Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elisabetta Baldi | -- | 3278 | 2023-03-17 09:27:11 | | | |
| 2 | Catherine Yang | Meta information modification | 3278 | 2023-03-17 09:31:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tamburrino, L.; Traini, G.; Marcellini, A.; Vignozzi, L.; Baldi, E.; Marchiani, S. Cryopreservation of Human Spermatozoa. Encyclopedia. Available online: https://encyclopedia.pub/entry/42298 (accessed on 01 March 2026).
Tamburrino L, Traini G, Marcellini A, Vignozzi L, Baldi E, Marchiani S. Cryopreservation of Human Spermatozoa. Encyclopedia. Available at: https://encyclopedia.pub/entry/42298. Accessed March 01, 2026.
Tamburrino, Lara, Giulia Traini, Arianna Marcellini, Linda Vignozzi, Elisabetta Baldi, Sara Marchiani. "Cryopreservation of Human Spermatozoa" Encyclopedia, https://encyclopedia.pub/entry/42298 (accessed March 01, 2026).
Tamburrino, L., Traini, G., Marcellini, A., Vignozzi, L., Baldi, E., & Marchiani, S. (2023, March 17). Cryopreservation of Human Spermatozoa. In Encyclopedia. https://encyclopedia.pub/entry/42298
Tamburrino, Lara, et al. "Cryopreservation of Human Spermatozoa." Encyclopedia. Web. 17 March, 2023.
Copy Citation
Cryopreservation is an expanding strategy to allow not only fertility preservation for individuals who need such procedures because of gonadotoxic treatments, active duty in dangerous occupations or social reasons and gamete donation for couples where conception is denied, but also for animal breeding and preservation of endangered animal species.
sperm cryopreservation
fertility preservation
sperm DNA damage
1. Introduction
The possibility to cryopreserve gametes and embryos represents an important advancement in reproductive biology. Such procedures are indeed essential for the maintenance of endangered animal species, for animal breeding via artificial insemination and, importantly, to give hope for future parenthood to individuals who must undergo therapies or surgery which can compromise gonadal function. In particular, according to the last edition of the WHO laboratory manual for the examination and processing of human semen [1], fertility preservation should be offered for autologous use to men before treatments with cytotoxic agents or radiotherapy [2], vasectomy, social freezing in cases of active duty in a dangerous occupation, male-to-female transsexual adults and adolescents before the initiation of hormonal therapies. In addition, semen cryopreservation can be advised to men before assisted reproduction techniques (ARTs) in the case of patients being unable to ejaculate, or with severe oligozoospermia or the inability to provide a fresh sample on the day of the ART procedure. Finally, the technique is used to cryopreserve spermatozoa from healthy donors for future use in couples where the male partner is azoospermic, to prevent the transmission of an inherited disorder, for women who wish to conceive but do not have a partner (the latter in those countries where the procedure is allowed) or for lesbian and transgender couples. Figure 1 reports the various conditions where cryopreservation is advised.

Figure 1. Schematic representation of categories of subjects to whom sperm cryopreservation is advised, cryoprotectants, cooling and thawing protocols which can be used and cryodamages that may occur.
From the first attempts to cryopreserve human male gametes, many advancements have been made, effective cryoprotectants have been discovered, the possibility to cryopreserve in liquid nitrogen has been developed and, nowadays, semen cryobanks are distributed widely around the world.
There are several procedures/protocols to cryopreserve semen and spermatozoa in liquid nitrogen or vapors (for review, see [3] and Figure 2). The research in this field has been focusing on finding solutions to minimize the generation of ice crystals within the cytoplasm, leading to the development and use of two types of cryoprotectants, permeating and non-permeating. The former (including DMSO, glycerol, ethylene glycol and others) creates an osmotic gradient to limit the formation of ice and stabilize the lipid bilayer. Non-permeating cryoprotectants (including sugars and lipoproteins) contribute to water leakage from the cytoplasm and protect membrane integrity. Cryoprotectants used nowadays usually include glycerol, a sugar and egg yolk mix used as a non-permeating cryoprotectant [4]. Antibiotics are also added to the mixture to fight the detrimental effect of microorganisms that may be present in semen.
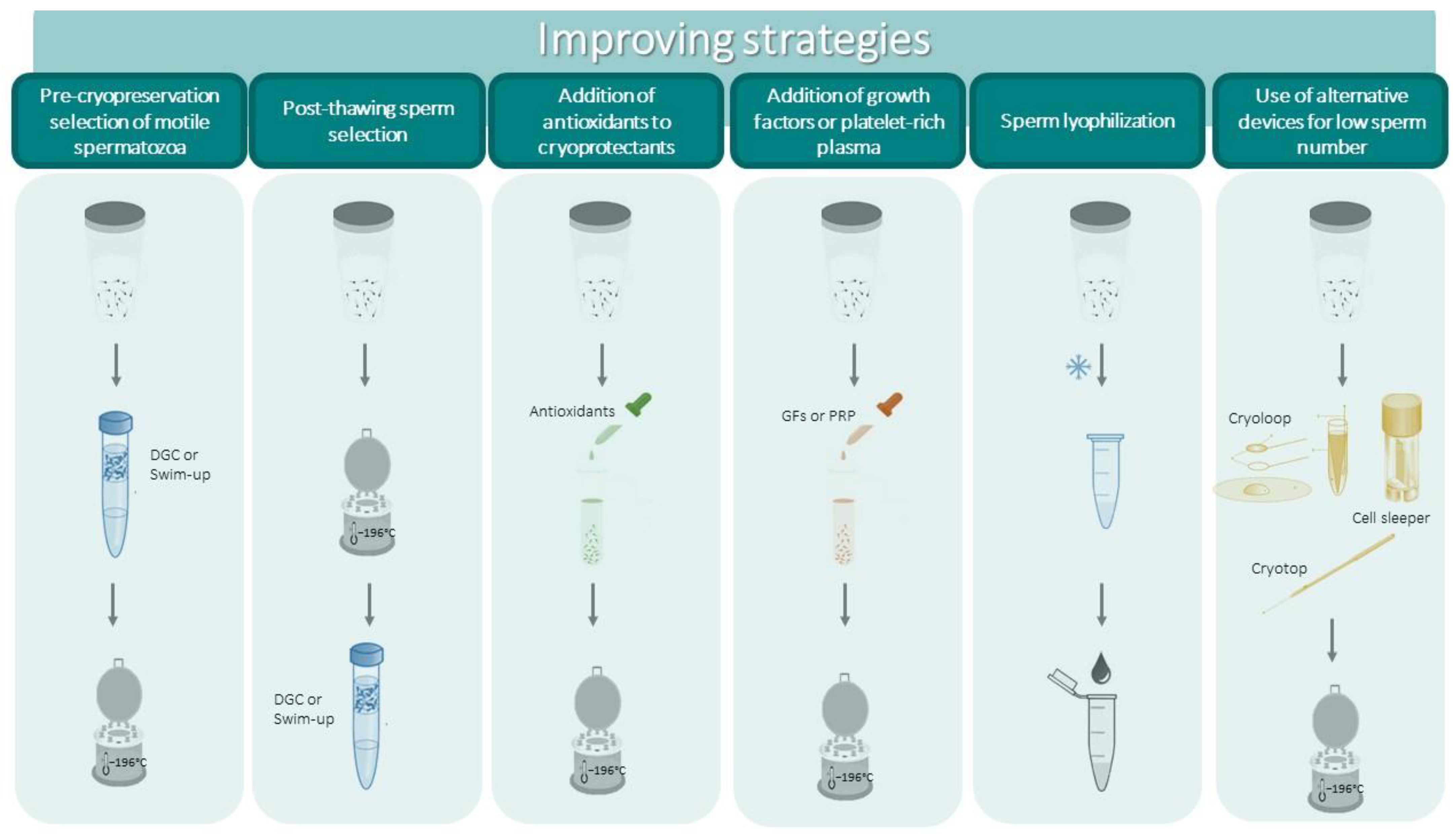
Figure 2. Schematic representation of strategies to improve the outcomes of cryopreservation and to cryopreserve low sperm numbers.
