Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Darwin Andrés Moreno-Pérez | -- | 2488 | 2023-03-16 16:38:42 | | | |
| 2 | Lindsay Dong | Meta information modification | 2488 | 2023-03-17 05:03:14 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2488 | 2023-03-17 05:03:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cuy-Chaparro, L.; Reyes, C.; Díaz-Guiot, E.V.; Moreno-Pérez, D.A.; Patarroyo, M.A. Anti-Babesia Vaccines. Encyclopedia. Available online: https://encyclopedia.pub/entry/42275 (accessed on 07 February 2026).
Cuy-Chaparro L, Reyes C, Díaz-Guiot EV, Moreno-Pérez DA, Patarroyo MA. Anti-Babesia Vaccines. Encyclopedia. Available at: https://encyclopedia.pub/entry/42275. Accessed February 07, 2026.
Cuy-Chaparro, Laura, César Reyes, Eliana Vanessa Díaz-Guiot, Darwin Andrés Moreno-Pérez, Manuel Alfonso Patarroyo. "Anti-Babesia Vaccines" Encyclopedia, https://encyclopedia.pub/entry/42275 (accessed February 07, 2026).
Cuy-Chaparro, L., Reyes, C., Díaz-Guiot, E.V., Moreno-Pérez, D.A., & Patarroyo, M.A. (2023, March 16). Anti-Babesia Vaccines. In Encyclopedia. https://encyclopedia.pub/entry/42275
Cuy-Chaparro, Laura, et al. "Anti-Babesia Vaccines." Encyclopedia. Web. 16 March, 2023.
Copy Citation
Bovine babesiosis is caused by the Apicomplexa parasites from the genus Babesia. It is one of the most important tick-borne veterinary diseases worldwide; Babesia bovis being the species associated with the most severe clinical signs of the disease and causing the greatest economic losses. Resistance to drugs targeting B. bovis or its transmitting vector has made vaccination against this parasite the main infection control method.
babesiosis
Babesia bovis
vaccine
synthetic vaccine
1. Introduction
The Apicomplexa phylum consists of a group of obligate intracellular pathogens that cause significant veterinary and human diseases, i.e., parasites from the genera Babesia, Theileria, Plasmodium and Toxoplasma [1]. Such parasites are characterised by having an apical complex consisting of the organelles (the rhoptries, the micronemes and the conoid organelles or the spherical bodies) which secrete molecules participating in the target cell adhesion/invasion and promote parasite development for producing the disease in a targeted host [2][3][4]. Such parasites are transmitted by the arthropod vectors, ticks being the main Babesia transmitting agent. More than 100 tick species have been reported to date, which are widely distributed throughout North America, South America, Africa, Asia and Australia [5][6][7]. Rhipicephalus (Boophilus) microplus infects a large variety of wild and domestic hosts (preferring cattle) and is a biological vector of the Babesia bovis species that causes bovine babesiosis in cows [8].
B. bovis has a complex life-cycle requiring a vector where the parasite’s sexual reproduction occurs and a vertebrate host where asexual multiplication takes place (Figure 1) [9]. A vector’s bite inoculates B. bovis sporozoites (Spz) into a host’s bloodstream; these directly invade the erythrocytes by establishing the pathogen-cell molecular interactions. Once inside a cell, the Spz become freely located in the cytoplasm where they become the trophozoites, a few hours later [8][10]. This marks the beginning of the merogony phase, i.e., the parasite asexually reproduces by replicating its own nucleus inside its host’s cell. This is followed by the parasite inducing the cell segmentation (i.e., dividing by binary fission) to produce two infective merozoites (Mrz) which lyse the erythrocytes to be released into the host circulation and invade new erythrocytes. The invasion process proceeds continuously, giving rise to successive and asynchronous Mrz production; this leads to different parasitic stages being found in a host’s bloodstream [8][11] and causes clinical signs 7 to 35 days after parasite inoculation.
