Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Magan-Fernandez | -- | 2329 | 2023-03-20 11:36:28 | | | |
| 2 | Rita Xu | -1 word(s) | 2328 | 2023-03-21 02:32:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mainas, G.; Ide, M.; Rizzo, M.; Magan-Fernandez, A.; Mesa, F.; Nibali, L. Systemic Impact of Periodontitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/42352 (accessed on 07 February 2026).
Mainas G, Ide M, Rizzo M, Magan-Fernandez A, Mesa F, Nibali L. Systemic Impact of Periodontitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/42352. Accessed February 07, 2026.
Mainas, Giuseppe, Mark Ide, Manfredi Rizzo, Antonio Magan-Fernandez, Francisco Mesa, Luigi Nibali. "Systemic Impact of Periodontitis" Encyclopedia, https://encyclopedia.pub/entry/42352 (accessed February 07, 2026).
Mainas, G., Ide, M., Rizzo, M., Magan-Fernandez, A., Mesa, F., & Nibali, L. (2023, March 20). Systemic Impact of Periodontitis. In Encyclopedia. https://encyclopedia.pub/entry/42352
Mainas, Giuseppe, et al. "Systemic Impact of Periodontitis." Encyclopedia. Web. 20 March, 2023.
Copy Citation
Periodontitis is a microbially driven host-mediated disease that leads to loss of periodontal attachment and bone. It is associated with elevation of systemic inflammatory markers and with the presence of systemic co-morbidities. Furthermore, periodontal treatment leads to a 24–48 h-long acute local and systemic inflammatory response.
periodontitis
inflammation
biomarkers
1. Introduction
Periodontitis is a microbially driven host-mediated disease that leads to loss of periodontal attachment and bone [1] At first stage, gingival inflammation (gingivitis) is caused by bacterial biofilm formation. Consequently, progression of periodontal disease to destructive periodontitis depends on microbial dysbiosis in response to nutrients from gingival inflammatory and tissue breakdown products favouring the growth of some bacterial species, and also in response to anti-bacterial mechanisms that try to contain the microbial challenge within the gingival sulcus [1]. According to the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions, severity and complexity of periodontitis are assessed following a staging process (Stage I, II, III and IV) that consider the initial clinical attachment level (CAL) whereas, the direct or indirect rate of progression is based on a grading scale (Grade A, B and C) that involves radiographic bone loss (RBL) and presence of risk factors such as smoking and diabetes [1].
However, periodontal inflammation is not just a local phenomenon. The possible link between periodontitis and systemic conditions has been extensively reported in 2013 in the Proceedings of a Workshop jointly held by the European Federation of Periodontology and the American Academy of Periodontology, where authors agreed that pro-inflammatory (infectious) events related to periodontitis may have a systemic impact and, vice-versa, some systemic disorders may influence periodontal outcomes [2][3][4].
Several studies have reported that both acute local and systemic inflammatory response is increased up to 24–48 h after periodontal treatment, either surgical or non-surgical [5][6]. Since this might be additional to the pre-existing inflammatory burden of patients with compromised medical history and/or uncontrolled systemic diseases, some have tried to investigate how to manage and modulate the impact of periodontal treatment on systemic inflammation.
2. Periodontitis and Systemic Diseases
Describing in detail the complex mechanisms concerning the connection between periodontitis and systemic disease is not the aim of the present research. For in-depth reading, researchers refer to the appropriate articles [7]. However, a brief introduction is provided in order to give the reader a general understanding of the subject (Figure 1).