Another critical point in the process of cryopreservation is represented by the cooling rate, which should be controlled, for instance by the use of programmable freezers or through a standardized manual fast vapor freezing method [1]. Similarly, the thawing process is also critical, and different thawing methods can be used. At present, the WHO manual advises to proceed with fast thawing at 37 °C [1]. It should also be noted that semen processing before cryopreservation should be performed in a sterile environment [1] according to good manufacturing practice (GMP) guidelines, to avoid additional contaminations to those already present in semen that may further compromise sperm performances.
Based on their particular structural and morphological characteristics, including the low amount of cytoplasm, spermatozoa are considered to be quite resistant to potential cryodamage [5]. Due to the particular lipid composition of the plasma membrane, which contains higher levels of cholesterol and a lower ratio of unsaturated vs. saturated fatty acids, human spermatozoa are less susceptible to cryodamage with respect to the spermatozoa of other mammals [6]. Despite this, human spermatozoa may be heavily damaged by the freezing/thawing procedure both at structural and functional levels (see below). There is evidence that the susceptibility to the deteriorating effects not only depends on the initial quality of semen [7] but, also, may differ from one subject to another and vary among the different types of pathology for which cryopreservation is indicated [7][8][9][10]. At present, the intrinsic sperm characteristics responsible for the different susceptibility to cryodamage are not known. Furthermore, the issues producing the damage should be better defined. Besides intracytoplasmic ice formation, the generation of reactive oxygen species (ROS) is considered to be one of the main causes responsible for the damage [11] but whether other toxic products are generated during the procedure is less known and poorly studied.
Although most studies agree that sperm damage is induced by freezing and thawing processes per se rather than by long storage in liquid nitrogen [12][13][14], there is at least one study reporting storage–time-dependent structural damages [15]. Importantly, the attainment of pregnancy with long-term cryopreserved spermatozoa has been reported [16][17] and a recent study demonstrated that storage up to 15 years does not affect clinical ART outcomes with donor spermatozoa [18].
Semen banks must use safety procedures in order to prevent infectious disease transmission with cryopreserved semen and the risks of cross-contamination inside storage tanks. For this reason, men should be screened for the main transmitted viral diseases (Hepatitis B or C, CMV and HIV) and other pathogens according to local legislations. For virus/pathogen positive samples, it is advised to use separate tanks and other strategies to avoid cross-contamination [1]. The recent SARS-CoV-2 pandemic raised some questions regarding semen cryopreservation safety for COVID-19-affected individuals [19][20]. However, the occurrence of SARS-Cov2 mRNA in human semen has been only occasionally reported [21][22], and whether the virus may be transmitted through semen remains to be defined. A recent study [23] reporting the results of a survey administered to 22 European semen banks showed that the majority of them did not adopt particular safety measures during the pandemic period and the most common strategy consisted of the administration of an anamnestic questionnaire to patients, as only half of the centers required a nasopharyngeal swab.
2. Possible Strategies to Prevent the Damage and New Approaches to Sperm Cryopreservation
Although cryopreservation is a valuable option for preserving male fertility when required, cryo-injury represents a problem for the future use of cryopreserved gametes, especially when basal semen characteristics do not guarantee the effectiveness of the procedure. In such situations, semen banks should adopt strategies aimed to prevent or to reduce the cryodamage.
One possible strategy is represented by the pre-cryopreservation selection of motile spermatozoa with standard procedures such as density gradient centrifugation (DGC) or swim up, which eliminate dead, immotile and morphologically abnormal spermatozoa as well as immature germ cells and leucocytes that can be present in whole semen. Such a strategy can be adopted in particular situations (for example, when high levels of leukocytes are present [1]) or in the case of elevated percentages of immotile spermatozoa. Leucocytes, apoptotic/damaged spermatozoa and immature germ cells may indeed produce high levels of ROS, aggravating the damage induced by cryopreservation. Alternatively, and when possible, the IVF laboratory may attempt post-thawing selection in order to enrich the sample with motile spermatozoa. It should be considered that selection procedures such as DGC and, to a lesser extent, swim up, may induce damage to DNA per se [24][25] and that removal of seminal fluid eliminates the protective effects of antioxidant substances present in semen [26]. As a matter of fact, an increase in post-thawing DNA damage has also been reported when swim up or DGC procedures were used to select spermatozoa before cryopreservation, and no improvement in sperm motility was observed [27]. However, a recent study demonstrated that performing DGC selection before cryopreservation resulted in better post-thaw parameters with respect to selection after thawing [28].
