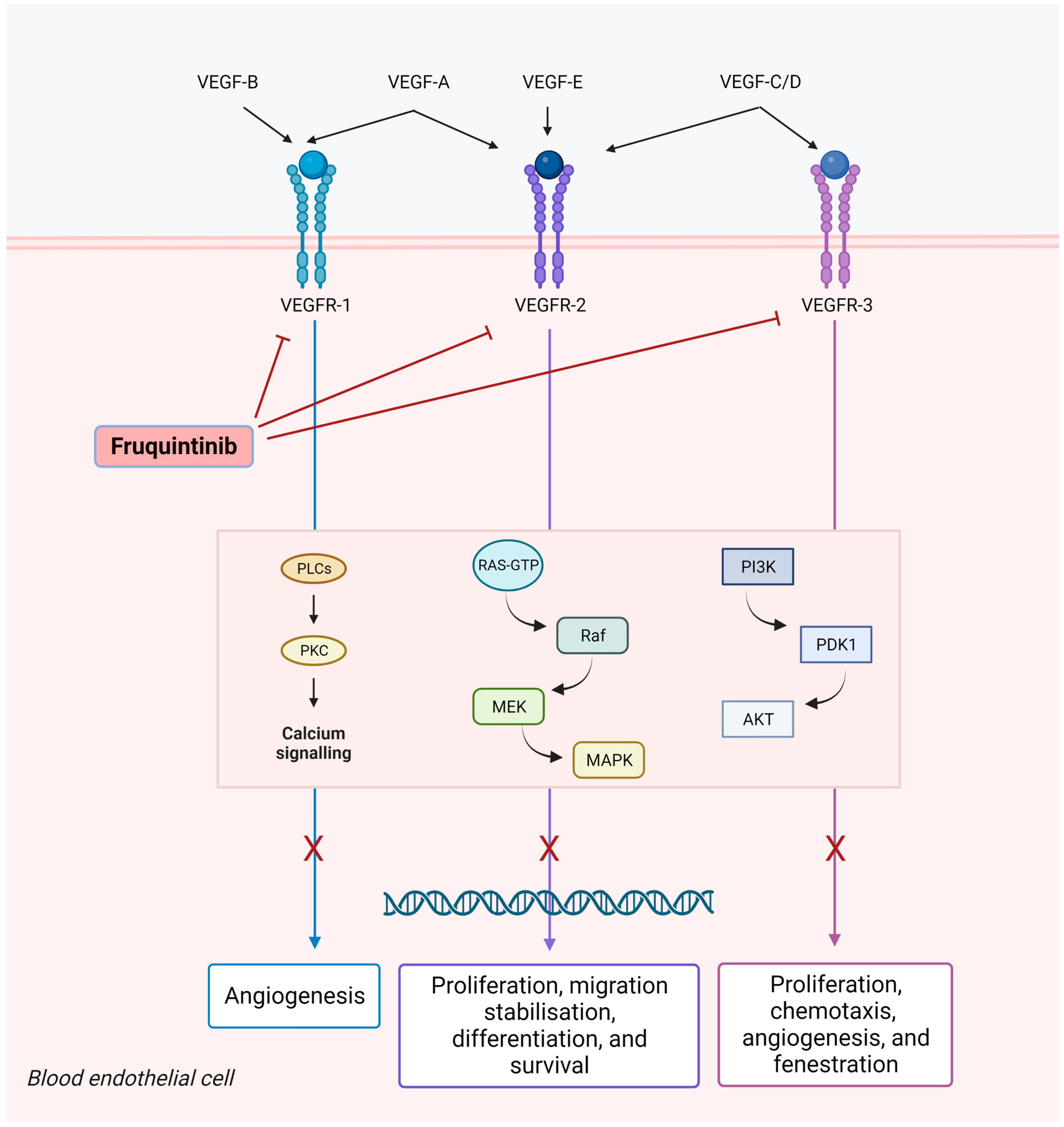
2. Pharmacodynamic Properties
3. Clinical Development
3.1. Phase 1–2
3.2. Phase 3
4. Summary
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2022, 72, 7–33.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32.
- Yoshino, T.; Watanabe, J.; Shitara, K.; Shitara, K.; Yamazaki, K.; Watanabe, J.; Oki, E.; Sato, T.; Naitoh, T.; Komatsu, Y.; et al. PARADIGM study: A multicenter, randomized, phase III study of mFOLFOX6 plus panitumumab or bevacizumab as first-line treatment in patients with RAS (KRAS/NRAS) wild-type metastatic colorectal cancer. J. Clin. Oncol. 2021, 39, 85.