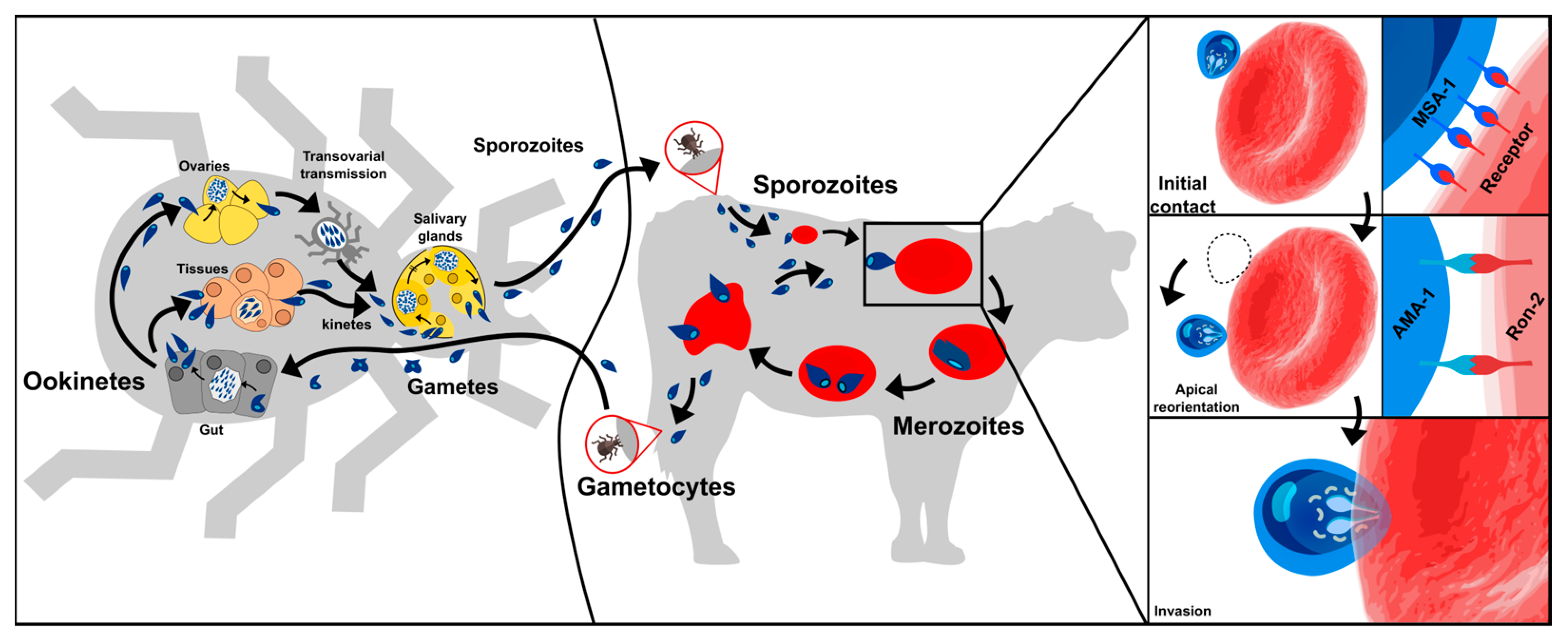
Figure 1. B. bovis life-cycle. Two hosts are involved; one is a vector (tick) in which the sexual part of its cycle takes place (left) and the other a vertebrate host (bovine) where asexual multiplication takes place (middle). Right panel shows proteins identified so far as participating in parasite initial contact and reorientation towards the apical pole.
2. Anti-Babesia Vaccines
The main vaccines developed and introduced in several countries for many years to date have involved using live attenuated parasites obtained from passages in splenectomised cattle (Figure 2) [12][13]. Other types of vaccine have also been studied, such as attenuated parasite-based vaccines obtained from in vitro cultures, or recombinant proteins/viral vectors or peptide-based vaccines targeting the B. bovis blood stage; however, these types of vaccines have only been used in scientific studies to date [14][15][16]. Even though these types of vaccines have induced considerable levels of protection (especially in calves under a year old), they have not been completely effective and the production-related difficulties have limited their commercialisation.
Figure 2. B. bovis vaccine design. The figure summarises the different anti-B. bovis vaccine types developed to date. Live attenuated vaccines are obtained by successive parasite passages in splenectomised cattle or in vitro cultured parasites that are subsequently attenuated by different methods. Protein-based vaccines have been obtained by size separation of crude parasite extracts, recombinant expression in E. coli and subsequent lysis and purification by affinity chromatography, or viral vectors engineered to express the protein if interest in vivo. Peptide-based vaccines are focused on the in silico identification of short regions containing B- or T-cell epitopes and subsequent chemical synthesis. Figure created with BioRender.com.