Improvements in sperm parameters, including decreased DNA damage, have been reported when DGC was followed by a more sophisticated sperm selection procedure such as annexin V-magnetic assisted cell sorting (MACS) both pre- or post-cryopreservation [29][30]. In particular, post-thawing selection procedures have been attempted in a few studies, reporting an improvement in sperm motility [31][32]. Successful live births after sperm sorting with annexin V-MACS of cryopreserved spermatozoa with high levels of sDF from a cancer patient survivor were reported [33]. The paucity of studies on pre- or post-cryopreservation sperm selection, however, does not allow one to draw firm conclusions regarding whether they can be applied on a large scale.
In view of the fact that ROS generation during the freezing/thawing process is the main thing responsible for cryodamage, several studies have evaluated the effects of the addition of natural agents with antioxidant properties (vitamins, endogenous substances, herbal extracts, antioxidant enzymes and others) to semen extenders (reviewed in [4][34]) with the aim of mitigating the possible toxic effects of extenders. Most of these studies reported some efficacy in sperm parameters and DNA integrity, but no clear-cut conclusions could be drawn and the need for further studies was evidenced [4][35]. Kumar et al. [36] have shown that mitoquinone, a mitochondrial-targeted antioxidant, both attenuates ultrastructural changes and protects several proteins involved in sperm key functions from alterations induced by vitrification. Emerging studies in the last few years have investigated the effects of the supplementation of freezing media with taurine and hypotaurine [37][38], melatonin [39][40] and gallic acid [41] as antioxidants. Some beneficial effects have been reported with taurine and its precursor hypotaurine which slightly but significantly improved sperm parameters, including DNA integrity, when supplemented to extenders both for standard cryopreservation [38] and vitrification [37] methods. Among the tested antioxidant agents, the most efficient in mitigating the cryodamage in human spermatozoa was melatonin, as reported in a recent meta-analysis [35]. Indeed, the addition of the hormone, a physiological regulator of the circadian rhythm, to cryoprotectants exerts a significant positive effect on sperm progressive motility and viability [35], is currently used for the cryopreservation of spermatozoa from several animal species [42][43][44] and has been also shown to improve the survivability of oocytes and embryos [40]. Such beneficial effects are not surprising considering that melatonin, besides showing antioxidant activity, is an anti-apoptotic and ROS scavenging agent [39].
A recent study has demonstrated that preconditioning sperm cells before cryopreservation with sublethal nitric oxide levels not only improves sperm motility, viability and fertilizing capability [45] but also maintains the redox balance without altering the metabolism of sperm proteins [46].
Another approach recently investigated concerns surrounding the addition of growth factor- or platelet-rich plasma to cryoprotectants. Mirzaei et al. [47] demonstrated that the addition of a plasma rich in growth factors at different percentages (from 1 to 10%) could significantly improve sperm parameters and DNA integrity with the best results at 1% concentration. The authors attribute such positive effects to the action exerted by growth factors on their receptors on the human sperm surface more than a direct antioxidant or ROS scavenging effect [47]. Minimal improving effects were observed with platelet-rich plasma [48][49] in a small number of samples. No studies so far have addressed the question of whether cryopreserved spermatozoa with enriched plasma retain the ability to fertilize and support embryo development.
Vitrification has been successfully used to cryopreserve oocytes and embryos [50]. The group of Isachenko first introduced this method [5][51][52][53] which is based on the direct exposure of the sample to liquid nitrogen, allowing for ultrarapid freezing that avoids or strongly reduces the formation of ice crystals in the cell. In the case of spermatozoa, both the direct plunge of semen (after dilution with an extender) in liquid nitrogen or after aspiration in closed devices (in straw vitrification) can be performed. Vitrification is easy to perform, is less time-consuming with respect to the standard procedure and can be applied both for whole semen and selected spermatozoa free of seminal plasma. Clinical studies have demonstrated that vitrified spermatozoa retains its fertilizing ability both in IVF, ICSI and IUI techniques, achieving live births [54]. A recent meta-analysis evidenced some advantages in post-thawing parameters after vitrification with respect to conventional methods [55]. In particular, progressive motility and morphology appear to be better preserved. Concerning DNA damage, although some studies promote vitrification [56], other authors have not observed differences in post-thaw DNA damage between the two methods [57][58][59][60]. The heterogeneity of studies does not allow one to draw firm conclusions on whether vitrification should be preferred to the standard cryopreservation [59][60][61][62] and, at present, vitrification for human spermatozoa should be considered to be experimental [1]. A recent study analyzed post-thawing parameters after vitrification vs. vapor fast freezing of low semen volumes in different experimental conditions including the use of cell sleepers [63] (also see below). They showed that vapor fast freezing better prevents cryodamage independently of the type of cryoprotectant and the support used. It should be noted that vitrification can present some disadvantages with respect to conventional cryopreservation, such as the higher concentration of cryoprotectants used (increasing their toxicity), a higher risk of potential contamination with pathogens (requiring sterilization of liquid nitrogen) and, finally, requiring skilled operators for manipulation procedures [64].
The attainment of the successful generation of embryos [65] and even live births [66][67][68] in some mammalian species after sperm lyophilization (freeze-drying) is certainly attractive for semen banks and ART centers. Lyophilization is indeed a more sustainable technique which would avoid the use of expensive liquid nitrogen, allowing easy storage, packaging and transfer of the samples. At present, only few studies, with conflicting results, have evaluated the eventual damaging effect of lyophilization on human spermatozoa. Kusakabe et al. [69] demonstrated that only a low percentage of sperm showed chromosomal alterations and Gianaroli et al. [70] did not find increased DNA damage after dry storage with respect to the standard procedure. However, lyophilization may harm cell membranes [71] and produce detrimental effects on the sperm head [72]. Considering that after lyophilization spermatozoa do not preserve viability or motility, the fact that they can support embryo development and live births after ICSI in some mammalian species (see above) indicates that the maintenance of DNA integrity [69][70] is an important achievement of the freeze-dry procedure. Whether non-viable spermatozoa may support embryo development and live birth in humans as well is presently poorly known, as only a case report on the attainment of live birth with an unviable testicular spermatozoon [73] is present in the literature. Clearly, further studies are needed regarding this interesting and sustainable method of sperm storage, which, if successful, could open important perspectives both for human and animal reproduction.
In view of the variety of studies regarding the additional components to be added to standard cryoprotectants and different procedures to freeze/thaw spermatozoa, it is not possible at present to define the optimal mixture of cryoprotectants and the best freezing procedure. Hopefully, further well-designed comparative studies or metanalyses will help to define a gold standard procedure for semen or sperm cryopreservation.