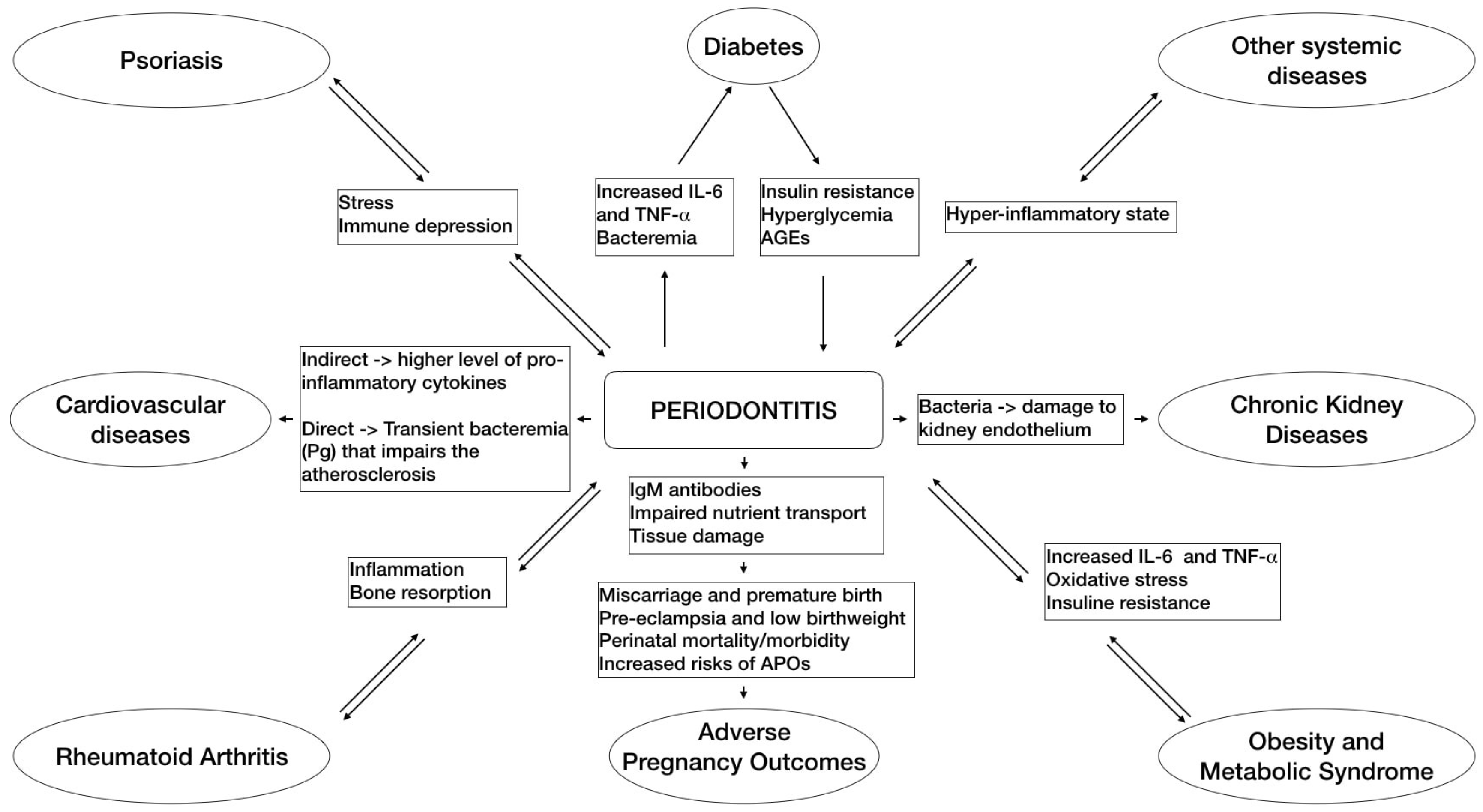
Figure 1. This flowchart schematically summarizes the main pathways of associations between periodontitis and some related systemic diseases based on the emerging evidence.
Two main simultaneous and not independent pathways, infection and inflammation, are involved in the pathogenic process of periodontitis–systemic connections [8]. The infectious pathway consists of oral micro-organisms that, together with putative pathogens located in periodontal pockets, may pass into the blood stream or respiratory tract causing a bacteremia that, in turn, can lead to complications for unhealthy and immunocompromised subjects, either systemically or possibly after selective colonization of distant sites and organs. On the other hand, the inflammatory pathway pertains to the systemic inflammation provoked by local and systemic effects of bacterial products and inflammatory molecules of periodontal origin that might be a risk factor for inflammatory associated systemic diseases in susceptible hosts [8].
