Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Usman | -- | 2949 | 2023-03-17 12:42:52 | | | |
| 2 | Rita Xu | -2 word(s) | 2947 | 2023-03-20 03:02:13 | | | | |
| 3 | Rita Xu | -7 word(s) | 2940 | 2023-03-20 03:04:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Usman, M. SAPO-34 Zeolite Membranes. Encyclopedia. Available online: https://encyclopedia.pub/entry/42304 (accessed on 07 February 2026).
Usman M. SAPO-34 Zeolite Membranes. Encyclopedia. Available at: https://encyclopedia.pub/entry/42304. Accessed February 07, 2026.
Usman, Muhammad. "SAPO-34 Zeolite Membranes" Encyclopedia, https://encyclopedia.pub/entry/42304 (accessed February 07, 2026).
Usman, M. (2023, March 17). SAPO-34 Zeolite Membranes. In Encyclopedia. https://encyclopedia.pub/entry/42304
Usman, Muhammad. "SAPO-34 Zeolite Membranes." Encyclopedia. Web. 17 March, 2023.
Copy Citation
In the zeolite family, the silicoaluminophosphate (SAPO)-34 zeolite has a unique chemical structure, distinctive pore size, adsorption characteristics, as well as chemical and thermal stability, has attracted much research attention. Increasing global carbon dioxide (CO2) emissions pose a serious environmental threat to humans, animals, plants, and the entire environment.
zeolites
SAPO-34
membranes
1. Introduction
Anthropogenic activities are one of the primary causes of global warming. The primary cause of climate change is the combustion of fossil fuels, which results in enormous CO2 concentration in the atmosphere. Recently, the CO2 levels in the atmosphere were recorded as 414 parts per million, which is several folds higher than before the industrial revolution [1][2]. This increasing level of CO2 causes the greenhouse effect, contributes to respiratory disease, acts as asphyxiant, causes ocean acidification, and acts as a major source of energy imbalance due to a rise in earth temperature. Several approaches have been developed to mitigate CO2 in the atmosphere, including geological sequestration, catalytic conversion to useful products, adsorption, and membrane separation [3][4][5][6]. One approach is to separate and capture CO2 from air and its originating sources.
Several gas separation strategies have been independently researched for CO2 capture and separation, including cryogenic distillation and post-combustion processes such as absorption, adsorption, hydrated-based systems, and membrane separation techniques [7][8][9]. Cryogenic distillation necessitates huge distillation columns and is a high-energy process. Due to its compact footprint, simplicity, and great energy efficiency, membrane gas separation technology has been considered to be one of the most promising technologies to replace older technologies such as amine scrubbing. The membrane separation technique has considerable advantages over other separation technologies because it is a continuous separation process that consumes less energy, and the materials can be recycled. Recently, as compared with polymeric membranes, the incorporation of zeolites into polymers has been shown to improve CO2 separation performance significantly.
In 1756, Swedish scientist Axel Fredrik Cronstedt invented the term “zeolite” [10]. Zeolites have a unique chemical composition, distinctive pore size distribution, and chemical, thermal, and ion exchange properties [11][12][13][14][15][16][17][18][19][20][21]. These materials have been employed for a range of applications, including capture, purification, and catalysis [22][23][24][25][26][27][28][29][30]. Among zeolites, Lok et al. [31] introduced the family of SAPO zeolite materials. The SAPO-34 unit cell has chabazite (CHA)-type topology related to other aluminophosphates (such as SAPO-15, SAPO-11, SAPO-16, and SAPO-31), aluminosilicate (low-silica CHA and high-silica SSZ-13) and pure silicate (all-silica CHA). All these low and high-silica zeolite materials have been explored for gas separation applications [32][33][34][35][36].
The SAPO-34 zeolite has intra-crystalline pore volumes and pore sizes ranging from 0.18 to 0.48 cm3/g and from 0.3 to 0.8 nm, respectively. The SAPO-34 structure is made up of eight-membered rings with a diameter of 9.4 (3.8 × 3.8), as shown in Figure 1. In addition to distinct pore size and volume, SAPO-34 material exhibits moderate to high hydrophobicity and has high thermal and hydrothermal stability. Due to these characteristic features, SAPO-34 has been extensively used in the methanol to olefin (MTO) process [37][38][39][40][41][42][43][44][45][46][47][48]. In recent years, SAPO-34 has been investigated for CO2 remediation, including CO2 capture [49][50][51], conversion [52][53][54], and separation [49]. Several studies have already covered a vast area of SAPO-34 materials research. Askari et al. [43] examined several synthetic procedures of SAPO-34. Ahmadi et al. summarized the deactivation of SAPO-34. Sun et al. looked at how to increase MTO performance in SAPO-34 by reducing the size of the zeolite using crystals and pore engineering. There has been a lot of research done on SAPO-34 membranes for CO2 separation, but no recent paper has been written about the role of SAPO-34 membranes in CO2 separation, especially from air (N2) and natural gas (CH4). Therefore, as a result of the rapidly developing research on SAPO-34, as shown in Figure 2, and its role in CO2 mitigation.
Figure 1. Schematic presentation of SAPO-34 membranes in CO2 separation.
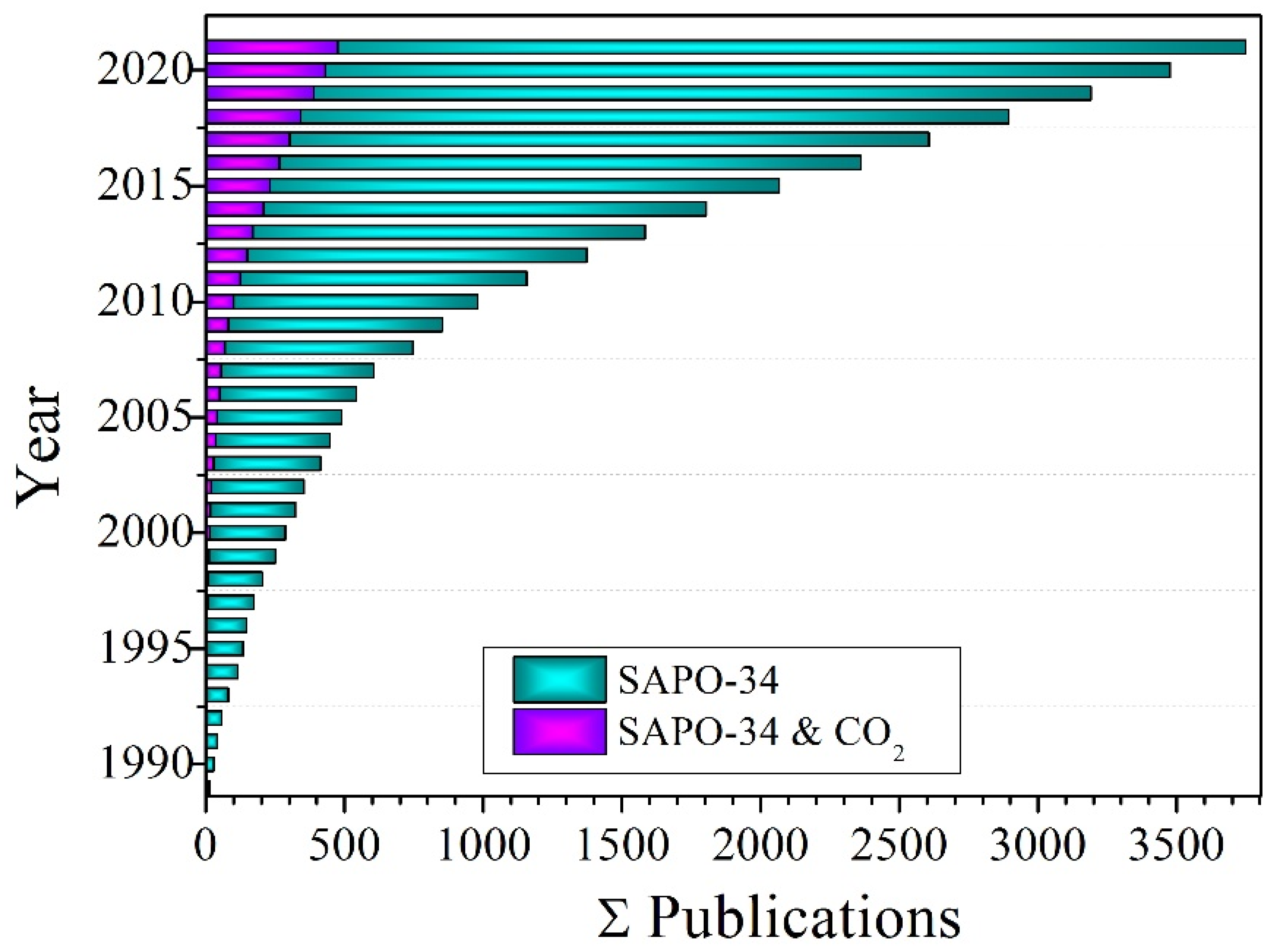
Figure 2. Histograms of SAPO-34 zeolite literature since 1990. Data were taken from SciFinder using keywords “SAPO-34” and “SAPO-34 and CO2”.