2.1. Live Attenuated Parasite Vaccines
This type of vaccine emerged from the observations made in 1899 concerning the fact that long-lasting immunity against new infection was induced in animals that had recovered from natural Babesia infection. Furthermore, subsequent evidence showed that blood from infection-recovered animals did not produce a severe form of disease in recipient animals [17]. The live attenuated parasite vaccine systems have involved using low virulence B. bovis strains that have been attenuated through successive passages in splenectomised cattle or by using in vitro cultures that were kept frozen for several days or by deep freezing for long periods in liquid nitrogen (Figure 2) [18]. B. bovis B, C and D strains attenuated using the aforementioned procedures (isolated from natural infection during babesiosis outbreaks in various regions of Australia) were evaluated around 1957, and demonstrated good efficacy and safety results [18]. However, it was reported that no more than 30 passages should be carried out in cattle since a greater amount considerably affected effectiveness in immunised cattle [19].
Developing a B. bovis in vitro culture has been another alternative used to facilitate attenuated live vaccine design and to evaluate its usefulness for inducing an immunogenic response during the experimental infection. For example, one study has shown that inoculating the B. bovis 429 strain (attenuated and adapted to grow in equine serum) into 4 splenectomised calves reduced the clinical signs of infection when the animals were challenged with the virulent B. bovis KB strain 44 and 78 days after the vaccination [20].
Such results suggest that an in vitro culture represents an alternative for obtaining antigens similar to those in in vivo culture which retain their functional properties [21][22]. The use of in vitro culture has advantages over the in vivo culture system given that it reduces animal usage by producing the attenuated strains in vitro; it also enables vaccines to be manufactured in more controlled and standardised conditions and implies less risk of pathogen co-transmission (due to measures such as gamma irradiation of the serum used for the parasite culture) [15][23].
Immunisation with live attenuated B. bovis microorganisms targeting this species has been used in Australia, Brazil, Israel, Mexico, Colombia, Argentina, South Africa and Uruguay. In addition to the aforementioned advantages, it must be stressed that this type of vaccine involves significant limitations related to its production and administration. For example, production of this type of vaccine requires fresh bovine erythrocytes and serum from specific donors requiring very strict production/maintenance conditions to ensure that they are pathogen-free (which can be difficult when the biological product is made in tick-endemic regions) and guaranteeing parasite viability from the beginning of preparation until the final product delivery. In vitro cultured parasite immunogenicity may be lost after long-term maintenance in such conditions; access to suitable laboratory equipment and the availability of trained personnel is necessary, given the complexity of maintaining a B. bovis culture for large-scale vaccine production [14][16]. Regarding administration, there are still difficulties related to standardising the dose required for the vaccination. There is also the potential risk of tick transmission of the vaccine strain that could recombine with the field strains, potentially leading to new virulent variants or virulent reversion [14][15]. The ideal moment for immunisation must be taken into account; administration to weaning age calves being preferred because adult cattle can be highly susceptible to vaccine components inducing serious reactions, such as abortion [24].
2.2. Protein-Based Vaccines
The main molecules activating an immune response are the parasite proteins expressed during the blood stages; there has thus been major interest in studying complete recombinant molecules or their fragments which can activate the cellular and the humoral immune responses controlling infection [25][26]. This approach has been useful, given that the parasite proteins/fragments can be obtained individually, involving low production costs using different expression systems (Escherichia coli being the most used), along with the relative ease of standardising the production and storage and a greater degree of safety compared to that for live vaccines [27].
Affinity chromatography purification and identification of the antigens obtained from the B. bovis crude extracts formed the first approach involving this type of vaccine. This assay involved the sequential vaccination/challenge experiments in adult cattle using crude parasite extracts systematically fractionated by chromatographic and electrophoretic procedures (Figure 2); fractions eliciting a protective response were used for purifying the antigens by pull-down assays (i.e., 11C5 and 12D3), using the monoclonal antibodies (Ab) developed against these parasite fractions.
The rhoptry associated protein 1 (RAP-1) has been confirmed as an antigen containing the immunodominant and conserved T-cell epitopes in its N-terminal region which can induce a response from the IFN-γ-producing CD4+ T-cells and increase IgG2 synthesis, thereby conferring partial protection in animals challenged with the parasite [28][29][30]. Nevertheless, such a response did not guarantee protective immunity against challenge with the virulent B. bovis strain, nor did it change the infection’s clinical course [31].