As summarized in the Proceedings of a Workshop jointly held by the European Federation of Periodontology and the American Academy of Periodontology [2], periodontal disease has been associated with diabetes [3][9][10], cardiovascular diseases (CDV) [11][12][13][14][15], adverse pregnancy outcomes [4][16][17], obesity [18][19], metabolic syndrome [20][21], chronic obstructive airways disease [22][23], cognitive impairment such as Alzheimer’s [24][25], rheumatoid arthritis [26], chronic kidney diseases [27][28], psoriasis [29], depression [30], osteoporosis [31][32] and cancers [7][33]. The understanding of these associations is further complicated by the fact that several of these relationships may be bidirectional or even interrelated between systemic outcomes.
3. Impact of Periodontitis on Quality of Life and Public Health
Oral health is a functional, structural, aesthetic, physiological and psychological state of wellbeing and is essential to an individual’s general health and quality of life [34]. Almost two decades ago, at the 2003 World Workshop on Emerging Science in Periodontology, it was recognized that patient-based outcomes (PBO) had to be considered a research priority [35]. It has been reported that periodontal disease impairs oral health-related quality of life (OHRQoL) [36] and, in addition, a correlation between extent and severity of periodontitis exists [37]. Two systematic reviews found that non-surgical periodontal treatment (NSPT), now termed professional mechanical plaque removal (PMPR), moderately improved the OHRQoL of adult patients in the short-term (1 week) and long-term (12 months) specifically by reducing pain, psychological discomfort and physical disability [38][39], whereas a surgical approach had a relatively lower impact [38]. Another important aspect is correlated to the devasting impact that periodontal disease has on public health [40]. Jeffcoat et al. analyzed medical and dental insurance claims data from a large cohort who simultaneously experienced periodontitis and one systemic condition, including coronary artery disease, cerebrovascular disease, pregnancy, diabetes mellitus type 2 and rheumatoid arthritis. They concluded that, with the exception of those with rheumatoid arthritis, patients who were periodontally treated generated lower medical costs and fewer hospitalizations, with cost reductions of 72.7%, 40.9%, 40.2% and 10.7% for pregnancy, cerebrovascular disease, diabetes mellitus type 2 and coronary artery disease care, respectively [41].
In 2021, an Economist Intelligence Unit’s report (promoted by the European Federation of Periodontology/EFP) across six European countries that share similar conditions (France, Germany, Italy, the Netherlands, Spain and UK) was released [42]. Based on the concepts of the economic burden and return-on-investment (ROI), it highlighted that home care by patients aiming at preventing periodontal disease was very cost effective for society and ultimately reduced the need for more difficult and expensive treatment. It was inferred that this may also then indirectly significantly lower costs related to other systemic co-morbidities [42].
4. Effects of Periodontal Treatment on Systemic Health in Both the Short- and Long-Term
Risk factor control, oral hygiene instructions and supra- and sub-gingival tooth debridement (professional mechanical plaque removal or PMPR) form the hallmark of initial periodontal treatment, now codified as step 1 and 2 of periodontal treatment [43]. The possible mechanisms that link periodontal treatment and systemic inflammation could be that both the bacteremia and the tissue damage (trauma) after subgingival debridement determine an increase of pro-inflammatory mediators, such as Interleukin 1-beta (IL-1β) and 6 (IL-6), which ultimately induce liver production of acute-phase proteins [5][6][44].