2. SAPO-34 Membranes for CO2 Separation
A crystalline hydrate aluminosilicate is distinguished by its uniform pore size (0.3–1.3 nm), as well as superior thermal, chemical, and mechanical stability. Zeolite is an ideal material for different applications, such as adsorption, ion exchange, and catalysis. Recently, it has become attractive for membrane applications due to its unique structure and excellent physicochemical properties [55]. Among more than 190 zeolite frameworks, a few have been distinguished for their promising separation performances. SAPO-34 is one of these structures that exhibits a significant separation performance, specifically removing CO2 from CH4 and N2 mixture. As shown in Figure 3, SAPO-34 membranes are mainly classified into two types: (1) Mixed matrix membranes (MMMs) where the SAPO-34 is incorporated in the polymer. These membranes are fabricated via various methods, including solution casting, phase inversion, solvent evaporation, and dip coating. (2) Pure SAPO-34 membranes where a substrate such as alumina, stainless steel, and silica are used as a support for the SAPO-34 membranes. Secondary seeded growth and in situ crystallization methods are the most frequent routes to fabricate these membrane types.
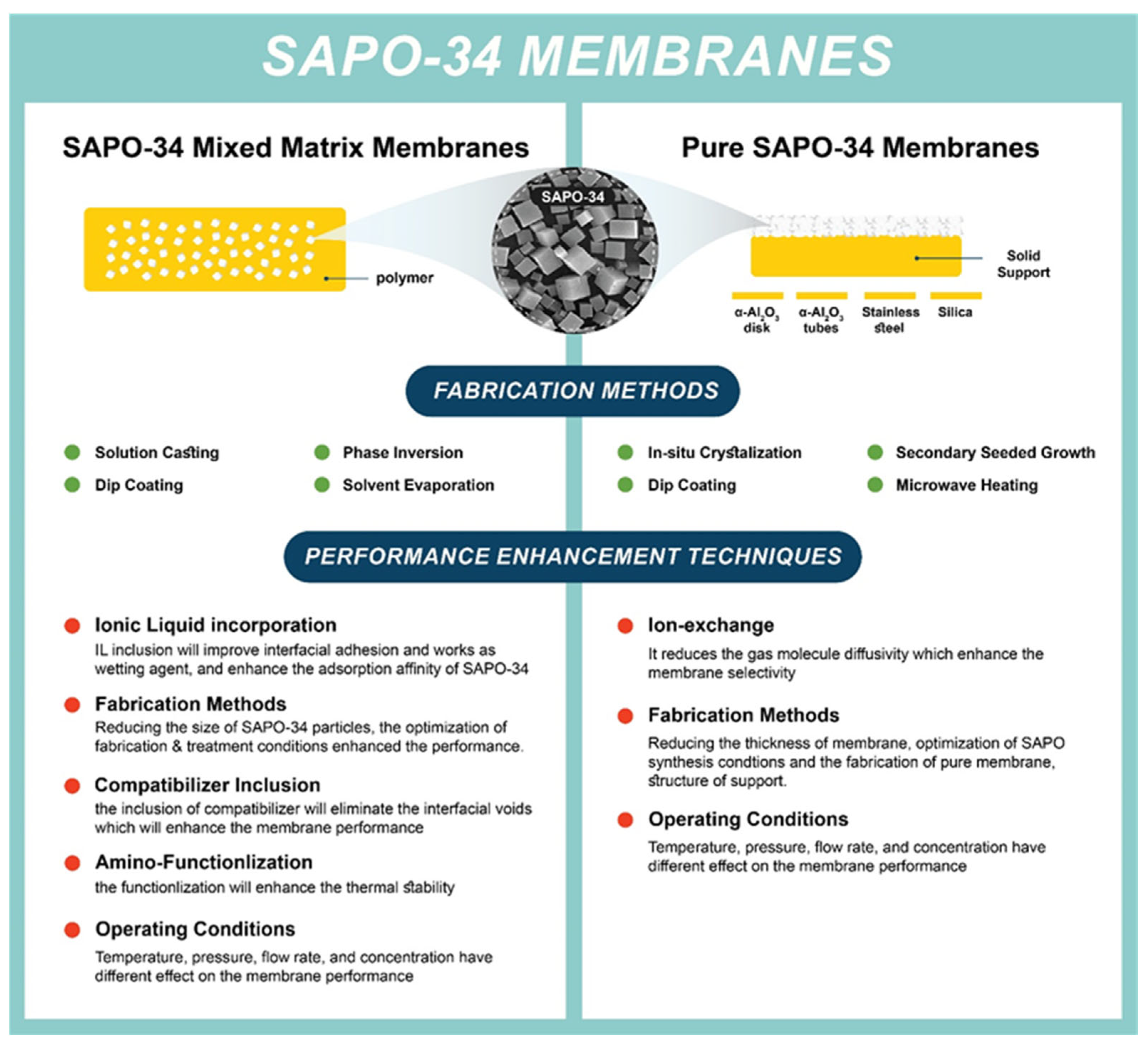
Figure 3. Schematic of fabrication method and performance advancement approaches for SAPO-34 membranes.
2.1. SAPO-34-Based Mixed Matrix Membranes
Several gas separation technologies have been investigated for CO2 capture, including cryogenic distillation, post-combustion process, and membrane separation processes. Membrane separation technology shows significant merits as compared with other separation technologies because it is a continuous separation process, requires low energy consumption, and the materials can be regenerated. As compared with the polymeric membranes, MMMs that combine the advantages of both polymeric and inorganic materials have become the focus for a next-generation gas separation membrane. MMMs could provide a solution to the permeability and selectivity trade-off in polymeric membranes and bridge the gap with pure inorganic membranes. MMMs also offer the physicochemical stability of a ceramic material while ensuring the desired morphology with higher permeability, selectivity, hydrophilicity, fouling resistance, as well as greater thermal, mechanical, and chemical strength over a wider temperature and pH range. In MMMs where the flexibility, processability, and scalability of polymeric membranes meet the exceptional separation performance, the chemical and thermal stability of inorganic fillers have become a trending focus of academia and industry.