Finally, it is worth mentioning that in the last edition of the WHO laboratory manual for examination and processing of human semen [1] it is stated that “as only a single spermatozoon is needed for ICSI of each oocyte, cryopreservation of any live spermatozoon is worthwhile”. The cryopreservation of small sperm numbers can be of clinical value for some male infertility factors such as severe oligozoosermia or criptozoospermia, cryptorchidism and obstructive azoospermia. Clearly, the use of standard procedures for very low sperm numbers is inadequate and may be time-consuming for the ICSI operators due to the dilution with the cryoprotectant, but also considering the cryodamage (see above). There are some alternative strategies to cryopreserve low sperm numbers, including the use of biological or non-biological carriers [74]. In particular, the latter (cryoloops, cell sleepers, cryotops and others) appear to be quite promising as they allow for the recovery of good percentages of motility and viability [63] and can also be used for spermatozoa recovered after TESE [75]. Such methods have been used in clinical settings demonstrating their efficiency in supporting live birth [76][77][78]. One important drawback of cryopreserving single spermatozoa is the necessity of using a micromanipulator with ICSI needles to pick single spermatozoa, requiring skilled operators, time and expensive instrumentation.
All of the possible strategies to prevent cryopreservation-induced damage and new alternative approaches are represented in Figure 2.
3. ART Outcomes after Use of Cryopreserved Spermatozoa
The usage rate of cryopreserved spermatozoa after cancer survival is quite low, estimated between 3 and 10% depending on the study and the length of follow up [79][80][81][82][83]. Such a rate is even lower for patients cryopreserving in a prevision of an ART procedure because, if present, fresh semen is always preferred. In most cancer cases, cryopreserved semen is destroyed after patient death, the attainment of a natural pregnancy or because of restored fertility after chemo- or radiotherapies. Regarding the latter point, studies on juvenile hematological cancers [84][85] indicate that most Hodgkin and non-Hodgkin lymphoma patients recover spermatogenesis 2 years after therapies, although the recovery highly depends on the therapy regimens (with worse results when chemotherapy is associated with radiotherapy) and is unpredictable. Similar results have been reported for testicular cancer patients [86]. One important aspect is related to the possible effects of chemo- and radiotherapies on sperm DNA integrity, also considering that there are studies reporting higher sperm DNA fragmentation levels in cancer patients before any therapy (see above). Most studies report an increase in sDF post-chemo or radiotherapies in testicular (reviewed in [87]) and hematological [88][89] cancer patients, which may persist for years after the end of the therapies. In consideration of the fact that cryopreservation may damage sperm DNA per se (see above), important clinical questions arise about the opportunity to use cryopreserved or fresh semen in cases of the recovery of spermatogenesis and when it is the right moment to attempt natural conception after cancer treatments, in order to avoid/decrease the risk of transmitting defective paternal genome to the offspring. Regarding the second question, as mentioned above, couples whose male partner regains fertility are requested to wait 1–2 years after the last cycle of therapy before attempting to conceive naturally or by ART using fresh semen. In any case, larger follow up studies are requested to give precise answers. Regarding the first question, it should be considered that the results of studies evaluating ART outcomes and the health of offspring with cryopreserved semen from cancer patients are highly conditioned and limited by the low usage rate of cryopreserved semen. In the bulk of them, these studies are quite reassuring about the attainment of clinical pregnancy and healthy offspring, with rates that do not differ or are only slightly lower with respect to control cycles [87][90][91][92].
Few studies have compared ART outcomes with fresh and frozen semen. In a randomized prospective study, Kuczynski et al. [93] demonstrated that the use of frozen spermatozoa from men with poor semen quality in ICSI cycles resulted in similar outcomes to freshly ejaculated spermatozoa and, actually, the rate of ongoing pregnancies was slightly, although insignificantly, higher in the frozen group. Similarly, a recent systematic review [94] on the use of fresh or frozen testicular spermatozoa from non-obstructive azoospermic men did not evidence significant differences in fertilization or pregnancy rates after ICSI. It should be noted, however, that Hauser et al. [95] reported, on average, lower implantation rates with frozen testicular spermatozoa. A retrospective study by Zhu et al. [96] compared the results of a consistent number of cycles from the fresh semen of normozoospermic men to those obtained with donor frozen semen. Clinical pregnancy and live birth rates after IVF were significantly higher and birth defects were reduced in the donor group.
Lower outcomes in terms of pregnancy rates are achieved when intrauterine insemination (IUI) is used in ART cycles. Botchan et al. [81], comparing the outcomes of ICSI and IUI cycles with the frozen spermatozoa of 184 cancer patients, found significantly higher pregnancy rates in the former (37.4 vs. 11.5%). Pregnancy rates per IUI cycle are also lower when cryopreserved donor semen samples are used [97][98][99]. Overall, these results suggest that IUI should be employed only in cases of attainment of adequate semen quality after thawing [81].
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021.
- Oktay, K.; Harvey, B.E.; Partridge, A.H.; Quinn, G.P.; Reinecke, J.; Taylor, H.S.; Wallace, W.H.; Wang, E.T.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1994–2001.
- Aliakbari, F.; Taghizabet, N.; Azizi, F.; Rezaei-Tazangi, F.; Samadee Gelehkolaee, K.; Kharazinejad, E. A review of methods for preserving male fertility. Zygote 2022, 30, 289–297.
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339.
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Dessole, S.; Nawroth, F. Vitrification of mammalian spermatozoa in the absence of cryoprotectants: From past practical difficulties to present success. Reprod. Biomed. Online 2003, 6, 191–200.
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7.
- Degl’Innocenti, S.; Filimberti, E.; Magini, A.; Krausz, C.; Lombardi, G.; Fino, M.G.; Rastrelli, G.; Maggi, M.; Baldi, E. Semen cryopreservation for men banking for oligospermia, cancers, and other pathologies: Prediction of post-thaw outcome using basal semen quality. Fertil. Steril. 2013, 100, 1555–1563.e1-3.
- Tamburrino, L.; Cambi, M.; Marchiani, S.; Manigrasso, I.; Degl’Innocenti, S.; Forti, G.; Maggi, M.; Baldi, E.; Muratori, M. Sperm DNA fragmentation in cryopreserved samples from subjects with different cancers. Reprod. Fertil. Dev. 2017, 29, 637–645.