Merozoite surface antigen-1 (MSA-1) has also been of interest given that it is mainly exposed to the immune system during natural infection. However, despite MSA-1 being able to induce neutralising Ab production in vitro [32], it did not protect calves immunised with three doses of recombinant saponin-emulsified MSA-1 after they were challenged with the B. bovis T2Bo strain [33]. Such findings could be explained by limitations such as the brief period of exposure to Mrz in serum, thereby limiting accessibility to neutralising epitopes [34], difficulties related to producing protective Abs (due to high MSA-1 variability inducing strain-specific responses and thus the lack of cross-protection), or the presence of other proteins from the B. bovis variable merozoite surface antigen (VMSA) family functionally replacing MSA-1 proteins.
The apical membrane antigen-1 (AMA-1) is a very interesting molecule as its evaluation in Plasmodium has shown that it plays an indispensable role in the host cell invasion. It has been found that the Ab production induced by the B. bovis recombinant protein encoded by the conserved central region (BbAMA-1 ectodomain I (DI)and II(DII)) significantly inhibited Mrz growth as well as the efficiency of in vitro Mrz invasion of the bovine erythrocytes by 70% after 6 h, suggesting that this region is a good candidate for inclusion in an anti-B. bovis vaccine [35].
2.3. Recombinant Viral Vector Vaccines
Recombinant viral vector vaccines seek to enhance humoral and cellular immune responses by using non-pathogenic viral vectors as expression platforms [36]. For example, the highly attenuated modified vaccinia virus Ankara (MVA) is a non-replicative vector used to express single or multiple immunodominant antigens in vivo (Figure 2). Once MVA has infected host cells, recombinant proteins are expressed and exhibited to the immunological system to trigger responses, mainly Th1-type [37].
2.4. Antigenic Peptide-Based Vaccines
The chemical synthesis of short sequences containing the T- and the B-cell recognition sites has been established as a strategy for vaccine development (Figure 2) as only these molecule’s fragments can induce an immune response [38]. This approach has been studied with proteins participating in the parasite-erythrocyte interaction and is based on evidence related to their antigenic role during natural infection.
Several peptides containing RAP-1, MSA-2c and AMA-1 B-cell epitopes were able to trigger neutralising Ab in cattle, inhibiting in vitro invasion by 34.7% on average (using RAP-1 P1 and P4), 6.28% (with AMA-1 P2) and 10.34% (MSA-2c P3 and P4) when used individually and 52.37% when evaluated as a pool. Interestingly, MSA2c P3 and AMA-1 P2 induced gamma interferon (IFN-γ) production in PBMCs from the vaccinated cattle after one year, thus providing a long-lasting Th1 immune response [39]. It has been shown that a recombinant epitope derived from the BbAMA-I-DI surface-exposed α-helix (specifically in the PAN motif) was able to elicit Ab in rabbits which significantly inhibited the in vitro parasite invasion by 60% within 4 h of incubation and Mrz growth by 50–70% on days 3 and 4 of culture [40].
3. Functional Approach
Knowledge about the biology of the parasites belonging to the Theileria, Plasmodium, Toxoplasma and Babesia genera has advanced with different intensity, Plasmodium being one of the most studied due to its significant impact on human health worldwide [41]. Plasmodium falciparum and Plasmodium vivax are the most important species causing the highest mortality and morbidity rates worldwide, whilst Plasmodium ovale, Plasmodium malariae and Plasmodium knowlesi have the lowest prevalence [42]. Different strategies have also been studied for developing a vaccine as the main disease control measure. However, a new and robust functional approach has been proposed during the last few years; it has used P. falciparum as a malaria disease model. Rather than identifying variable regions, it is based on selecting critical protein-derived conserved regions related to parasite adhesion [43]. These regions were initially identified in P. falciparum proteins by screening the erythrocyte binding properties of 20-residue-long, non-overlapping peptides spanning the entire molecules. A sensitive and specific radio-iodination-based binding assay was used.