Since the first report of MMMs in 1970 [56], extensive research has been conducted to improve the separation performance and industrial implementation for a range of applications such as hydrogen recovery, treatment of natural gas, and air separation [57][58][59]. Different types of polymer matrices have been reported with various fillers, such as MOFs [60][61][62][63][64], COFs [65][66][67][68][69], carbon materials [70], zeolites [56], and other materials [71][72]. Zeolite materials with their sieving properties and cost-effectiveness on a large scale make them better candidates for gas separation. The most suitable zeolite filler for CO2 separation is SAPO-34 due to its unique structure and CO2 adsorption affinity [73].
2.1.1. SAPO-34 MMMs
Peydayesh et al. [74] fabricated SAPO-34/Matrimid 5218 MMMs. They showed 55% and 97% enhancements in CO2 permeability and CO2/CH4 selectivity, respectively, indicating the good adhesion of the filler in the polymer matrix. Wu et al. [75] reported an MMMs obtained by the inclusion of SAPO-34 nanoparticles within a polyethersulfone (PESU) polymer. The separation performance increased with increasing SAPO-34 loading. In addition, the nanoparticle size was investigated, where 100 nm particles resulted in defective membranes. In contrast, 200 nm SAPO-34 showed fewer defects with a continuous interface and higher permselectivity than smaller particles. Carter et al. [76] reported three types of filler: SAPO-34, silica, and ZIF-8. Among all the fabricated MMMs, ZIF-8 showed the best performance owing to the strong interaction between the filler and polymer matrix and surface diffusion transport. In addition, the study claimed that pore size was the most influential factor in gas permeability, as it increased permeability. As a result of the reduced interfacial voids and chain mobility, the SPAO-34 MMMs showed high ideal selectivity. Messaoud et al. [77] reported a dip-coating route for fabricating SAPO-34/polyetherimide MMMs. This study investigated the effects of two solvents, N-methyl-2-pyrrolidone (NMP) and dichloroethane (DCE), on membrane fabrication. DCE resulted in better performance properties related to the entrapping of small DCE molecules in SAPO-34 particles, which induced the sealing of SAPO-34 pores. The best molecular sieving performance was achieved with 5 wt% SAPO-34 MMMs with 4.41 × 10−10 mol m−2 s−1 Pa−1 and 60 CO2/CH4 selectivity. Particle agglomeration was observed with 10 wt% MMMs. Zhao et al. [78] reported SAPO-34/Pebax1657 MMMs fabricated by solvent evaporation. The inclusion of SAPO significantly enhanced the CO2 permeability as compared with that of the neat membrane, whereas the selectivity remained constant. The effect of pressure was studied, and as a reason for plasticization, the permeation of MMMs increased with pressure. Junaidi et al. [79] conducted two studies on MMMs. First, asymmetric SAPO-34/PSf MMMs were prepared using the phase inversion method. The highest performance was achieved with 10 wt% MMMs with ideal selectivity 28.1 and 26.2 for CO2/CH4 and CO2/N2, respectively. When the filler loading was increased to >20 wt%, it led to poor interaction between filler and matrix which caused interfacial voids. They modified the SAPO-34 particles with the coupling agent APMS using two solvents, isopropanol and ethanol, before being added to the polymer matrix to overcome the previously reported challenge. The study showed that modified SAPO-34 MMMs exhibited better performance than unmodified and neat membranes owing to the reduction in interfacial voids [80]. An experimental and modeling study was reported by Santaniello et al. [73] where 200 nm SAPO-34 was incorporated, for the first time, in a polyhexafluoropropylene PHFP matrix. The MMMs with 24.6 v% and 36 v% showed an enhancement in the permeability and CH4/CO2 selectivity as compared with the neat membrane, which was ascribed to the increased polymer-free volume. The modeling part of gas transport confirmed the experimental results that 200 nm SAPO-34 particles provoked a polymer-free volume of 24.6 v%.
2.1.2. SAPO-34 Functionalized MMMs
The functionalization strategy offers the prospect of enhancing membrane performance. Cakal et al. [81] reported the influence of compatibilizer additives on the permeation performance of SAPO-34/HMA/PES membranes. The elimination of interfacial voids in the membrane is the main role of the HMA compatibilizer. The improvement in CO2/CH4 selectivity for SAPO-34 (20 wt%)/HMA (10%)/PES as compared with neat PES was attributed to the reduction in the diffusion pathway of CH4. The effect of temperature was expected, as the permeability of all gases was enhanced as the temperature increased [82]. The effect of the functionalization of SAPO-34 with ethylenediamine (EDA) and hexylamine (HA) organic amino cations on the gas permeation, morphology, and pore size of SAPO-34/PES MMMs was investigated. The MMMs fabricated with modified SAPO-34 with the EDA agent showed better performance than the HA agent owing to higher amino grafting, which enhanced the filler/polymer adhesion, resulting in a better CO2/CH4 ideal selectivity [83]. Amino functionalization and ionic liquid inclusion were studied by Nasir et al. [84]. The study revealed that the improvement of the particles/polymer interphase was due to the incorporation of [emim][Tf2N] ionic liquids. Simultaneously, the amino functionalization of the SAPO-34 surface by EDA and HA enhanced the thermal stability of the MMMs. In addition, the membrane with the modified SAPO-34 and [emim][Tf2N] IL exhibited the best CO2/CH4 selectivity as compared with that of the neat membrane. The hydrophobicity of MMMs is a key factor for industrial applications. Functionalization of SAPO-34 using 1H,1H,2H,2H-perflourodecyltriethoxysilane (HFDS) fluorocarbons was reported by Junaidi et al. [85]. In this study, functionalized SAPO-34 particles were embedded in a PSf polymer to overcome the competitive adsorption of moisture under wet conditions. Among the fabricated MMMs, the SAPO-34 10 wt% + 0.1 HFDS/PSF membrane showed the best performance (CO2 permeance = 278 GPU) and (CO2/CH4 = 38.9) as compared with a bare polymer. The incorporation of modified SAPO-34 enhanced the membrane 17.64% hydrophobicity and showed better filler/polymer adhesion. In addition, SAPO-34 10 wt% + 0.1 HFDS/PSf membrane showed excellent stability for long-term stability tests under wet and dry conditions, whereas the unmodified membrane lost 90% of its performance under wet conditions.
Incorporating the third component in the MMM plays a vital role in improving SAPO-34 membrane performance. Nawar et al. reported the synergetic influence of ionic liquid (IL) inclusions on the separation of SAPO-34 MMMs [86]. In this study, 5 wt% SAPO-34 particles were incorporated into the polysulfone matrix, and the resulting membrane was immersed in 1-ethyl-3-methylimidazolium bis(tri-fluoromethylsulfonyl)imide IL. The membrane with the 0.2 M ionic liquid showed enhanced membrane performance as compared with the unmodified membrane, which was ascribed to interfacial defect reduction due to ionic liquid inclusion. Increasing the amount of ionic liquid caused a reduction in permeance and selectivity owing to pore and filler blockage. Ahmad et al. [87] used an [emim][TF2N] IL. Increasing the immersion time of SAPO-34 membranes led to an enhancement in the adsorption affinity of SAPO-34 for CO2 and filler/polymer interfacial adhesion, and SAPO-34 + IL/PSF showed the best performance as compared with neat PSF, with ideal selectivity of 20.35 and 18.82 for CO2/CH4 and CO2/N2, respectively. Mohshim et al. [88] reported the use of Tf2N in SAPO-34/PES. This work also proved that ionic liquids improve interfacial adhesion and function as wetting agents. The performance of the modified MMMs was significantly enhanced as compared with bare PES. A modeling study was conducted to study the effect of incorporating (emim [Tf2N]) and (emim[CF3SO3]) ionic liquids in a polymer matrix using the Maxwell, Lewis–Nielson, and Maxwell–Wagner–Sillar (MWS) gas separation models. The study showed a local agglomeration of SAPO-34 particles and a high deviation from the experimental results. Modification of the MWS model to include the wet phase factor showed good agreement with the experimental results [89]. Sen et al. [90] investigated the impregnation of carbon in polyetherimide using in situ carbonization to tailor SAPO-34 MMMs. Owing to the incompatibility, the impregnated carbon particles redecorated the interfacial pores formed between the filler and polymer. This approach minimized the interfacial pores/defects, which enhanced membrane performance.