A very interesting study described the kinetics of serum inflammatory markers following non-surgical (full-mouth) and surgical periodontal treatment in 14 patients with chronic periodontitis [5]. C-reactive protein (CRP), leucocyte counts, serum amyloid-A (SAA), D-dimers and cystatin C (renal function) were studied at 1, 7, 30, 90 and 180 days. A consistent increase in the serum level of CRP and SAA was observed at day 1 after PMPR and a smaller increase after the first surgery; D-dimer plasma levels significantly increased 24 h following PMPR but decreased after surgery, whereas cystatin C presented a moderate initial increase followed by an important reduction 12 months after treatment completion. Therefore, within the limitations of the study. such as small sample size and absence of control group, authors concluded that (i) both surgical and non-surgical periodontal treatment lead to systemic inflammation; however, (ii) healthy patients usually do not present any complications [5]. One of the most interesting aspect of these findings is that CRP serum levels were higher after PMPR compared to after surgical interventions, which might be counter-intuitive given the clinical perception of a greater trauma caused by surgery. A possible explanation might be the fact that the traumatized area is smaller in surgery than the full-mouth non-causal therapy, with a consequent reduced systemic inflammation due to a smaller post-operative wound. Another hypothesis is that subgingival debridement provokes a greater bacteremia, considering that the surgical treatment is provided after the same PMPR has reduced and modified the pathogens’ habitat, including number and quality of pathogens. Alternatively, a prerequisite for surgical intervention is the achievement of good oral hygiene, and this may also impact on local tissue inflammation, permeability and the degree of bacteremia. These short-term effects have been used as an experimental model to illustrate what might happen at a lower grade, more chronic and frequent exposure event every day in periodontitis patients, as well as an example to study the systemic acute-phase response [6].
Moreover, in the short- and long-term, other events have been detected [45][46]. A randomized clinical trial comparing the standard cycle of supragingival mechanical scaling and polishing and full-mouth intensive subgingival debridement observed that intensive treatment provoked an acute systemic inflammation (significantly higher levels of CRP, IL-6, soluble E-selectin and von Willebrand factor) and endothelial dysfunction (lower flow-mediated dilatation) at 24 h. Nevertheless, at 3- and 6 months, intensive treatment showed reduced indexes of periodontal disease severity and significantly better endothelial function [46]. These finding were corroborated by an observational study that found moderate acute systemic inflammation and endothelial dysfunction 1- and 7 days after providing an intensive periodontal treatment [45]. In addition, the same authors found an alteration of the hemostatic system in terms of increasing blood coagulability (significant increase of D-dimer levels) [45].
A very recent systematic review confirmed the aforementioned findings. The authors reported that conventional and, mainly, intensive PMPR provokes changes in CRP including a sharp increase over the first week [47].
4.1. Diabetes
Diabetes mellitus and periodontitis have a very complex bidirectional relationship that involves genetic factors and pro-inflammatory mediators [3]. Presence of periodontitis might impair insulin resistance which appears as hyperglycemia that produces advanced glycation end products (AGEs). In turn, AGEs lead to overproduction of IL-6, IL-1 and Tumor Necrosis Factor alpha (TNF-α). As a consequence, presence of AGEs in tissues causes impaired wound healing (improper collagen turnover in fibroblasts) and excessive neutrophil response to periodontal bacteria [48].
Considering the effect of inflammation in Diabetes mellitus type 2, it is thought that periodontal therapy may have a positive effect in reducing insulin resistance with following improvement of glycemic control [49]. Another study, with the aim of assessing the effects of PMPR on obese type 2 diabetes mellitus patients, evaluated inflammatory biomarkers such as CRP, IL-4, IL-6, IL-8, IL-10, TNF-α and fibrinogen. After 3 months, TNF-a and fibrinogen decreased significantly and, in general, the other biomarkers presented a trend towards reduction, even if not statistically significant [50]. A systematic review and meta-analysis found a statistically significant mean difference in favor of patients undergoing PMPR compared with the control group for CRP (−1.28 mg/L) and TNF-a (−1.33 pg/mL) after a follow-up period of from 1–12 months [51]. Another more recent systematic review including studies with follow-ups ranging between 3–6 months reported that PMPR contributes to the reduction of IL-6 serum levels in patients with type 2 diabetes mellitus [52].
4.2. Cardiovascular Diseases
Periodontal disease and atherosclerotic vascular disease (the main cause of cardiovascular disease) present an established association, as was stated in the 2009 Consensus by the editors of the American Journal of Cardiology and Journal of Periodontology, and in the 2012 Statement by the American Heart Association [53][54]. Several mechanisms have been proposed to explain this very close association, such as the infection of atherosclerotic plaques by periodontal pathogens, the pro-atherogenic effect on the lipid profile, and the systemic dissemination of pro-inflammatory mediators [55].