- MacKenna, A.; Crosby, J.; Huidobro, C.; Correa, E.; Duque, G. Semen quality before cryopreservation and after thawing in 543 patients with testicular cancer. JBRA Assist. Reprod. 2017, 21, 31–34.
- Hamano, I.; Hatakeyama, S.; Nakamura, R.; Fukuhara, R.; Noro, D.; Tanaka, T.; Yoneyama, T.; Yamamoto, H.; Yoneyama, T.; Hashimoto, Y.; et al. Differences in semen characteristics between patients with testicular cancer and other malignancies using various cut-off values. Int. J. Urol. 2018, 25, 817–824.
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol. Reprod. 1998, 59, 1037–1046.
- Clarke, G.N.; Liu, D.Y.; Baker, H.W. Recovery of human sperm motility and ability to interact with the human zona pellucida after more than 28 years of storage in liquid nitrogen. Fertil. Steril. 2006, 86, 721–722.
- Edelstein, A.; Yavetz, H.; Kleiman, S.E.; Botchan, A.; Hauser, R.; Paz, G.; Yogev, L. Deoxyribonucleic acid-damaged sperm in cryopreserved-thawed specimens from cancer patients and healthy men. Fertil. Steril. 2008, 90, 205–208.
- Yogev, L.; Kleiman, S.E.; Shabtai, E.; Botchan, A.; Paz, G.; Hauser, R.; Lehavi, O.; Yavetz, H.; Gamzu, R. Long-term cryostorage of sperm in a human sperm bank does not damage progressive motility concentration. Hum. Reprod. 2010, 25, 1097–1103.
- Desrosiers, P.; Légaré, C.; Leclerc, P.; Sullivan, R. Membranous and structural damage that occur during cryopreservation of human sperm may be time-related events. Fertil. Steril. 2006, 85, 1744–1752.
- Horne, G.; Atkinson, A.D.; Pease, E.H.; Logue, J.P.; Brison, D.R.; Lieberman, B.A. Live birth with sperm cryopreserved for 21 years prior to cancer treatment: Case report. Hum. Reprod. 2004, 19, 1448–1449.
- Szell, A.Z.; Bierbaum, R.C.; Hazelrigg, W.B.; Chetkowski, R.J. Live births from frozen human semen stored for 40 years. J. Assist. Reprod. Genet. 2013, 30, 743–744.
- Huang, C.; Lei, L.; Wu, H.L.; Gan, R.X.; Yuan, X.B.; Fan, L.Q.; Zhu, W.B. Long-term cryostorage of semen in a human sperm bank does not affect clinical outcomes. Fertil. Steril. 2019, 112, 663–669.e1.
- Corona, G.; Baldi, E.; Isidori, A.M.; Paoli, D.; Pallotti, F.; De Santis, L.; Francavilla, F.; La Vignera, S.; Selice, R.; Caponecchia, L.; et al. SARS-CoV-2 infection, male fertility and sperm cryopreservation: A position statement of the Italian Society of Andrology and Sexual Medicine (SIAMS) (Società Italiana di Andrologia e Medicina della Sessualità). J. Endocrinol. Investig. 2020, 43, 1153–1157.
- Anifandis, G.; Taylor, T.H.; Messini, C.I.; Chatzimeletiou, K.; Daponte, A.; Ioannou, D.; Tempest, H.G. The Impact of SARS-CoV-2 on Sperm Cryostorage, Theoretical or Real Risk? Medicina 2021, 57, 946.
- Banihani, S.A. Human semen quality as affected by SARS-CoV-2 infection: An up-to-date review. Andrologia 2022, 54, e14295.
- Corona, G.; Vena, W.; Pizzocaro, A.; Pallotti, F.; Paoli, D.; Rastrelli, G.; Baldi, E.; Cilloni, N.; Gacci, M.; Semeraro, F.; et al. Andrological effects of SARS-Cov-2 infection: A systematic review and meta-analysis. J. Endocrinol. Investig. 2022, 45, 2207–2219.
- Marchiani, S.; Dabizzi, S.; Degl’Innocenti, S.; Fino, M.G.; Torcia, M.G.; Paoli, D.; Lombardo, F.; Ciccone, N.; Pollini, S.; Rossolini, G.M.; et al. Safety issues in semen banks during the COVID-19 pandemic: Data from a European survey. J. Endocrinol. Investig. 2022, 45, 973–980.
- Muratori, M.; Tarozzi, N.; Cambi, M.; Boni, L.; Iorio, A.L.; Passaro, C.; Luppino, B.; Nadalini, M.; Marchiani, S.; Tamburrino, L.; et al. Variation of DNA Fragmentation Levels During Density Gradient Sperm Selection for Assisted Reproduction Techniques: A Possible New Male Predictive Parameter of Pregnancy? Medicine 2016, 95, e3624.
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492.
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368.
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Cryopreservation of human semen and prepared sperm: Effects on motility parameters and DNA integrity. Fertil. Steril. 2001, 76, 892–900.
- Androni, D.A.; Dodds, S.; Tomlinson, M.; Maalouf, W.E. Is pre-freeze sperm preparation more advantageous than post-freeze? Reprod. Fertil. 2021, 2, 17–25.
- Grunewald, S.; Paasch, U.; Said, T.M.; Rasch, M.; Agarwal, A.; Glander, H.J. Magnetic-activated cell sorting before cryopreservation preserves mitochondrial integrity in human spermatozoa. Cell Tissue Bank. 2006, 7, 99–104.
- González-Ravina, C.; Santamaría-López, E.; Pacheco, A.; Ramos, J.; Carranza, F.; Murria, L.; Ortiz-Vallecillo, A.; Fernández-Sánchez, M. Effect of Sperm Selection by Magnetic-Activated Cell Sorting in D-IUI: A Randomized Control Trial. Cells 2022, 11, 1794.
- Allamaneni, S.S.; Agarwal, A.; Rama, S.; Ranganathan, P.; Sharma, R.K. Comparative study on density gradients and swim-up preparation techniques utilizing neat and cryopreserved spermatozoa. Asian J. Androl. 2005, 7, 86–92.
- Palomar Rios, A.; Gascón, A.; Martínez, J.V.; Balasch, S.; Molina Botella, I. Sperm preparation after freezing improves motile sperm count, motility, and viability in frozen-thawed sperm compared with sperm preparation before freezing-thawing process. J. Assist. Reprod. Genet. 2018, 35, 237–245.