2.1.3. SAPO-34 MMMs and Operating Conditions
The operating conditions are one of the key factors affecting membrane performance. Sodeifian et al. [91] investigated the influence of pressure and inclusion of SAPO-34 nanoparticles. The study showed that increasing the pressure (0.4–1.4 MPa) caused an increase in CO2 permeability, whereas CH4 and N2 permeability remained constant. Increasing SAPO-34 within the polyurethane matrix decreases the permeability of CO2 and CH4 and enhanced the ideal selectivity of CO2/N2 and CO2/CH4, which indicated the benefit of SAPO-34 particle incorporation in the PU matrix, as shown in Figure 4.
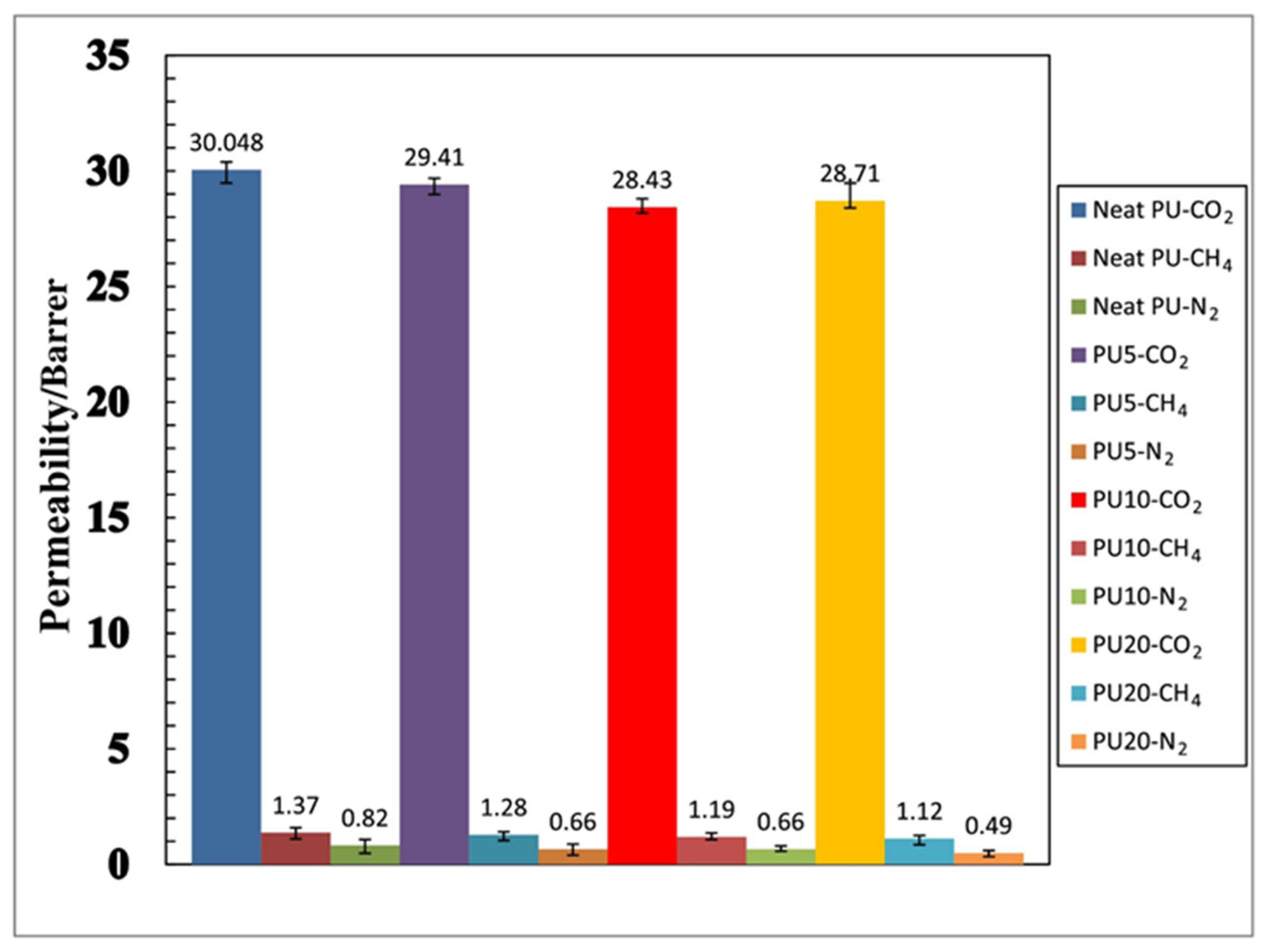
Figure 4. Effect of SAPO-34 content in the gas permeation properties of polyurethane–SAPO-34 membranes on the permeability of CO2, CH4, and N2 gases in 1.2 MPa pressure and selectivity of CO2/CH4 and CO2/N2 gases in 1.2 MPa pressure.
Rabiee et al. [92] investigated the effects of temperature and pressure on the separation performance of SAPO-34/Pebax MMMs. The study showed that the incorporation of SAPO-34 led to an enhancement in gas permeability, and the membranes exhibited diffusion-dominant behavior, and indicated the molecular sieving effect of SAPO-34. An increase in operating conditions and pressure (4–24 bar) led to an enhancement in gas permeation, increasing the driving force and solution diffusion mechanism. The temperature alternation also showed the same behavior, which increased the Pebax chain mobility around the filler. The above discussions are summarized in Table 1.
Table 1. Summary of separation performance for SAOP-34 MMMs.