Two linking pathways have been extensively investigated: indirect and direct. The indirect pathway was described as the systemic inflammation provoked by periodontitis that leads to higher levels of several pro-inflammatory cytokines [56]. The direct pathway consists of periodontal pathogens that enter into the circulatory system and cause a transient bacteremia (e.g., toothbrushing, chewing, etc.) that can affect cardiovascular mediators and contribute to impair the atherosclerosis; Porphyromonas gingivalis (Pg), in particular, is capable of invading host cells such as macrophages, epithelial cells and fibroblasts [57].
Several systematic reviews have been carried out regarding the effect of periodontal treatment on cardiovascular disease markers [11][58][59]. One reported a significant reduction in almost each biomarker assessed in favor of treatment group (PMPR) compared to control group (no periodontal treatment) and, in addition, a sub-analysis related to patients with co-morbidities (diabetes and CVD) revealed that periodontal treatment reduced CVD risk factors [58]. Concerning CRP levels, a statistically mean difference of −0.231 mg/L, eight weeks after PMPR, was observed [59]. Other authors found that short-term periodontal treatment leads to local and systemic inflammatory response with disruption of the hemostatic system and endothelial function. After 6 months, a progressive decrease in CVD risk biomarkers including CRP, lipids, fibrinogen and E-selectin was observed [11].
Furthermore, two randomized clinical trials assessing the importance of an adequate and immediate PMPR on CVD surrogate markers have been published [46][60]. Compared to patients that only received supragingival mechanical scaling and polishing, patients who received an intense full-mouth subgingival plaque-removal treatment presented, at 24 h, a significant reduction in flow-mediated dilatation (the vasodilation of the brachial artery to assess the endothelial function), and a significant increase in the levels of CRP, IL-6, and the endothelial-activation markers, soluble E-selectin and von Willebrand factor. Six months later, however, intensive-treatment patients presented greater flow-mediated dilatation and lower E-selectin levels than the other group. The remaining parameters and biomarkers showed no statistical difference at 6 months. Therefore, the 1authors concluded that benefits in oral health are provided over time and lead to an improved endothelial function [46]. In the other study, periodontal patients with nonresponsive arterial hypertension were divided in two groups: the test group received immediate non-causal therapy, whereas the control group was treated 3 months later. It was found that the biomarkers assessed (CPR, IL-6 and fibrinogen) simultaneously and significantly decreased in plasma level, 3 months after the treatment [60]. Very interestingly, a prospective study on a Korean cardiovascular health population showed that presence of periodontal disease was associated with an increased risk of future stroke, acute myocardial infarction, hearth failure (major cardiovascular events) and death [61]. These findings were confirmed in a recent comprehensive systematic review and meta-analysis [62].
Other authors have focused on the potential pro-atherogenic alterations in plasma lipids and lipoproteins, since dyslipidemia is a common feature of patients with periodontitis; a systematic review and meta-analysis of published studies revealed that periodontitis is associated with higher plasma levels of total- and LDL-cholesterol and triglycerides, with a concomitant decrease of HDL-cholesterol concentrations [63]. Yet, beyond the increased concentrations of LDL-cholesterol, untreated periodontitis is also associated with an altered LDL subclass profile, with a predominance of atherogenic small dense LDL [64]; this means, therefore, that patients with periodontitis have alterations in both the quantity and the quality of LDL, which represents a well-known mechanism for the development of pro-atherogenic alterations and the enhancement of cardiovascular risk [65].
References
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172.
- Linden, G.J.; Herzberg, M.C. Periodontitis and systemic diseases: A record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S20–S23.
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84, S135–S152.
- Ide, M.; Papapanou, P.N. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes–systematic review. J. Periodontol. 2013, 84, S181–S194.
- Graziani, F.; Cei, S.; Tonetti, M.; Paolantonio, M.; Serio, R.; Sammartino, G.; Gabriele, M.; D’Aiuto, F. Systemic inflammation following non-surgical and surgical periodontal therapy. J. Clin. Periodontol. 2010, 37, 848–854.