- Herrero, M.B.; Delbes, G.; Chung, J.T.; Son, W.Y.; Holzer, H.; Buckett, W.; Chan, P. Case report: The use of annexin V coupled with magnetic activated cell sorting in cryopreserved spermatozoa from a male cancer survivor: Healthy twin newborns after two previous ICSI failures. J. Assist. Reprod. Genet. 2013, 30, 1415–1419.
- Hungerford, A.; Bakos, H.W.; Aitken, R.J. Sperm cryopreservation: Current status and future developments. Reprod. Fertil. Dev. 2023, 35, 265–281.
- Bahmyari, R.; Zare, M.; Sharma, R.; Agarwal, A.; Halvaei, I. The efficacy of antioxidants in sperm parameters and production of reactive oxygen species levels during the freeze-thaw process: A systematic review and meta-analysis. Andrologia 2020, 52, e13514.
- Kumar, P.; Wang, M.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Wang, W.; von Brandenstein, M.; Isachenko, V. Unraveling Subcellular and Ultrastructural Changes During Vitrification of Human Spermatozoa: Effect of a Mitochondria-Targeted Antioxidant and a Permeable Cryoprotectant. Front. Cell Dev. Biol. 2021, 9, 672862.
- Seify, M.; Zarabadipour, M.; Ghaleno, L.R.; Alizadeh, A.; Rezazadeh Valojerdi, M. The anti-oxidant roles of Taurine and Hypotaurine on acrosome integrity, HBA and HSPA2 of the human sperm during vitrification and post warming in two different temperature. Cryobiology 2019, 90, 89–95.
- Pons-Rejraji, H.; Vorilhon, S.; Difrane, A.; Dollet, S.; Bourgne, C.; Berger, M.; Chaput, L.; Pereira, B.; Bouche, C.; Drevet, J.R.; et al. Beneficial effects of hypotaurine supplementation in preparation and freezing media on human sperm cryo-capacitation and DNA quality. Basic Clin. Androl. 2021, 31, 26.
- Marcantonini, G.; Bartolini, D.; Zatini, L.; Costa, S.; Passerini, M.; Rende, M.; Luca, G.; Basta, G.; Murdolo, G.; Calafiore, R.; et al. Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin. Molecules 2022, 27, 3254.
- Choi, H.W.; Jang, H. Application of Nanoparticles and Melatonin for Cryopreservation of Gametes and Embryos. Curr. Issues Mol. Biol. 2022, 44, 4028–4044.
- Akbarzadeh-Jahromi, M.; Jafari, F.; Parsanezhad, M.E.; Alaee, S. Evaluation of supplementation of cryopreservation medium with gallic acid as an antioxidant in quality of post-thaw human spermatozoa. Andrologia 2022, 54, e14571.
- Tamay, E.; Palacios, P.; Peláez, G.; Saa, L.R.; Dorado, J.; Santiago-Moreno, J.; Galarza, D.A. Effect of Melatonin and Caffeine Supplementation to Freezing Medium on Cryosurvival of Peruvian Paso Horse Sperm Using a Two-Step Accelerating Cooling Rate. Biopreserv. Biobank. 2022; published online ahead of print.
- Monteiro, K.S.; Motta, N.C.; Cardoso, A.C.P.; Souza, S.P.; Murgas, L.D.S. Melatonin Supplementation for the Cryopreservation of Canine Sperm. Biopreserv. Biobank. 2022; published online ahead of print.
- Ustuner, B.; Ustuner, H.; Gokce, E.; Onder, N.T.; Yilmaz, M.M.; Huraydin, O.; Toker, M.B. The Combined Effect of Melatonin Implant and Removal of Buck Seminal Plasma on Cryopreservation During the Nonbreeding Season. Biopreserv. Biobank. 2022; ahead of print.
- Hezavehei, M.; Kouchesfahani, H.M.; Shahverdi, A.; Sharafi, M.; Salekdeh, G.H.; Eftekhari-Yazdi, P. Preconditioning of sperm with sublethal nitrosative stress: A novel approach to improve frozen-thawed sperm function. Reprod. Biomed. Online 2019, 38, 413–425.
- Hezavehei, M.; Mirzaei, M.; Sharafi, M.; Wu, Y.; Gupta, V.; Fitzhenry, M.; Kouchesfahani, H.M.; Eftekhari-Yazdi, P.; Baharvand, H.; Dalman, A.; et al. Proteomics study reveals the molecular mechanisms underlying cryotolerance induced by mild sublethal stress in human sperm. Cell Tissue Res. 2022, 387, 143–157.
- Mirzaei, J.; Movahedin, M.; Halvaei, I. Plasma-Rich in Growth Factors Ameliorates Detrimental Effects of Cryopreservation on Human Sperm: A Prospective Study. Cell J. 2022, 24, 330–336.
- Yan, B.; Zhang, Y.; Tian, S.; Hu, R.; Wu, B. Effect of autologous platelet-rich plasma on human sperm quality during cryopreservation. Cryobiology 2021, 98, 12–16.
- Nabavinia, M.S.; Yari, A.; Ghasemi-Esmailabad, S.; Gholoobi, A.; Gholizadeh, L.; Nabi, A.; Lotfi, M.; Khalili, M.A. Improvement of human sperm properties with platelet-rich plasma as a cryoprotectant supplement. Cell Tissue Bank. 2022; published online ahead of print.
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155.
- Nawroth, F.; Isachenko, V.; Dessole, S.; Rahimi, G.; Farina, M.; Vargiu, N.; Mallmann, P.; Dattena, M.; Capobianco, G.; Peters, D.; et al. Vitrification of human spermatozoa without cryoprotectants. Cryo Lett. 2002, 23, 93–102.
- Isachenko, E.; Isachenko, V.; Katkov, I.I.; Rahimi, G.; Schöndorf, T.; Mallmann, P.; Dessole, S.; Nawroth, F. DNA integrity and motility of human spermatozoa after standard slow freezing versus cryoprotectant-free vitrification. Hum. Reprod. 2004, 19, 932–939.
- Isachenko, V.; Isachenko, E.; Montag, M.; Zaeva, V.; Krivokharchenko, I.; Nawroth, F.; Dessole, S.; Katkov, I.I.; van der Ven, H. Clean technique for cryoprotectant-free vitrification of human spermatozoa. Reprod. Biomed. Online 2005, 10, 350–354.