| Filler | Substrate | CO2 Permeance | CO2/CH4 Selectivity | CO2/N2 Selectivity | Ref. |
|---|---|---|---|---|---|
| Neat | Matrimid 5218 | 4.4 Barrer | 34 | - | [74] |
| SAPO-34 2 wt% | Matrimid 5218 | 4.5 Barrer | 41.98 | - | [74] |
| SAPO-34 5 wt% | Matrimid 5218 | 4.6 Barrer | 44.24 | - | [74] |
| SAPO-34 10 wt% | Matrimid 5218 | 5.3 Barrer | 50.82 | - | [74] |
| SAPO-34 15 wt% | Matrimid 5218 | 5.9 Barrer | 58.14 | - | [74] |
| SAPO-34 20 wt% | Matrimid 5218 | 6.9 Barrer | 66.99 | - | [74] |
| Neat | Polyethersulfone (PESU) | 6.7 Barrer | 37.8 | - | [75] |
| SAPO-34 NP 20 wt% | Polyethersulfone (PESU) | 8.2 Barrer | 42.6 | - | [75] |
| SAPO-34 NP 30 wt% | Polyethersulfone (PESU) | 8.9 Barrer | 48.3 | - | [75] |
| Neat | Matrimid 5218 | 9.5 ± 1.07 GPU | 29.81 | 13.63 | [76] |
| SAPO-34 10 wt% uncalcined | Matrimid 5218 | 7.63 ± 0.81 GPU | 31.79 | 26.31 | [76] |
| SAPO-34 10 wt% calcined | Matrimid 5218 | 12.5 ± 1.3 GPU | 9.32 | 10.50 | [76] |
| Neat | Polyetherimide | 6 × 10−10 mol/(m2 s Pa) | 0.02 | - | [77] |
| SAPO-34 5 wt% | Polyetherimide | 4.4 × 10−10 mol/(m2 s Pa) | 60 | - | [77] |
| SAPO-34 10 wt% | Polyetherimide | 6 × 10−10 mol/(m2 s Pa) | 8 | - | [77] |
| Neat | Pebax 1657 | 100 Barrer | 16.7 | 53.8 | [78] |
| SAPO-34 23 wt% | Pebax 1657 | 134 Barrer | 21.7 | 55.2 | [78] |
| SAPO-34 33 wt% | Pebax 1657 | 252 Barrer | 17 | 55 | [78] |
| SAPO-34 50 wt% | Pebax 1657 | 339 Barrer | 16.8 | 53.2 | [78] |
| Neat | Polysulfone (Asymmetric) | 22.0 ± 3.42 GPU | 17.3 | 16.5 | [79] |
| SAPO-34 5 wt% | Polysulfone (Asymmetric) | 205.9 ± 7.26 GPU | 22.5 | 21.4 | [79] |
| SAPO-34 10 wt% | Polysulfone (Asymmetric) | 314.0 ± 4.65 GPU | 28.2 | 26.1 | [79] |
| SAPO-34 20 wt% | Polysulfone (Asymmetric) | 281.18 ± 6.92 GPU | 10.9 | 10.7 | [79] |
| SAPO-34 30 wt% | Polysulfone (Asymmetric) | 232. ± 3.21 GPU | 3 | 2.9 | [79] |
| Neat | Polysulfone (Asymmetric) | 105 GPU | 15 | 13 | [80] |
| SAPO-34 10 wt% | Polysulfone (Asymmetric) | 459 GPU | 27 | 21 | [80] |
| SAPO-34E 10 wt% | Polysulfone (Asymmetric) | 706 GPU | 31 | 28 | [80] |
| SAPO-34I 10 wt% | Polysulfone (Asymmetric) | 775 GPU | 28 | 22 | [80] |
| Neat | Polyhexafluoropropylene (PHFP) | 290 Barrer | 14.1 | - | [73] |
| SAPO-34 NP 24.6 v% | Polyhexafluoropropylene (PHFP) | 468 Barrer | 15.8 | - | [73] |
| SAPO-34 NP 36 v% | Polyhexafluoropropylene (PHFP) | 437 Barrer | 17.5 | - | [73] |
| Neat | PES | 4.45 Barrer | 33.2 | - | [81] |
| HMA 10% | PES | 0.8 Barrer | 32.3 | - | [81] |
| SAPO-34 20 wt% | PES | 5.7 Barrer | 37 | - | [81] |
| SAPO-34 20 wt% + HMA 10% | PES | 1.3 Barrer | 44.7 | - | [81] |
| HMA 4% | PES | 5.1 Barrer | 39.3 | - | [82] |
| SAPO-34 20 wt% | PES | 13.8 Barrer | 32.7 | - | [82] |
| SAPO-34 20 wt% + HMA 4% | PES | 7.8 Barrer | 41.6 | - | [82] |
| SAPO-34 | PES | 18 GPU | 1.2 | - | [83] |
| SAPO-34 20 wt% | PES | 30 GPU | 1.3 | - | [83] |
| SAPO-34 20 wt% m-EDA | PES | 10.0 GPU | 12.14 | - | [83] |
| SAPO-34 20 wt% | PES | 50 GPU | 2.5 | - | [84] |
| SAPO-34 20 wt%/IL | PES | 0.03 GPU | 4.9 | - | [84] |
| SAPO-34 20 wt% m-EDA/IL | PES | 0.09 GPU | 26.5 | - | [84] |
| SAPO-34 20 wt% m-HA/IL | PES | 0.045 GPU | 37.2 | - | [84] |
| Neat | Polysulfone (PSf) | 21.3 ± 2.8 GPU | 17.2 | - | [85] |
| SAPO-34 10 wt% | Polysulfone (PSf) | 317.0 ± 3.5 GPU | 27.9 | - | [85] |
| SAPO-34 20 wt% | Polysulfone (PSf) | 283.0 ± 2.2 GPU | 10.8 | - | [85] |
| SAPO-34 10 wt% + 0.5 wt%HFDS | Polysulfone (PSf) | 310.4 ± 1.7 GPU | 30.4 | - | [85] |
| SAPO-34 10 wt% + 1 wt%HFDS | Polysulfone (PSf) | 278.8 ± 2.1 GPU | 38.9 | - | [85] |
| SAPO-34 10 wt% + 1.5 wt%HFDS | Polysulfone (PSf) | 259.7 ± 4.2 GPU | 37.3 | - | [85] |
| SAPO-34 20 wt% + 0.5 wt%HFDS | Polysulfone (PSf) | 332.1 ± 5.5 GPU | 11.9 | - | [85] |
| SAPO-34 20 wt% + 1 wt%HFDS | Polysulfone (PSf) | 293.7 ± 4.9 GPU | 27.5 | - | [85] |
| SAPO-34 20 wt% + 1.5 wt%HFDS | Polysulfone (PSf) | 306.8 ± 5.2 GPU | 24.8 | - | [85] |
| SAPO-34 5 wt% | Polysulfone (PSf) | 6.1 GPU | 4.9 | 5.1 | [87] |
| SAPO-34 5 wt%/IL(0.2 M) | Polysulfone (PSf) | 24.89 GPU | 35.06 | 40.15 | [87] |
| Neat | Polysulfone (PSf) | 5.60 ± 0.75 GPU | 3.24 | 6.15 | [88] |
| SAPO-34 5 wt% | Polysulfone (PSf) | 6.53 ± 1.22 GPU | 3.47 | 5.67 | [88] |
| SAPO-34 5 wt%/IL(0.4 M) | Polysulfone (PSf) | 4.82 ± 1.28 GPU | 4.86 | 8.04 | [88] |
| SAPO-34 5 wt%/IL(0.6 M) | Polysulfone (PSf) | 7.24 ± 1.78 GPU | 20.35 | 18.82 | [88] |
| SAPO-34 20 wt% | Polyethersulfone (PES) | 85.7 GPU | 20.67 | - | [89] |
| SAPO-34 20 wt% + IL 5 wt% | Polysulfone (PSf) | 230.8 GPU | - | 46.20 | [89] |
| SAPO-34 20 wt% + IL 10 wt% | Polysulfone (PSf) | 255.69 GPU | - | 58.83 | [89] |
| SAPO-34 20 wt% + IL 15 wt% | Polysulfone (PSf) | 279.2 GPU | - | 60.62 | [89] |
| SAPO-34 20 wt% + IL 20 wt% | Polysulfone (PSf) | 300.0 GPU | - | 62.58 | [89] |
| Neat | Polyetherimide | 3.8 × 10−10 mol/(m2 s Pa) | - | 2.23 | [91] |
| SAPO-34 10 wt% | Polyetherimide | 2.8 × 10−8 mol/(m2 s Pa) | - | 2.54 | [91] |
| SAPO-34 25 wt% + Carbonization | Polyetherimide | 8.42 × 10−8 mol/(m2 s Pa) | - | 6.47 | [91] |
| SAPO-34 40 wt% | Polyetherimide | 9.1 × 10−7 mol/(m2 s Pa) | - | 5.05 | [91] |
| Neat | Polyurethane | 30.05 Barrer | 21.93 | 36.64 | [86] |
| SAPO-34 NP 5 wt% | Polyurethane | 29.41 Barrer | 22.97 | 44.56 | [86] |
| SAPO-34 NP 10 wt% | Polyurethane | 28.43 Barrer | 23.89 | 54.67 | [86] |
| SAPO-34 NP 20 wt% | Polyurethane | 28.71 Barrer | 25.63 | 58.59 | [86] |
| Neat | Pebax 1074 | 120 Barrer | 17.5 | 60.3 | [92] |
| SAPO-34 5 wt% | Pebax 1074 | 123 Barrer | 18.5 | 61 | [92] |
| SAPO-34 10 wt% | Pebax 1074 | 130 Barrer | 22 | 62.5 | [92] |
| SAPO-34 20 wt% | Pebax 1074 | 152 Barrer | 29 | 68 | [92] |
| SAPO-34 30 wt% | Pebax 1074 | 156 Barrer | 35 | 69 | [92] |
References
- Burns, T.D.; Pai, K.N.; Subraveti, S.G.; Collins, S.P.; Krykunov, M.; Rajendran, A.; Woo, T.K. Prediction of MOF Performance in Vacuum Swing Adsorption Systems for Postcombustion CO2 Capture Based on Integrated Molecular Simulations, Process Optimizations, and Machine Learning Models. Environ. Sci. Technol. 2020, 54, 4536–4544.