- D’Aiuto, F.; Nibali, L.; Mohamed-Ali, V.; Vallance, P.; Tonetti, M.S. Periodontal therapy: A novel non-drug-induced experimental model to study human inflammation. J. Periodontal Res. 2004, 39, 294–299.
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440.
- Van Dyke, T.E.; van Winkelhoff, A.J. Infection and inflammatory mechanisms. J. Periodontol. 2013, 84, S1–S7.
- Chapple, I.L.; Genco, R. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S106–S112.
- Taylor, J.J.; Preshaw, P.M.; Lalla, E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J. Clin. Periodontol. 2013, 40, S113–S134.
- D’Aiuto, F.; Orlandi, M.; Gunsolley, J.C. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J. Periodontol. 2013, 84, S85–S105.
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Periodontol. 2013, 84, S70–S84.
- Schenkein, H.A.; Loos, B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Periodontol. 2013, 84, S51–S69.
- Tonetti, M.S.; Van Dyke, T.E. Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S24–S29.
- Romandini, M.; Laforí, A.; Romandini, P.; Baima, G.; Cordaro, M. Periodontitis and platelet count: A new potential link with cardiovascular and other systemic inflammatory diseases. J. Clin. Periodontol. 2018, 45, 1299–1310.
- Madianos, P.N.; Bobetsis, Y.A.; Offenbacher, S. Adverse pregnancy outcomes (APOs) and periodontal disease: Pathogenic mechanisms. J. Periodontol. 2013, 84, S170–S180.
- Sanz, M.; Kornman, K. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Clin. Periodontol. 2013, 40, S164–S169.
- Keller, A.; Rohde, J.F.; Raymond, K.; Heitmann, B.L. Association between periodontal disease and overweight and obesity: A systematic review. J. Periodontol. 2015, 86, 766–776.
- Martinez-Herrera, M.; Silvestre-Rangil, J.; Silvestre, F.J. Association between obesity and periodontal disease. A systematic review of epidemiological studies and controlled clinical trials. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e708–e715.
- Gobin, R.; Tian, D.; Liu, Q.; Wang, J. Periodontal Diseases and the Risk of Metabolic Syndrome: An Updated Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 336.
- Lamster, I.B.; Pagan, M. Periodontal disease and the metabolic syndrome. Int. Dent. J. 2017, 67, 67–77.
- Sapey, E.; Yonel, Z.; Edgar, R.; Parmar, S.; Hobbins, S.; Newby, P.; Crossley, D.; Usher, A.; Johnson, S.; Walton, G.M.; et al. The clinical and inflammatory relationships between periodontitis and chronic obstructive pulmonary disease. J. Clin. Periodontol. 2020, 47, 1040–1052.
- Shi, Q.; Zhang, B.; Xing, H.; Yang, S.; Xu, J.; Liu, H. Patients with Chronic Obstructive Pulmonary Disease Suffer from Worse Periodontal Health-Evidence from a Meta-Analysis. Front. Physiol. 2018, 9, 33.
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Russo, L.L.; Muzio, L.L. The Role of Periodontitis and Periodontal Bacteria in the Onset and Progression of Alzheimer’s Disease: A Systematic Review. J. Clin. Med. 2020, 9, 495.
- Beydoun, M.A.; Beydoun, H.A.; Hossain, S.; El-Hajj, Z.W.; Weiss, J.; Zonderman, A.B. Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J. Alzheimers Dis. 2020, 75, 157–172.
- Araújo, V.M.; Melo, I.M.; Lima, V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediat. Inflamm. 2015, 2015, 259074.
- Chambrone, L.; Foz, A.M.; Guglielmetti, M.R.; Pannuti, C.M.; Artese, H.P.; Feres, M.; Romito, G.A. Periodontitis and chronic kidney disease: A systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J. Clin. Periodontol. 2013, 40, 443–456.
- Wahid, A.; Chaudhry, S.; Ehsan, A.; Butt, S.; Ali Khan, A. Bidirectional Relationship between Chronic Kidney Disease & Periodontal Disease. Pak. J. Med. Sci. 2013, 29, 211–215.