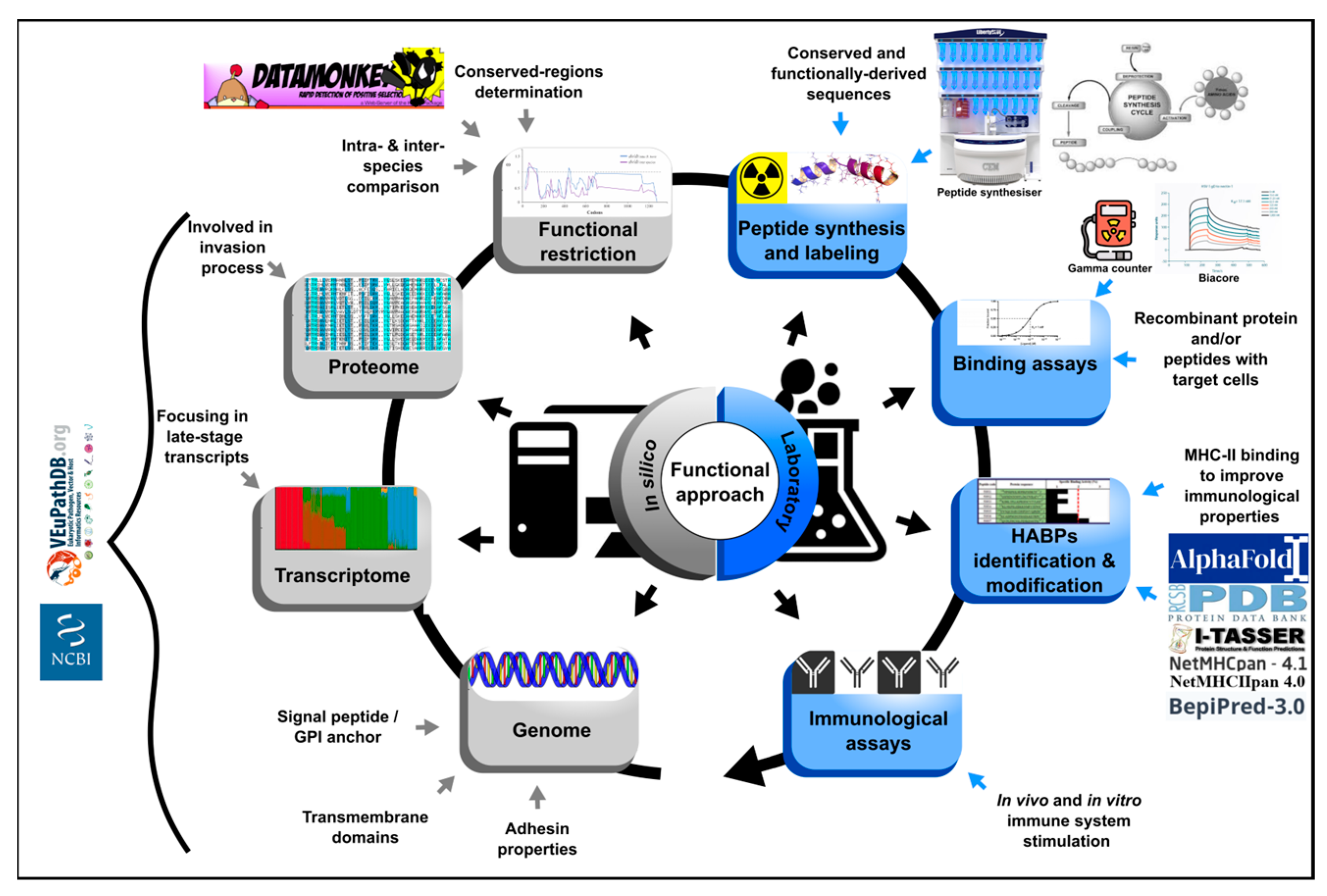
This approach has been used and adapted for identifying minimal regions involved in P. vivax binding to the reticulocytes (Figure 3) [44]. Omic study results have been compiled as a strategy for filtering those regions expressed during the parasite’s life-cycle, that have the potential of participating in target cell entry. Bioinformatics tools are used to prioritise the candidate selection based on their in silico characteristics (such as the signal peptides, the membrane anchor sequences, the functional domains, the stage-specific expression profiles and the specific location pattern) and intra- and inter-species conservation level, the latter determined by natural selection analysis [45]. The protein’s target cell binding role is then evaluated using only 20-residue-long, non-overlapping peptides derived from the regions under functional constraint by the protein-cell interaction assays, like those performed for P. falciparum. This has enabled finding P. vivax cHABPs to target cells; their role in cell invasion must be confirmed in vitro and in vivo assays using modified peptides in the latter to improve their antigenicity. Unlike results obtained by the classical approach to designing vaccines based on proteins and peptides, in vitro results obtained by the functional approach are expected to be consistent with those in vivo, as confirmed for P. falciparum.