- Usman, M.; Iqbal, N.; Noor, T.; Zaman, N.; Asghar, A.; Abdelnaby, M.M.; Galadima, A.; Helal, A. Advanced strategies in Metal-Organic Frameworks for CO2 Capture and Separation. Chem. Rec. 2021.
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2021, 9, 104756.
- Dindi, A.; Quang, D.V.; Vega, L.F.; Nashef, E.; Abu-Zahra, M.R.M. Applications of fly ash for CO2 capture, utilization, and storage. J. CO2 Util. 2019, 29, 82–102.
- Khan, S.; Khulief, Y.A.; Al-Shuhail, A.A. Effects of reservoir size and boundary conditions on pore-pressure buildup and fault reactivation during CO2 injection in deep geological reservoirs. Environ. Earth Sci. 2020, 79, 294.
- Khan, S.; Khulief, Y.A.; Al-Shuhail, A.A. Numerical Modeling of the Geomechanical Behavior of Biyadh Reservoir Undergoing CO2 Injection. Int. J. Geomech. 2017, 17, 04017039.
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sust. Energ. Rev. 2019, 101, 265–278.
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351.
- Tengku Hassan, T.N.A.; Shariff, A.M.; Mohd Pauzi, M.M.i.; Khidzir, M.S.; Surmi, A. Insights on Cryogenic Distillation Technology for Simultaneous CO2 and H2S Removal for Sour Gas Fields. Molecules 2022, 27, 1424.
- Breck, D.W.; Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons: New York, NY, USA, 1973.
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278.
- Usman, M.; Humayun, M.; Shah, S.S.; Ullah, H.; Tahir, A.A.; Khan, A.; Ullah, H. Bismuth-Graphene Nanohybrids: Synthesis, Reaction Mechanisms, and Photocatalytic Applications—A Review. Energies 2021, 14, 2281.
- Usman, M.; Humayun, M.; Garba, M.D.; Ullah, L.; Zeb, Z.; Helal, A.; Suliman, M.H.; Alfaifi, B.Y.; Iqbal, N.; Abdinejad, M.; et al. Electrochemical Reduction of CO2: A Review of Cobalt Based Catalysts for Carbon Dioxide Conversion to Fuels. Nanomaterials 2021, 11, 2029.
- Israf Ud, D.; Qazi, N.; Mustapha, D.G.; Abdulrahman, I.A.; Mshari, A.A.; Muhammad, U. A Review of Preparation Methods for Heterogeneous Catalysts. Mini-Rev. Org. Chem. 2022, 19, 92–110.
- Humayun, M.; Zada, A.; Li, Z.; Xie, M.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B 2016, 180, 219–226.
- Humayun, M.; Ullah, H.; Tahir, A.A.; bin Mohd Yusoff, A.R.; Mat Teridi, M.A.; Nazeeruddin, M.K.; Luo, W. An Overview of the Recent Progress in Polymeric Carbon Nitride Based Photocatalysis. Chem. Rec. 2021.
- Humayun, M.; Ullah, H.; Usman, M.; Habibi-Yangjeh, A.; Tahir, A.A.; Wang, C.; Luo, W. Perovskite-type lanthanum ferrite based photocatalysts: Preparation, properties, and applications. J. Energy Chem. 2022, 66, 314–338.
- Helal, A.; Usman, M.; Arafat, M.E.; Abdelnaby, M.M. Allyl functionalized UiO-66 metal-organic framework as a catalyst for the synthesis of cyclic carbonates by CO2 cycloaddition. J. Ind. Eng. Chem. 2020, 89, 104–110.
- Yaqoob, L.; Noor, T.; Iqbal, N.; Nasir, H.; Sohail, M.; Zaman, N.; Usman, M. Nanocomposites of cobalt benzene tricarboxylic acid MOF with rGO: An efficient and robust electrocatalyst for oxygen evolution reaction (OER). Renew. Energy 2020, 156, 1040–1054.
- Ullah, L.; Zhao, G.; Xu, Z.; He, H.; Usman, M.; Zhang, S. 12-Tungstophosphoric acid niched in Zr-based metal-organic framework: A stable and efficient catalyst for Friedel-Crafts acylation. Sci. China Chem. 2018, 61, 402–411.
- Ullah, L.; Zhao, G.; Ma, J.-X.; Usman, M.; Khan, R.; Hedin, N. Pd-promoted heteropolyacid on mesoporous zirconia as a stable and bifunctional catalyst for oxidation of thiophenes. Fuel 2022, 310, 122462.
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919.
- Arslan, M.T.; Qureshi, B.A.; Gilani, S.Z.A.; Cai, D.; Ma, Y.; Usman, M.; Chen, X.; Wang, Y.; Wei, F. Single-Step Conversion of H2-Deficient Syngas into High Yield of Tetramethylbenzene. ACS Catal. 2019, 9, 2203–2212.
- Zhu, J.; Li, Y.; Muhammad, U.; Wang, D.; Wang, Y. Effect of alkene co-feed on the MTO reactions over SAPO-34. Chem. Eng. J. 2017, 316, 187–195.
- Ma, Y.; Cai, D.; Li, Y.; Wang, N.; Muhammad, U.; Carlsson, A.; Tang, D.; Qian, W.; Wang, Y.; Su, D.; et al. The influence of straight pore blockage on the selectivity of methanol to aromatics in nanosized Zn/ZSM-5: An atomic Cs-corrected STEM analysis study. RSC Adv. 2016, 6, 74797–74801.
- Usman, M.; Li, D.; Razzaq, R.; Yaseen, M.; Li, C.; Zhang, S. Novel MoP/HY catalyst for the selective conversion of naphthalene to tetralin. J. Ind. Eng. Chem. 2015, 23, 21–26.
- Wang, H.; Cao, Y.; Li, D.; Muhammad, U.; Li, C.; Li, Z.; Zhang, S. Catalytic hydrorefining of tar to liquid fuel over multi-metals (W-Mo-Ni) catalysts. J. Renew. Sustain. Energy 2013, 5, 053114.
- Zhang, H.H.; Cao, Y.M.; Usman, M.; Li, L.J.; Li, C.S. Study on the Hydrotreating Catalysts Containing Phosphorus of Coal Tar to Clean Fuels. Adv. Mat. Res. 2012, 531, 263–267.
- Kan, T.; Sun, X.; Wang, H.; Li, C.; Muhammad, U. Production of Gasoline and Diesel from Coal Tar via Its Catalytic Hydrogenation in Serial Fixed Beds. Energy Fuels 2012, 26, 3604–3611.
- Li, D.; Zhang, H.; Usman, M.; Li, Z.; Han, L.; Li, C.; Zhang, S. Study on the hydrotreatment of C9 aromatics over supported multi-metal catalysts on γ-Al2O3. J. Renew. Sustain. Energy 2014, 6, 033132.
- Lok, B.M.; Messina, C.A.; Patton, R.L.; Gajek, R.T.; Cannan, T.R.; Flanigen, E.M. Silicoaluminophosphate molecular sieves: Another new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1984, 106, 6092–6093.