- Preus, H.R.; Khanifam, P.; Kolltveit, K.; Mørk, C.; Gjermo, P. Periodontitis in psoriasis patients: A blinded, case-controlled study. Acta Odontol. Scand. 2010, 68, 165–170.
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200.
- Lin, T.H.; Lung, C.C.; Su, H.P.; Huang, J.Y.; Ko, P.C.; Jan, S.R.; Sun, Y.H.; Nfor, O.N.; Tu, H.P.; Chang, C.S.; et al. Association between periodontal disease and osteoporosis by gender: A nationwide population-based cohort study. Medicine 2015, 94, e553.
- Bullon, P.; Goberna, B.; Guerrero, J.M.; Segura, J.J.; Perez-Cano, R.; Martinez-Sahuquillo, A. Serum, saliva, and gingival crevicular fluid osteocalcin: Their relation to periodontal status and bone mineral density in postmenopausal women. J. Periodontol. 2005, 76, 513–519.
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233.
- Myers-Wright, N.; Lamster, I.B. A New Practice Approach for Oral Health Professionals. J. Evid. Based Dent. Pract. 2016, 16, 43–51.
- Tonetti, M.S.; Fourmousis, I.; Suvan, J.; Cortellini, P.; Brägger, U.; Lang, N.P. Healing, post-operative morbidity and patient perception of outcomes following regenerative therapy of deep intrabony defects. J. Clin. Periodontol. 2004, 31, 1092–1098.
- Patel, R.R.; Richards, P.S.; Inglehart, M.R. Periodontal health, quality of life, and smiling patterns—An exploration. J. Periodontol. 2008, 79, 224–231.
- Needleman, I.; McGrath, C.; Floyd, P.; Biddle, A. Impact of oral health on the life quality of periodontal patients. J. Clin. Periodontol. 2004, 31, 454–457.
- Shanbhag, S.; Dahiya, M.; Croucher, R. The impact of periodontal therapy on oral health-related quality of life in adults: A systematic review. J. Clin. Periodontol. 2012, 39, 725–735.
- Khan, S.; Khalid, T.; Bettiol, S.; Crocombe, L.A. Non-surgical periodontal therapy effectively improves patient-reported outcomes: A systematic review. Int. J. Dent. Hyg. 2021, 19, 18–28.
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462.
- Jeffcoat, M.K.; Jeffcoat, R.L.; Gladowski, P.A.; Bramson, J.B.; Blum, J.J. Impact of periodontal therapy on general health: Evidence from insurance data for five systemic conditions. Am. J. Prev. Med. 2014, 47, 166–174.
- Bouchard, S.C.P.; Carter, N.; Cortellini, P.; Kocher, T.; Listl, S.; Loos, B.; Marcenes, W.; Nart, J.; Needleman, I.; Papapanou, P.; et al. Time to Take Gum Disease Seriously. Available online: https://impact.economist.com/perspectives/sites/default/files/eiu-efp-oralb-gum-disease.pdf (accessed on 24 September 2021).
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Aass, A.M.; Aimetti, M.; et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47, 4–60.
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636.
- D’Aiuto, F.; Parkar, M.; Tonetti, M.S. Acute effects of periodontal therapy on bio-markers of vascular health. J. Clin. Periodontol. 2007, 34, 124–129.
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920.
- Machado, V.; Botelho, J.; Escalda, C.; Hussain, S.B.; Luthra, S.; Mascarenhas, P.; Orlandi, M.; Mendes, J.J.; D’Aiuto, F. Serum C-Reactive Protein and Periodontitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 706432.
- Bascones-Martinez, A.; Matesanz-Perez, P.; Escribano-Bermejo, M.; González-Moles, M.; Bascones-Ilundain, J.; Meurman, J.H. Periodontal disease and diabetes-Review of the Literature. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, e722–e729.
- Engebretson, S.; Kocher, T. Evidence that periodontal treatment improves diabetes outcomes: A systematic review and meta-analysis. J. Periodontol. 2013, 84, S153–S169.