- Schulz, M.; Risopatrón, J.; Uribe, P.; Isachenko, E.; Isachenko, V.; Sánchez, R. Human sperm vitrification: A scientific report. Andrology 2020, 8, 1642–1650.
- Li, Y.X.; Zhou, L.; Lv, M.Q.; Ge, P.; Liu, Y.C.; Zhou, D.X. Vitrification and conventional freezing methods in sperm cryopreservation: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 233, 84–92.
- O’Neill, H.C.; Nikoloska, M.; Ho, H.; Doshi, A.; Maalouf, W. Improved cryopreservation of spermatozoa using vitrification: Comparison of cryoprotectants and a novel device for long-term storage. J. Assist. Reprod. Genet. 2019, 36, 1713–1720.
- Darvishnia, H.; Lakpour, N.; Lahijani, M.S.; Heidari-Vala, H.; Akhondi, M.A.; Zeraati, H.; Sadeghi, M.R. Effects of very rapid versus vapor phase freezing on human sperm parameters. Cell Tissue Bank. 2013, 14, 679–685.
- Agha-Rahimi, A.; Khalili, M.A.; Nabi, A.; Ashourzadeh, S. Vitrification is not superior to rapid freezing of normozoospermic spermatozoa: Effects on sperm parameters, DNA fragmentation and hyaluronan binding. Reprod. Biomed. Online 2014, 28, 352–358.
- Ali Mohamed, M.S. Slow cryopreservation is not superior to vitrification in human spermatozoa; an experimental controlled study. Iran J. Reprod. Med. 2015, 13, 633–644.
- Tongdee, P.; Sukprasert, M.; Satirapod, C.; Wongkularb, A.; Choktanasiri, W. Comparison of Cryopreserved Human Sperm between Ultra Rapid Freezing and Slow Programmable Freezing: Effect on Motility, Morphology and DNA Integrity. J. Med. Assoc. Thail. 2015, 98 (Suppl. 4), S33–S42.
- Saritha, K.R.; Bongso, A. Comparative evaluation of fresh and washed human sperm cryopreserved in vapor and liquid phases of liquid nitrogen. J. Androl. 2001, 22, 857–862.
- Chang, H.J.; Lee, J.R.; Chae, S.J.; Jee, B.C.; Suh, C.S.; Kim, S.H. Comparative study of two cryopreservation methods of human spermatozoa: Vitrification versus slow freezing. Fertil. Steril. 2008, 90, S280.
- Arciero, V.; Ammar, O.; Maggi, M.; Vignozzi, L.; Muratori, M.; Dabizzi, S. Vapour fast freezing with low semen volumes can highly improve motility and viability or DNA quality of cryopreserved human spermatozoa. Andrology 2022, 10, 1123–1133.
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18.
- Keskintepe, L.; Pacholczyk, G.; Machnicka, A.; Norris, K.; Curuk, M.A.; Khan, I.; Brackett, B.G. Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biol. Reprod. 2002, 67, 409–415.
- Liu, J.L.; Kusakabe, H.; Chang, C.C.; Suzuki, H.; Schmidt, D.W.; Julian, M.; Pfeffer, R.; Bormann, C.L.; Tian, X.C.; Yanagimachi, R.; et al. Freeze-dried sperm fertilization leads to full-term development in rabbits. Biol. Reprod. 2004, 70, 1776–1781.
- Hirabayashi, M.; Kato, M.; Ito, J.; Hochi, S. Viable rat offspring derived from oocytes intracytoplasmically injected with freeze-dried sperm heads. Zygote 2005, 13, 79–85.
- Hochi, S.; Watanabe, K.; Kato, M.; Hirabayashi, M. Live rats resulting from injection of oocytes with spermatozoa freeze-dried and stored for one year. Mol. Reprod. Dev. 2008, 75, 890–894.
- Kusakabe, H.; Yanagimachi, R.; Kamiguchi, Y. Mouse and human spermatozoa can be freeze-dried without damaging their chromosomes. Hum. Reprod. 2008, 23, 233–239.
- Gianaroli, L.; Magli, M.C.; Stanghellini, I.; Crippa, A.; Crivello, A.M.; Pescatori, E.S.; Ferraretti, A.P. DNA integrity is maintained after freeze-drying of human spermatozoa. Fertil. Steril. 2012, 97, 1067–1073.e1.
- Zhu, W.J.; Jing, L.I.; Xiao, L.J. Changes on membrane integrity and ultrastructure of human sperm after freeze-drying. J. Reprod. Contracept. 2016, 27, 76–81.
- Bossi, R.L.; Cabral, M.; Oliveira, M.; Lopes, S.; Hurtado, R.; Sampaio, M.; Geber, S. Ultrastructural analysis of Lyophilized Human Spermatozoa. JBRA Assist. Reprod. 2021, 25, 473–479.
- Stecher, A.; Bach, M.; Neyer, A.; Vanderzwalmen, P.; Zintz, M.; Zech, N.H. Case report: Live birth following ICSI with non-vital frozen-thawed testicular sperm and oocyte activation with calcium ionophore. J. Assist. Reprod. Genet. 2011, 28, 411–414.
- Liu, S.; Li, F. Cryopreservation of single-sperm: Where are we today? Reprod. Biol. Endocrinol. 2020, 18, 41.
- Coetzee, K.; Ozgur, K.; Berkkanoglu, M.; Bulut, H.; Isikli, A. Reliable single sperm cryopreservation in Cell Sleepers for azoospermia management. Andrologia 2016, 48, 203–210.
- Herbemont, C.; Mnallah, S.; Bennani-Smires, B.; Peigne, M.; Cedrin-Durnerin, I.; Grynberg, M.; Sifer, C. Cryopreservation of small numbers of human spermatozoa in a Stripper tip: Report of the first live-birth worldwide. Cryobiology 2021, 99, 103–105.
- Endo, Y.; Fujii, Y.; Motoyama, H. Clinical and neonatal outcomes of individually vitrified human sperm with Cryotop and Cell Sleeper. Cryobiology 2022, 108, 78–81.
- Huang, C.; Tang, Y.L.; Hu, J.L.; Zhou, W.J.; Huang, Z.H.; Luo, X.F.; Li, Z.; Zhu, W.B. Update on techniques for cryopreservation of human spermatozoa. Asian J. Androl. 2022, 24, 563–569.