- Song, S.; Gao, F.; Zhang, Y.; Li, X.; Zhou, M.; Wang, B.; Zhou, R. Preparation of SSZ-13 membranes with enhanced fluxes using asymmetric alumina supports for N2/CH4 and CO2/CH4 separations. Sep. Purif. Technol. 2019, 209, 946–954.
- Liu, H.; Gao, X.; Wang, S.; Hong, Z.; Wang, X.; Gu, X. SSZ-13 zeolite membranes on four-channel α-Al2O3 hollow fibers for CO2 separation. Sep. Purif. Technol. 2021, 267, 118611.
- Kalipcilar, H.; Bowen, T.C.; Noble, R.D.; Falconer, J.L. Synthesis and Separation Performance of SSZ-13 Zeolite Membranes on Tubular Supports. Chem. Mater. 2002, 14, 3458–3464.
- Hasegawa, Y.; Abe, C.; Natsui, M.; Ikeda, A. Gas Permeation Properties of High-Silica CHA-Type Zeolite Membrane. Membranes 2021, 11, 249.
- Liang, L.; Zhu, M.; Chen, L.; Zhong, C.; Yang, Y.; Wu, T.; Wang, H.; Kumakiri, I.; Chen, X.; Kita, H. Single Gas Permeance Performance of High Silica SSZ-13 Zeolite Membranes. Membranes 2018, 8, 43.
- Dai, W.; Wang, C.; Dyballa, M.; Wu, G.; Guan, N.; Li, L.; Xie, Z.; Hunger, M. Understanding the Early Stages of the Methanol-to-Olefin Conversion on H-SAPO-34. ACS Catal. 2015, 5, 317–326.
- Nishiyama, N.; Kawaguchi, M.; Hirota, Y.; Van Vu, D.; Egashira, Y.; Ueyama, K. Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Appl. Catal. A-Gen. 2009, 362, 193–199.
- Sun, Q.; Xie, Z.; Yu, J. The state-of-the-art synthetic strategies for SAPO-34 zeolite catalysts in methanol-to-olefin conversion. Natl. Sci. Rev. 2017, 5, 542–558.
- Yang, Z.; Zhang, L.; Zhou, Y.; Wang, H.; Wen, L.; Kianfar, E. Investigation of effective parameters on SAPO-34 nanocatalyst in the methanol-to-olefin conversion process: A review. Rev. Inorg. Chem. 2020, 40, 91–105.
- Yang, G.; Han, J.; Huang, Y.; Chen, X.; Valtchev, V. Busting the efficiency of SAPO-34 catalysts for the methanol-to-olefin conversion by post-synthesis methods. Chin. J. Chem. Eng. 2020, 28, 2022–2027.
- Ahmad, M.S.; Cheng, C.K.; Bhuyar, P.; Atabani, A.E.; Pugazhendhi, A.; Chi, N.T.L.; Witoon, T.; Lim, J.W.; Juan, J.C. Effect of reaction conditions on the lifetime of SAPO-34 catalysts in methanol to olefins process—A review. Fuel 2021, 283, 118851.
- Askari, S.; Bashardoust Siahmard, A.; Halladj, R.; Miar Alipour, S. Different techniques and their effective parameters in nano SAPO-34 synthesis: A review. Powder Technol. 2016, 301, 268–287.
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Al-Shammari, T.K.; Yokoi, T. OSDA-free chabazite (CHA) zeolite synthesized in the presence of fluoride for selective methanol-to-olefins. Micrpor. Mesopor. Mater. 2019, 274, 277–285.
- Nasser, G.A.; Muraza, O.; Nishitoba, T.; Malaibari, Z.; Yamani, Z.H.; Al-Shammari, T.K.; Yokoi, T. Microwave-Assisted Hydrothermal Synthesis of CHA Zeolite for Methanol-to-Olefins Reaction. Ind. Eng. Chem. Res. 2019, 58, 60–68.
- Salih, H.A.; Muraza, O.; Abussaud, B.; Al-Shammari, T.K.; Yokoi, T. Catalytic Enhancement of SAPO-34 for Methanol Conversion to Light Olefins Using in Situ Metal Incorporation. Ind. Eng. Chem. Res. 2018, 57, 6639–6646.
- Nasser, G.A.; Al-Qadri, A.A.; Jamil, A.K.; Bakare, I.A.; Sanhoob, M.A.; Muraza, O.; Yamani, Z.H.; Yokoi, T.; Saleem, Q.; Alsewdan, D. Conversion of Methanol to Olefins over Modified OSDA-Free CHA Zeolite Catalyst. Ind. Eng. Chem. Res. 2021, 60, 12189–12199.
- Liang, J.; Li, H.; Zhao, S.; Guo, W.; Wang, R.; Ying, M. Characteristics and performance of SAPO-34 catalyst for methanol-to-olefin conversion. Appl. Catal. 1990, 64, 31–40.
- Yu, L.; Nobandegani, M.S.; Hedlund, J. Industrially relevant CHA membranes for CO2/CH4 separation. J. Membr. Sci. 2022, 641, 119888.
- Wang, B.; Huang, W.; Zhu, Y.; Zhou, R.; Xing, W. Ultra-permeable high-selective SAPO-34 membranes for efficient CO2 capture. J. Membr. Sci. 2022, 650, 120420.
- Wang, B.; Wang, N.; Li, X.; Zhou, R.; Xing, W. Exfoliation of lamellar SAPO-34 zeolite to nanosheets and synthesis of thin SAPO-34 membranes by a nanosheet-seeded secondary growth approach. J. Membr. Sci. 2022, 645, 120177.
- Ahmed, A.; Ishiguro, S.; Seshimo, M.; Subramanian, B.; Matsukata, M. Synthesis of SAPO-34 Membrane and Its Application to the Separation of Water/Acetic Acid Mixtures by Vapor Permeation. J. Chem. Eng. Jpn. 2022, 55, 97–104.
- Liu, Z.; Xu, S.; Hao, J.; Song, L.; Chong, M.; Cheng, D.-G.; Chen, F. Bifunctional catalysts composed of low silicon-content SAPO-34 nanosheets and In2O3/ZrO2 with improved performance for CO2 hydrogenation. Greenh. Gases Sci. Technol. 2022, 12, 305–320.
- Usman, M.; Ghanem, A.S.; Ali Shah, S.N.; Garba, M.D.; Khan, M.Y.; Khan, S.; Humayun, M.; Khan, A.L. A review on SAPO-34 zeolite materials for CO2 capture and conversion. Chem. Rec. 2022, e202200039.
- Perez, E.V.; Kalaw, G.J.D.; Ferraris, J.P.; Balkus, K.J.; Musselman, I.H. Amine-functionalized (Al) MIL-53/VTEC™ mixed-matrix membranes for H2/CO2 mixture separations at high pressure and high temperature. J. Membr. Sci. 2017, 530, 201–212.
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393.
- Davoodabadi, A.; Mahmoudi, A.; Ghasemi, H. The potential of hydrogen hydrate as a future hydrogen storage medium. iScience 2021, 24, 101907.
- Ackley, M.W. Medical oxygen concentrators: A review of progress in air separation technology. Adsorption 2019, 25, 1437–1474.
- Marwat, M.A.; Humayun, M.; Afridi, M.W.; Zhang, H.; Abdul Karim, M.R.; Ashtar, M.; Usman, M.; Waqar, S.; Ullah, H.; Wang, C.; et al. Advanced Catalysts for Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2021, 4, 12007–12031.
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88.
- Shafiq, S.; Al-Maythalony, B.A.; Usman, M.; Ba-Shammakh, M.S.; Al-Shammari, A.A. ZIF-95 as a filler for enhanced gas separation performance of polysulfone membrane. RSC Adv. 2021, 11, 34319–34328.
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly Efficient Permeation and Separation of Gases with Metal–Organic Frameworks Confined in Polymeric Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001.