- Lavacchi, D.; Fancelli, S.; Roviello, G.; Castiglione, F.; Caliman, E.; Rossi, G.; Venturini, J.; Pellegrini, E.; Brugia, M.; Vannini, A.; et al. Mutations matter: An observational study of the prognostic and predictive value of KRAS mutations in metastatic colorectal cancer. Front. Oncol. 2022, 12, 1055019.
- Ciardiello, D.; Chiarazzo, C.; Famiglietti, V.; Damato, A.; Pinto, C.; Zampino, M.G.; Castellano, G.; Gervaso, L.; Zaniboni, A.; Oneda, E.; et al. Clinical efficacy of sequential treatments in KRASG12C-mutant metastatic colorectal cancer: Findings from a real-life multicenter Italian study (CRC-KR GOIM). ESMO Open 2022, 7, 100567.
- Cremolini, C.; Antoniotti, C.; Rossini, D.; Lonardi, S.; Loupakis, F.; Pietrantonio, F.; Bordonaro, R.; Latiano, T.P.; Tamburini, E.; Santini, D.; et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020, 21, 497–507.
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729.
- Lenz, H.J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170.
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218.
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027.
- Fallah, A.; Sadeghinia, A.; Kahroba, H.; Samadi, A.; Heidari, H.R.; Bradaran, B.; Zeinali, S.; Molavi, O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2018, 110, 775–785.
- Semenza, G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 2003, 3, 721–732.
- Shinkaruk, S.; Bayle, M.; Laïn, G.; Déléris, G. Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy. Curr. Med. Chem. Agents 2003, 3, 95–117.
- Hansen, T.F.; Qvortrup, C.; Pfeiffer, P. Angiogenesis Inhibitors for Colorectal Cancer. A Review of the Clinical Data. Cancers 2021, 13, 1031.
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342.
- Giantonio, B.J.; Catalano, P.J.; Meropol, N.J.; O’Dwyer, P.J.; Mitchell, E.P.; Alberts, S.R.; Schwartz, M.A.; Benson, A.B., 3rd; Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007, 25, 1539–1544.
- Masi, G.; Salvatore, L.; Boni, L.; Loupakis, F.; Cremolini, C.; Fornaro, L.; Schirripa, M.; Cupini, S.; Barbara, C.; Safina, V.; et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: Final results of the randomized BEBYP trial. Ann. Oncol. 2015, 26, 724–730.
- Fan, F.; Samuel, S.; Gaur, P.; Lu, J.; Dallas, N.A.; Xia, L.; Bose, D.; Ramachandran, V.; Ellis, L.M. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br. J. Cancer 2011, 104, 1270–1277.
- Itatani, Y.; Kawada, K.; Yamamoto, T.; Sakai, Y. Resistance to Anti-Angiogenic Therapy in Cancer-Alterations to Anti-VEGF Pathway. Int. J. Mol. Sci. 2018, 19, 1232.
- Hayashi, H.; Arao, T.; Matsumoto, K.; Kimura, H.; Togashi, Y.; Hirashima, Y.; Horita, Y.; Iwasa, S.; Okita, N.T.; Honma, Y.; et al. Biomarkers of reactive resistance and early disease progression during chemotherapy plus bevacizumab treatment for colorectal carcinoma. Oncotarget 2014, 5, 2588–2595.
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of Aflibercept to Fluorouracil, Leucovorin, and Irinotecan Improves Survival in a Phase III Randomized Trial in Patients with Metastatic Colorectal Cancer Previously Treated with an Oxaliplatin-Based Regimen. J. Clin. Oncol. 2012, 30, 3499–3506.
- Lavacchi, D.; Roviello, G.; Giommoni, E.; Dreoni, L.; Derio, S.; Brugia, M.; Amedei, A.; Pillozzi, S.; Antonuzzo, L. Aflibercept Plus FOLFIRI as Second-Line Treatment for Metastatic Colorectal Cancer: A Single-Institution Real-Life Experience. Cancers 2021, 13, 3863.
- Grothey, A.; Cutsem, E.V.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312.
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919.
- Loupakis, F.; Antonuzzo, L.; Bachet, J.-B.; Kuan, F.C.; Macarulla, T.; Pietrantonio, F.; Xu, R.H.; Taniguchi, H.; Winder, T.; Yuki, S.; et al. Practical considerations in the use of regorafenib in metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2020, 12.
- Cosso, F.; Lavacchi, D.; Fancelli, S.; Caliman, E.; Brugia, M.; Rossi, G.; Winchler, C.; Pillozzi, S.; Antonuzzo, L. Long-term response of more than 9 years to regorafenib in a heavily pretreated patient with metastatic colorectal cancer. Anti-Cancer Drugs 2022, 34, 451–454.