- Bizet, P.; Saias-Magnan, J.; Jouve, E.; Grillo, J.M.; Karsenty, G.; Metzler-Guillemain, C.; Perrin, J. Sperm cryopreservation before cancer treatment: A 15-year monocentric experience. Reprod. Biomed. Online 2012, 24, 321–330.
- Muller, I.; Oude Ophuis, R.J.; Broekmans, F.J.; Lock, T.M. Semen cryopreservation and usage rate for assisted reproductive technology in 898 men with cancer. Reprod. Biomed. Online 2016, 32, 147–153.
- Botchan, A.; Karpol, S.; Lehavi, O.; Paz, G.; Kleiman, S.E.; Yogev, L.; Yavetz, H.; Hauser, R. Preservation of sperm of cancer patients: Extent of use and pregnancy outcome in a tertiary infertility center. Asian J. Androl. 2013, 15, 382–386.
- Vomstein, K.; Reiser, E.; Pinggera, G.M.; Toerzsoek, P.; Deininger, S.; Kriesche, T.; Biasio, W.; Lusuardi, L.; Toth, B. Sperm banking before gonadotoxic treatment: Is it worth the effort? Asian J. Androl. 2021, 23, 490–494.
- Ferrari, S.; Paffoni, A.; Reschini, M.; Noli, S.; Dallagiovanna, C.; Guarneri, C.; Filippi, F.; Somigliana, E. Variables affecting long-term usage rate of sperm samples cryopreserved for fertility preservation in cancer patients. Andrology 2021, 9, 204–211.
- Paoli, D.; Rizzo, F.; Fiore, G.; Pallotti, F.; Pulsoni, A.; Annechini, G.; Lombardo, F.; Lenzi, A.; Gandini, L. Spermatogenesis in Hodgkin’s lymphoma patients: A retrospective study of semen quality before and after different chemotherapy regimens. Hum. Reprod. 2016, 31, 263–272.
- Pallotti, F.; Pelloni, M.; Faja, F.; Di Chiano, S.; Di Rocco, A.; Lenzi, A.; Lombardo, F.; Paoli, D. Semen quality in non-Hodgkin lymphoma survivors: A monocentric retrospective study. Hum. Reprod. 2021, 36, 16–25.
- Gandini, L.; Sgrò, P.; Lombardo, F.; Paoli, D.; Culasso, F.; Toselli, L.; Tsamatropoulos, P.; Lenzi, A. Effect of chemo- or radiotherapy on sperm parameters of testicular cancer patients. Hum. Reprod. 2006, 21, 2882–2889.
- Paoli, D.; Pallotti, F.; Lenzi, A.; Lombardo, F. Fatherhood and Sperm DNA Damage in Testicular Cancer Patients. Front Endocrinol 2018, 9, 506.
- Ståhl, O.; Eberhard, J.; Cavallin-Ståhl, E.; Jepson, K.; Friberg, B.; Tingsmark, C.; Spanò, M.; Giwercman, A. Sperm DNA integrity in cancer patients: The effect of disease and treatment. Int. J. Androl. 2009, 32, 695–703.
- O’Flaherty, C.; Vaisheva, F.; Hales, B.F.; Chan, P.; Robaire, B. Characterization of sperm chromatin quality in testicular cancer and Hodgkin’s lymphoma patients prior to chemotherapy. Hum. Reprod. 2008, 23, 1044–1052.
- Pening, D.; Constant, M.; Bruynbroeck, M.; Delbaere, A.; Demeestere, I. Impact of cancer on cryopreserved sperm quality and fertility: A cohort study. Health Sci. Rep. 2022, 5, e726.
- Ferrari, S.; Paffoni, A.; Filippi, F.; Busnelli, A.; Vegetti, W.; Somigliana, E. Sperm cryopreservation and reproductive outcome in male cancer patients: A systematic review. Reprod. Biomed. Online 2016, 33, 29–38.
- Fernández-González, M.J.; Radauer-Plank, A.C.; Stelzer, C.; Geiger, W.; Goranova, I.; Borgmann-Staudt, A.; Balcerek, M.; Wilkemeyer, I. Sperm and testicular tissue cryopreservation and assisted reproductive technology outcomes in male cancer patients: A 15-year experience. J. Cancer Res. Clin. Oncol. 2022; published online ahead of print.
- Kuczyński, W.; Dhont, M.; Grygoruk, C.; Grochowski, D.; Wołczyński, S.; Szamatowicz, M. The outcome of intracytoplasmic injection of fresh and cryopreserved ejaculated spermatozoa--a prospective randomized study. Hum. Reprod. 2001, 16, 2109–2113.
- Amer, M.; Fakhry, E. Fresh vs frozen testicular sperm for assisted reproductive technology in patients with non-obstructive azoospermia: A systematic review. Arab. J. Urol. 2021, 19, 247–254.
- Hauser, R.; Yogev, L.; Amit, A.; Yavetz, H.; Botchan, A.; Azem, F.; Lessing, J.B.; Ben-Yosef, D. Severe hypospermatogenesis in cases of nonobstructive azoospermia: Should we use fresh or frozen testicular spermatozoa? J. Androl. 2005, 26, 772–778.
- Zhu, Y.; Zhang, F.; Chen, H.; Sun, X.; Jiang, F. The use of frozen embryos and frozen sperm have complementary IVF outcomes: A retrospective analysis in couples experiencing IVF/Donor and IVF/Husband. BMC Pregnancy Childbirth 2022, 22, 776.
- Subak, L.L.; Adamson, G.D.; Boltz, N.L. Therapeutic donor insemination: A prospective randomized trial of fresh versus frozen sperm. Am. J. Obstet. Gynecol. 1992, 166, 1597–1604; discussion 1604–1606.
- Botchan, A.; Hauser, R.; Gamzu, R.; Yogev, L.; Paz, G.; Yavetz, H. Results of 6139 artificial insemination cycles with donor spermatozoa. Hum. Reprod. 2001, 16, 2298–2304.
- Thijssen, A.; Creemers, A.; Van der Elst, W.; Creemers, E.; Vandormael, E.; Dhont, N.; Ombelet, W. Predictive factors influencing pregnancy rates after intrauterine insemination with frozen donor semen: A prospective cohort study. Reprod. Biomed. Online 2017, 34, 590–597.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
847
Revisions:
2 times
(View History)
Update Date:
17 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