Figure 3. Functional approach strategy. Flow diagram describing the steps for prioritising in silico selection and laboratory experiments for identifying ideal vaccine component peptides. The strategy involves identifying the entire gene repertoire (using genome, transcriptome and proteome data mining) and their encoding sequence analysis by bioinformatics tools based on in silico characteristics and their intra (from 5 genomes)- and inter-species (phylogenetically related) conservation level [45]. Peptides encoded by regions under functional constraint are then chemically synthesised and target cell binding activity must be tested by radio-iodination-based peptide-cell interaction and competition assays [46]. Identified HABPs must be evaluated in in vitro culture and then modified to fit inside MHC-II to evaluate their role as vaccine component in in vivo tests in the pertinent experimental model. The figure shows the required resources for carrying out the functional approach.
4. Conclusions
Anti-B. bovis vaccine development has been focused on live-attenuated parasites, recombinant proteins and antigenic peptides. However, such traditional strategies have highlighted the variability of these approaches, along with the many limitations related to their success. This has emphasised the need for adopting safe alternative vaccine design approaches, capable of inducing a long-lasting protection-inducing immune response. A functional approach is thus worth considering, taking into account the implicit advantages of safety, efficiency, ease of maintenance and production.
References
- Arisue, N.; Hashimoto, T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015, 64, 254–259.
- Shen, B.; Sibley, L.D. The moving junction, a key portal to host cell invasion by apicomplexan parasites. Curr. Opin. Microbiol. 2012, 15, 449–455.
- Bargieri, D.; Lagal, V.; Andenmatten, N.; Tardieux, I.; Meissner, M.; Menard, R. Host cell invasion by apicomplexan parasites: The junction conundrum. PLOS Pathog. 2014, 10, e1004273.
- Lobo, C.A.; Rodriguez, M.; Cursino-Santos, J.R. Babesia and red cell invasion. Curr. Opin. Hematol. 2012, 19, 170–175.
- Marques, R.; Kruger, R.F.; Peterson, A.T.; de Melo, L.F.; Vicenzi, N.; Jimenez-Garcia, D. Climate change implications for the distribution of the babesiosis and anaplasmosis tick vector, Rhipicephalus (Boophilus) microplus. Vet. Res. 2020, 51, 81.
- Nyangiwe, N.; Horak, I.G.; Van der Mescht, L.; Matthee, S. Range expansion of the economically important Asiatic blue tick, Rhipicephalus microplus, in South Africa. J. South Afr. Vet. Assoc. 2017, 88, e1–e7.
- Sonenshine, D.E. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Environ. Res. Public Health 2018, 15, 478.
- Chauvin, A.; Moreau, E.; Bonnet, S.; Plantard, O.; Malandrin, L. Babesia and its hosts: Adaptation to long-lasting interactions as a way to achieve efficient transmission. Vet. Res. 2009, 40, 37.
- Rathinasamy, V.; Poole, W.A.; Bastos, R.G.; Suarez, C.E.; Cooke, B.M. Babesiosis Vaccines: Lessons Learned, Challenges Ahead, and Future Glimpses. Trends Parasitol. 2019, 35, 622–635.
- Jalovecka, M.; Sojka, D.; Ascencio, M.; Schnittger, L. Babesia Life Cycle—When Phylogeny Meets Biology. Trends Parasitol. 2019, 35, 356–368.
- Jalovecka, M.; Bonsergent, C.; Hajdusek, O.; Kopacek, P.; Malandrin, L. Stimulation and quantification of Babesia divergens gametocytogenesis. Parasites Vectors 2016, 9, 439.
- Moreno, J.M. Evaluación Clínica e Inmunológica de una Vacuna Anabasan Limor Contra Hemoparásitos Bovinos; Universidad de La Salle: Bogotá, Colombia, 1998.
- Vacuna Contra la Anaplasmosis y la Babesiosis Bovina. Available online: https://www.argentina.gob.ar/inta/tecnologias/vacuna-contra-la-anaplasmosis-y-la-babesiosis-bovina-0 (accessed on 24 November 2022).
- Florin-Christensen, M.; Suarez, C.E.; Rodriguez, A.E.; Flores, D.A.; Schnittger, L. Vaccines against bovine babesiosis: Where we are now and possible roads ahead. Parasitology 2014, 141, 1563–1592.
- Shkap, V.; de Vos, A.J.; Zweygarth, E.; Jongejan, F. Attenuated vaccines for tropical theileriosis, babesiosis and heartwater: The continuing necessity. Trends Parasitol. 2007, 23, 420–426.
- Alvarez, J.A.; Rojas, C.; Figueroa, J.V. An Overview of Current Knowledge on in vitro Babesia Cultivation for Production of Live Attenuated Vaccines for Bovine Babesiosis in Mexico. Front. Vet. Sci. 2020, 7, 364.