- Antonuzzo, L.; Lunghi, A.; Giommoni, E.; Brugia, M.; Di Costanzo, F. Regorafenib Also Can Cause Osteonecrosis of the Jaw. JNCI J. Natl. Cancer Inst. 2016, 108, djw002.
- Antonuzzo, L.; Lunghi, A.; Petreni, P.; Brugia, M.; Laffi, A.; Giommoni, E.; Mela, M.M.; Mazzoni, F.; Balestri, V.; Costanzo, F.D. Osteonecrosis of the Jaw and Angiogenesis inhibitors: A Revival of a Rare but Serous Side Effect. Curr. Med. Chem. 2017, 24, 3068–3076.
- Sun, Q.; Zhou, J.; Zhang, Z.; Guo, M.; Liang, J.; Zhou, F.; Long, J.; Zhang, W.; Yin, F.; Cai, H.; et al. Discovery of fruquintinib, a potent and highly selective small molecule inhibitor of VEGFR 1, 2, 3 tyrosine kinases for cancer therapy. Cancer Biol. Ther. 2014, 15, 1635–1645.
- Gu, Y.; Wang, J.; Li, K.; Zhang, L.; Ren, H.; Guo, L.; Sai, Y.; Zhang, W.; Su, W. Preclinical pharmacokinetics and disposition of a novel selective VEGFR inhibitor fruquintinib (HMPL-013) and the prediction of its human pharmacokinetics. Cancer Chemother. Pharmacol. 2014, 74, 95–115.
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625.
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105.
- Zeng, H.; Dvorak, H.F.; Mukhopadhyay, D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J. Biol. Chem. 2001, 276, 26969–26979.
- Martins, S.F.; Garcia, E.A.; Luz, M.A.; Pardal, F.; Rodrigues, M.; Filho, A.L. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom. Proteom. 2013, 10, 55–67.
- Bui, H.M.; Enis, D.; Robciuc, M.R.; Nurmi, H.J.; Cohen, J.; Chen, M.; Yang, Y.; Dhillon, V.; Johnson, K.; Zhang, H.; et al. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J. Clin. Investig. 2016, 126, 2167–2180.
- Shirley, M. Fruquintinib: First Global Approval. Drugs 2018, 78, 1757–1761.
- Ren, Y.; Sun, Q.; Long, J.; Fan, S.; Tang, R.; Zhang, W.; Ge, X.; Tang, J.; Wang, L.; Shi, D.; et al. Abstract 2089: Evaluation of fruquintinib, a potent and selective oral VEGFR inhibitor, in combination with targeted therapies or immune checkpoint inhibitors in preclinical tumor models’. Cancer Res 2017, 77, 2089.
- Li, Q.; Cheng, X.; Zhou, C.; Tang, Y.; Li, F.; Zhang, B.; Huang, T.; Wang, J.; Tu, S. Fruquintinib Enhances the Antitumor Immune Responses of Anti-Programmed Death Receptor-1 in Colorectal Cancer. Front. Oncol. 2022, 12, 841977.
- Cao, J.; Zhang, J.; Peng, W.; Chen, Z.; Fan, S.; Su, W.; Li, K.; Li, J. A Phase I study of safety and pharmacokinetics of fruquintinib, a novel selective inhibitor of vascular endothelial growth factor receptor-1, -2, and -3 tyrosine kinases in Chinese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2016, 78, 259–269.
- Xu, R.H.; Li, J.; Bai, Y.; Xu, J.; Liu, T.; Shen, L.; Wang, L.; Pan, H.; Cao, J.; Zhang, D.; et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: A phase Ib study and a randomized double-blind phase II study. J. Hematol. Oncol. 2017, 10, 22.
- Zhang, Y.; Zou, J.Y.; Wang, Z.; Wang, Y. Fruquintinib: A novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag. Res. 2019, 11, 7787–7803.
- Grothey, A.; Sargent, D.; Goldberg, R.M.; Schmoll, H.J. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J. Clin. Oncol. 2004, 22, 1209–1214.
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422.
- Goldberg, R.M.; Rothenberg, M.L.; Van Cutsem, E.; Benson, A.B.; Blanke, C.D.; Diasio, R.B.; Grothey, A.; Lenz, H.J.; Meropol, N.J.; Ramanathan, R.K.; et al. The continuum of care: A paradigm for the management of metastatic colorectal cancer. Oncologist 2007, 12, 38–50.