- Correa, F.O.; Gonçalves, D.; Figueredo, C.M.; Bastos, A.S.; Gustafsson, A.; Orrico, S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010, 37, 53–58.
- Artese, H.P.; Foz, A.M.; Rabelo, M.d.S.; Gomes, G.H.; Orlandi, M.; Suvan, J.; D’Aiuto, F.; Romito, G.A. Periodontal therapy and systemic inflammation in type 2 diabetes mellitus: A meta-analysis. PLoS ONE 2015, 10, e0128344.
- Lima, R.P.E.; Belém, F.V.; Abreu, L.G.; Cunha, F.A.; Cota, L.O.M.; da Costa, J.E.; Costa, F.O. Effect of Periodontal Therapy on Serum Levels of IL-6 in Type 2 Diabetics: A Systematic Review. Int. J. Periodontics Restor. Dent. 2019, 39, e1–e10.
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: Periodontitis and atherosclerotic cardiovascular disease. J. Periodontol. 2009, 80, 1021–1032.
- Zhang; Pickett, F.A. AHA statement on periodontal disease and heart disease. J. Can. Dent. Assoc. 2012, 77, c54.
- Mesa, F.; Magan-Fernandez, A.; Castellino, G.; Chianetta, R.; Nibali, L.; Rizzo, M. Periodontitis and mechanisms of cardiometabolic risk: Novel insights and future perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 476–484.
- Lockhart, P.B.; Bolger, A.F.; Papapanou, P.N.; Osinbowale, O.; Trevisan, M.; Levison, M.E.; Taubert, K.A.; Newburger, J.W.; Gornik, H.L.; Gewitz, M.H.; et al. Periodontal disease and atherosclerotic vascular disease: Does the evidence support an independent association?: A scientific statement from the American Heart Association. Circulation 2012, 125, 2520–2544.
- Reyes, L.; Herrera, D.; Kozarov, E.; Roldá, S.; Progulske-Fox, A. Periodontal bacterial invasion and infection: Contribution to atherosclerotic pathology. J. Periodontol. 2013, 84, S30–S50.
- Teeuw, W.J.; Slot, D.E.; Susanto, H.; Gerdes, V.E.; Abbas, F.; D’Aiuto, F.; Kastelein, J.J.; Loos, B.G. Treatment of periodontitis improves the atherosclerotic profile: A systematic review and meta-analysis. J. Clin. Periodontol. 2014, 41, 70–79.
- Freitas, C.O.; Gomes-Filho, I.S.; Naves, R.C.; Filho, G.D.R.N.; Cruz, S.S.; Santos, C.A.; Dunningham, L.; Miranda, L.F.; Barbosa, M.D. Influence of periodontal therapy on C-reactive protein level: A systematic review and meta-analysis. J. Appl. Oral Sci. 2012, 20, 1–8.
- Vidal, F.; Figueredo, C.M.; Cordovil, I.; Fischer, R.G. Periodontal therapy reduces plasma levels of interleukin-6, C-reactive protein, and fibrinogen in patients with severe periodontitis and refractory arterial hypertension. J. Periodontol. 2009, 80, 786–791.
- Park, S.Y.; Kim, S.H.; Kang, S.H.; Yoon, C.H.; Lee, H.J.; Yun, P.Y.; Youn, T.J.; Chae, I.H. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: A population-based study from Korea. Eur. Heart J. 2019, 40, 1138–1145.
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49.
- Xu, J.; Duan, X. Association between periodontitis and hyperlipidaemia: A systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1861–1873.
- Rizzo, M.; Cappello, F.; Marfil, R.; Nibali, L.; Marino Gammazza, A.; Rappa, F.; Bonaventura, G.; Galindo-Moreno, P.; O’Valle, F.; Zummo, G.; et al. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: A pilot study. Cell Stress Chaperones 2012, 17, 399–407.
- Rizzo, M.; Rini, G.B.; Berneis, K. The clinical relevance of LDL size and subclasses modulation in patients with type-2 diabetes. Exp. Clin. Endocrinol. Diabetes 2007, 115, 477–482.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
771
Revisions:
2 times
(View History)
Update Date:
21 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