- Connoway, J.W.; Francines, M. Texas fever: Experiments made by the Texas Experiment Station in immunizing Northern breeding cattle against Texas fever. In Station; Texas Agricultural Experiment Station; J.J. Pastoriza Printing & Litho. Co.: Houston, TX, USA, 1902; pp. 56–106.
- Callow, L.L.; Mellors, L.T.; McGregor, W. Reduction in virulence of Babesia bovis due to rapid passage in splenectomized cattle. Int. J. Parasitol. 1979, 9, 333–338.
- Callow, L.A.; Dalgliesh, R. The development of effective, safe vaccination against babesiosis and anaplasmosis in Australia. In Proceedings of the 56th Annual Conference of the Australian Veterinary Association, Townsville, Australia, 14–18 May 1979.
- Yunker, C.E.; Kuttler, K.L.; Johnson, L.W. Attenuation of Babesia bovis by in vitro cultivation. Vet. Parasitol. 1987, 24, 7–13.
- Mangold, A.J.; Vanzini, V.R.; Echaide, I.E.; de Echaide, S.T.; Volpogni, M.M.; Guglielmone, A.A. Viability after thawing and dilution of simultaneously cryopreserved vaccinal Babesia bovis and Babesia bigemina strains cultured in vitro. Vet. Parasitol. 1996, 61, 345–348.
- Alarcón, J.; Alvaréz, J.; Ramírez, E.; Aragón, J.; Gualito, J. Protection against bovine babesiosis with a mixed vaccine of Babesia bovis and Babesia bigemina derived from in vitro culture under field confrontation. Immun. Dis. Free. Area 2003, 34, 11.
- Rojas, C.; Figueroa, J.V.; Alvarado, A.; Mejia, P.; Mosqueda, J.J.; Falcon, A.; Vega, C.A.; Alvarez, A. Bovine babesiosis live vaccine production: Use of gamma irradiation on the substrate. Ann. N. Y. Acad. Sci. 2006, 1081, 405–416.
- Fish, L.; Leibovich, B.; Krigel, Y.; McElwain, T.; Shkap, V. Vaccination of cattle against B. bovis infection with live attenuated parasites and non-viable immunogens. Vaccine 2008, 26 (Suppl. S6), G29–G33.
- Brown, W.C.; Palmer, G.H. Designing blood-stage vaccines against Babesia bovis and B. bigemina. Parasitol. Today 1999, 15, 275–281.
- Brown, W.C.; Norimine, J.; Goff, W.L.; Suarez, C.E.; McElwain, T.F. Prospects for recombinant vaccines against Babesia bovis and related parasites. Parasite Immunol. 2006, 28, 315–327.
- Nascimento, I.P.; Leite, L.C. Recombinant vaccines and the development of new vaccine strategies. Braz. J. Med. Biol. Res. 2012, 45, 1102–1111.
- Brown, W.C.; McElwain, T.F.; Ruef, B.J.; Suarez, C.E.; Shkap, V.; Chitko-McKown, C.G.; Tuo, W.; Rice-Ficht, A.C.; Palmer, G.H. Babesia bovis rhoptry-associated protein 1 is immunodominant for T helper cells of immune cattle and contains T-cell epitopes conserved among geographically distant B. bovis strains. Infect. Immun. 1996, 64, 3341–3350.
- Norimine, J.; Suarez, C.E.; McElwain, T.F.; Florin-Christensen, M.; Brown, W.C. Immunodominant epitopes in Babesia bovis rhoptry-associated protein 1 that elicit memory CD4(+)-T-lymphocyte responses in B. bovis-immune individuals are located in the amino-terminal domain. Infect. Immun. 2002, 70, 2039–2048.
- Brown, W.C.; McElwain, T.F.; Palmer, G.H.; Chantler, S.E.; Estes, D.M. Bovine CD4(+) T-lymphocyte clones specific for rhoptry-associated protein 1 of Babesia bigemina stimulate enhanced immunoglobulin G1 (IgG1) and IgG2 synthesis. Infect. Immun. 1999, 67, 155–164.
- Norimine, J.; Mosqueda, J.; Suarez, C.; Palmer, G.H.; McElwain, T.F.; Mbassa, G.; Brown, W.C. Stimulation of T-helper cell gamma interferon and immunoglobulin G responses specific for Babesia bovis rhoptry-associated protein 1 (RAP-1) or a RAP-1 protein lacking the carboxy-terminal repeat region is insufficient to provide protective immunity against virulent B. bovis challenge. Infect. Immun. 2003, 71, 5021–5032.