- Husna, A.; Hossain, I.; Jeong, I.; Kim, T.-H. Mixed Matrix Membranes for Efficient CO2 Separation Using an Engineered UiO-66 MOF in a Pebax Polymer. Polymers 2022, 14, 655.
- Tara, N.; Shamair, Z.; Habib, N.; Craven, M.; Bilad, M.R.; Usman, M.; Tu, X.; Khan, A.L. Simultaneous increase in CO2 permeability and selectivity by BIT-72 and modified BIT-72 based mixed matrix membranes. Chem. Eng. Res. Des. 2022, 178, 136–147.
- Xiong, S.; Li, L.; Dong, L.; Tang, J.; Yu, G.; Pan, C. Covalent-organic frameworks (COFs)-based membranes for CO2 separation. J. CO2 Util. 2020, 41, 101224.
- Khan, N.A.; Humayun, M.; Usman, M.; Ghazi, Z.A.; Naeem, A.; Khan, A.; Khan, A.L.; Tahir, A.A.; Ullah, H. Structural Characteristics and Environmental Applications of Covalent Organic Frameworks. Energies 2021, 14, 2267.
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285.
- Duan, K.; Wang, J.; Zhang, Y.; Liu, J. Covalent organic frameworks (COFs) functionalized mixed matrix membrane for effective CO2/N2 separation. J. Membr. Sci. 2019, 572, 588–595.
- Wang, X.; Shi, X.; Wang, Y. In Situ Growth of Cationic Covalent Organic Frameworks (COFs) for Mixed Matrix Membranes with Enhanced Performances. Langmuir 2020, 36, 10970–10978.
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25.
- Kiran, M.D.; Govindaraju, H.K.; Jayaraju, T.; Kumar, N. Review-Effect of Fillers on Mechanical Properties of Polymer Matrix Composites. Mater. Today Proc. 2018, 5, 22421–22424.
- Cai, W.; Xie, J.; Luo, J.; Chen, X.; Wang, M.; Wang, Y.; Li, J. n-Octyltrichlorosilane Modified SAPO-34/PDMS Mixed Matrix Membranes for Propane/Nitrogen Mixture Separation. Separations 2022, 9, 64.
- Santaniello, A.; Di Renzo, A.; Di Maio, F.; Belov, N.A.; Yampolskii, Y.P.; Golemme, G. Competing non ideal behaviour of SAPO-34 and Poly(hexafluoropropylene) in mixed matrix membranes. Micrpor. Mesopor. Mater. 2020, 303, 110241.
- Peydayesh, M.; Asarehpour, S.; Mohammadi, T.; Bakhtiari, O. Preparation and characterization of SAPO-34—Matrimid® 5218 mixed matrix membranes for CO2/CH4 separation. Chem. Eng. Res. Des. 2013, 91, 1335–1342.
- Wu, T.; Liu, Y.S.; Kumakiri, I.; Tanaka, K.; Chen, X.S.; Kita, H. Preparation and Permeation Properties of PESU-Based Mixed Matrix Membranes with Nano-Sized CHA Zeolites. J. Chem. Eng. Jpn. 2019, 52, 514–520.
- Carter, D.; Tezel, F.H.; Kruczek, B.; Kalipcilar, H. Investigation and comparison of mixed matrix membranes composed of polyimide matrimid with ZIF-8, silicalite, and SAPO-34. J. Membr. Sci. 2017, 544, 35–46.
- Belhaj Messaoud, S.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Mixed matrix membranes using SAPO-34/polyetherimide for carbon dioxide/methane separation. Sep. Purif. Technol. 2015, 148, 38–48.
- Zhao, D.; Ren, J.Z.; Li, H.; Hua, K.S.; Deng, M.C. Poly(amide-6-b-ethylene oxide)/SAPO-34 mixed matrix membrane for CO2 separation. J. Energy Chem. 2014, 23, 227–234.
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Kamal, S.N.M.; Chew, T.L. Carbon dioxide separation using asymmetric polysulfone mixed matrix membranes incorporated with SAPO-34 zeolite. Fuel Process. Technol. 2014, 118, 125–132.
- Junaidi, M.U.M.; Khoo, C.P.; Leo, C.P.; Ahmad, A.L. The effects of solvents on the modification of SAPO-34 zeolite using 3-aminopropyl trimethoxy silane for the preparation of asymmetric polysulfone mixed matrix membrane in the application of CO2 separation. Micrpor. Mesopor. Mater. 2014, 192, 52–59.
- Cakal, U.; Yilmaz, L.; Kalipcilar, H. Effect of feed gas composition on the separation of CO2/CH4 mixtures by PES-SAPO 34-HMA mixed matrix membranes. J. Membr. Sci. 2012, 417, 45–51.
- Oral, E.E.; Yilmaz, L.; Kalipcilar, H. Effect of Gas Permeation Temperature and Annealing Procedure on the Performance of Binary and Ternary Mixed Matrix Membranes of Polyethersulfone, SAPO-34, and 2-Hydroxy 5-Methyl Aniline. J. Appl. Polym. Sci. 2014, 131, 40679.
- Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F.; Nasir, R.; Man, Z. Effect of different organic amino cations on SAPO-34 for PES/SAPO-34 mixed matrix membranes toward CO2/CH4separation. J. Appl. Polym. Sci. 2016, 133, 43387.
- Nasir, R.; Ahmad, N.N.R.; Mukhtar, H.; Mohshim, D.F. Effect of ionic liquid inclusion and amino–functionalized SAPO-34 on the performance of mixed matrix membranes for CO2/CH4 separation. J. Environ. Chem. Eng 2018, 6, 2363–2368.
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Ahmad, N.A. Fluorocarbon functionalized SAPO-34 zeolite incorporated in asymmetric mixed matrix membranes for carbon dioxide separation in wet gases. Micrpor. Mesopor. Mater. 2015, 206, 23–33.
- Nawar, A.; Ghaedi, H.; Ali, M.; Zhao, M.; Iqbal, N.; Khan, R. Recycling waste-derived marble powder for CO2 capture. Process Saf. Environ. Prot. 2019, 132, 214–225.
- Ahmad, N.N.R.; Leo, C.P.; Mohammad, A.W.; Ahmad, A.L. Modification of gas selective SAPO zeolites using imidazolium ionic liquid to develop polysulfone mixed matrix membrane for CO2 gas separation. Micrpor. Mesopor. Mater. 2017, 244, 21–30.
- Mohshim, D.F.; Mukhtar, H.; Man, Z. The effect of incorporating ionic liquid into polyethersulfone-SAPO34 based mixed matrix membrane on CO2 gas separation performance. Sep. Purif. Technol. 2014, 135, 252–258.
- Mohshim, D.F.; Mukhtar, H.; Dutta, B.K.; Man, Z. Predicting CO2 Permeation through an Enhanced Ionic Liquid Mixed Matrix Membrane (IL3M). Int. J. Chem. Eng. 2019, 2019, 1–10.
- Sen, M.; Das, N. In situ carbon deposition in polyetherimide/SAPO-34 mixed matrix membrane for efficient CO2/CH4 separation. J. Appl. Polym. Sci. 2017, 134, 45508.
- Sodeifian, G.; Raji, M.; Asghari, M.; Rezakazemi, M.; Dashti, A. Polyurethane-SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. Chin. J. Chem. Eng. 2019, 27, 322–334.
- Rabiee, H.; Meshkat Alsadat, S.; Soltanieh, M.; Mousavi, S.A.; Ghadimi, A. Gas permeation and sorption properties of poly(amide-12-b-ethyleneoxide)(Pebax1074)/SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. J. Ind. Eng. Chem. 2015, 27, 223–239.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
3 times
(View History)
Update Date:
20 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