- Hines, S.A.; Palmer, G.H.; Jasmer, D.P.; McGuire, T.C.; McElwain, T.F. Neutralization-sensitive merozoite surface antigens of Babesia bovis encoded by members of a polymorphic gene family. Mol. Biochem. Parasitol. 1992, 55, 85–94.
- Hines, S.A.; Palmer, G.H.; Jasmer, D.P.; Goff, W.L.; McElwain, T.F. Immunization of cattle with recombinant Babesia bovis merozoite surface antigen-1. Infect. Immun. 1995, 63, 349–352.
- Saul, A. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 1987, 9, 1–9.
- Terkawi, M.A.; Ratthanophart, J.; Salama, A.; AbouLaila, M.; Asada, M.; Ueno, A.; Alhasan, H.; Guswanto, A.; Masatani, T.; Yokoyama, N.; et al. Molecular characterization of a new Babesia bovis thrombospondin-related anonymous protein (BbTRAP2). PLoS ONE 2013, 8, e83305.
- Jaramillo Ortiz, J.M.; Del Medico Zajac, M.P.; Zanetti, F.A.; Molinari, M.P.; Gravisaco, M.J.; Calamante, G.; Wilkowsky, S.E. Vaccine strategies against Babesia bovis based on prime-boost immunizations in mice with modified vaccinia Ankara vector and recombinant proteins. Vaccine 2014, 32, 4625–4632.
- Gomez, C.E.; Najera, J.L.; Krupa, M.; Esteban, M. The poxvirus vectors MVA and NYVAC as gene delivery systems for vaccination against infectious diseases and cancer. Curr. Gene Ther. 2008, 8, 97–120.
- Francis, M.J. Recent Advances in Vaccine Technologies. Vet. Clin. N. Am. Small Anim. Pract. 2018, 48, 231–241.
- Hidalgo-Ruiz, M.; Mejia-Lopez, S.; Perez-Serrano, R.M.; Zaldivar-Lelo de Larrea, G.; Ganzinelli, S.; Florin-Christensen, M.; Suarez, C.E.; Hernandez-Ortiz, R.; Mercado-Uriostegui, M.A.; Rodriguez-Torres, A.; et al. Babesia bovis AMA-1, MSA-2c and RAP-1 contain conserved B and T-cell epitopes, which generate neutralizing antibodies and a long-lasting Th1 immune response in vaccinated cattle. Vaccine 2022, 40, 1108–1115.
- Rittipornlertrak, A.; Nambooppha, B.; Muenthaisong, A.; Punyapornwithaya, V.; Tiwananthagorn, S.; Chung, Y.T.; Tuvshintulga, B.; Sivakumar, T.; Yokoyama, N.; Sthitmatee, N. Structural and immunological characterization of an epitope within the PAN motif of ectodomain I in Babesia bovis apical membrane antigen 1 for vaccine development. PeerJ 2021, 9, e11765.
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018.
- Dayananda, K.K.; Achur, R.N.; Gowda, D.C. Epidemiology, drug resistance, and pathophysiology of Plasmodium vivax malaria. J. Vector Borne Dis. 2018, 55, 1–8.
- Patarroyo, M.E.; Arevalo-Pinzon, G.; Reyes, C.; Moreno-Vranich, A.; Patarroyo, M.A. Malaria Parasite Survival Depends on Conserved Binding Peptides’ Critical Biological Functions. Curr. Issues Mol. Biol. 2016, 18, 57–78.
- Patarroyo, M.A.; Arevalo-Pinzon, G.; Moreno-Perez, D.A. From a basic to a functional approach for developing a blood stage vaccine against Plasmodium vivax. Expert Rev. Vaccines 2020, 19, 195–207.
- Garzon-Ospina, D.; Forero-Rodriguez, J.; Patarroyo, M.A. Inferring natural selection signals in Plasmodium vivax-encoded proteins having a potential role in merozoite invasion. Infect. Genet. Evol. 2015, 33, 182–188.
- Patarroyo, M.E.; Bermudez, A.; Patarroyo, M.A. Structural and immunological principles leading to chemically synthesized, multiantigenic, multistage, minimal subunit-based vaccine development. Chem. Rev. 2011, 111, 3459–3507.
More
Information
Subjects:
Veterinary Sciences; Parasitology; Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
874
Revisions:
3 times
(View History)
Update Date:
17 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




