
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aurelia Scarano | -- | 2883 | 2023-03-16 10:19:35 | | | |
| 2 | Rita Xu | -4 word(s) | 2879 | 2023-03-16 10:33:20 | | |
Video Upload Options
Many studies have widely examined the effects of dietary polyphenols on human health. Polyphenols are well known for their antioxidant properties and for their chelating abilities, by which they can be potentially employed in cases of pathological conditions, such as iron overload. The iron-binding abilities of dietary polyphenols can be important in inflammatory/immunomodulatory responses, especially involving macrophages and dendritic cells, and they also might contribute to reshape the gut microbiota into a healthy profile. As the axes “polyphenol–iron metabolism–inflammatory responses” and “polyphenol–iron availability–gut microbiota” have not been very well explored so far, there is the need for further investigation to exploit such a potential to prevent or counteract pathological conditions.
1. Introduction
2. Anti/Pro-Oxidant Activities of Polyphenols
3. Iron-Chelating Abilities of Polyphenols
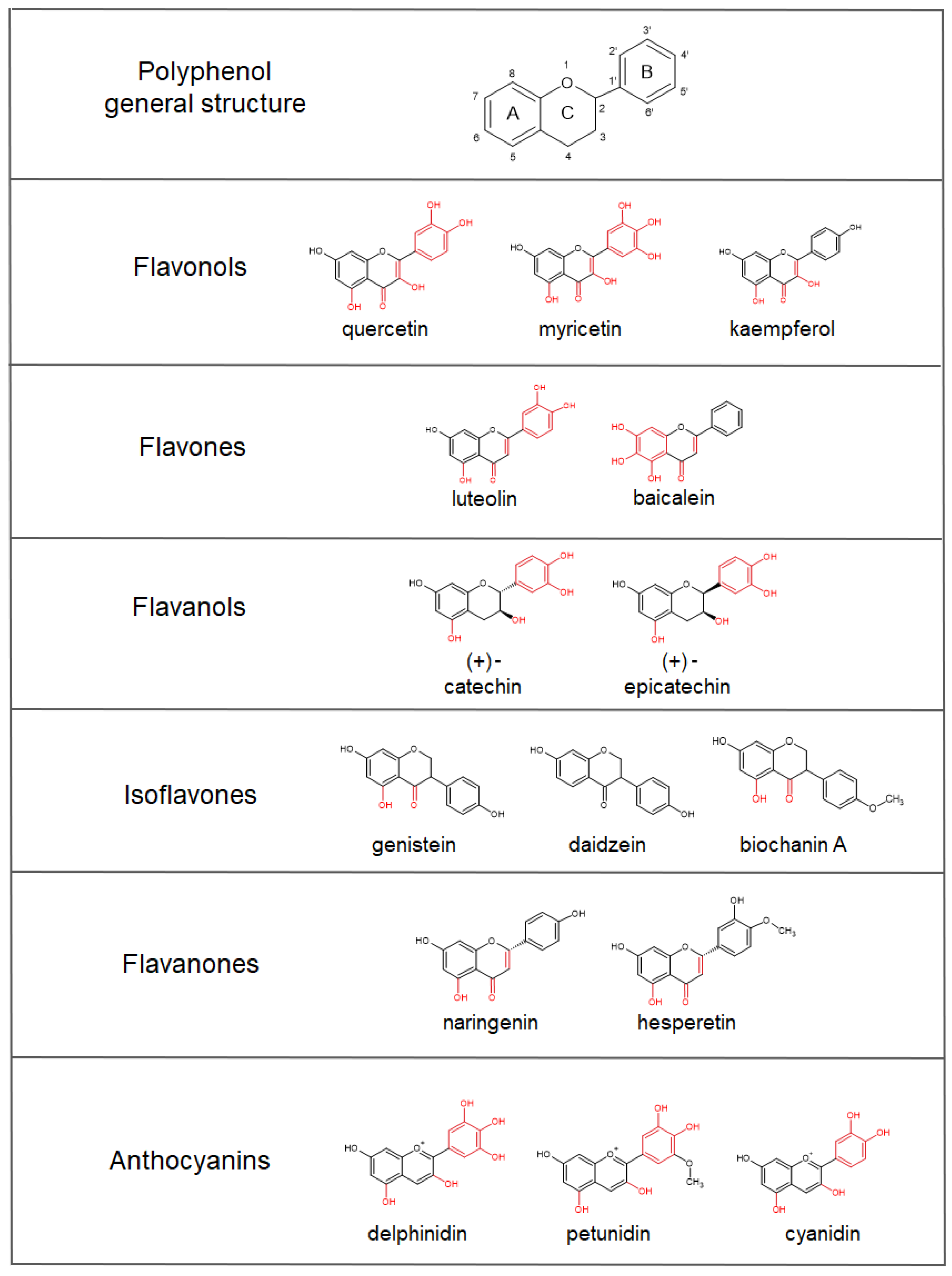
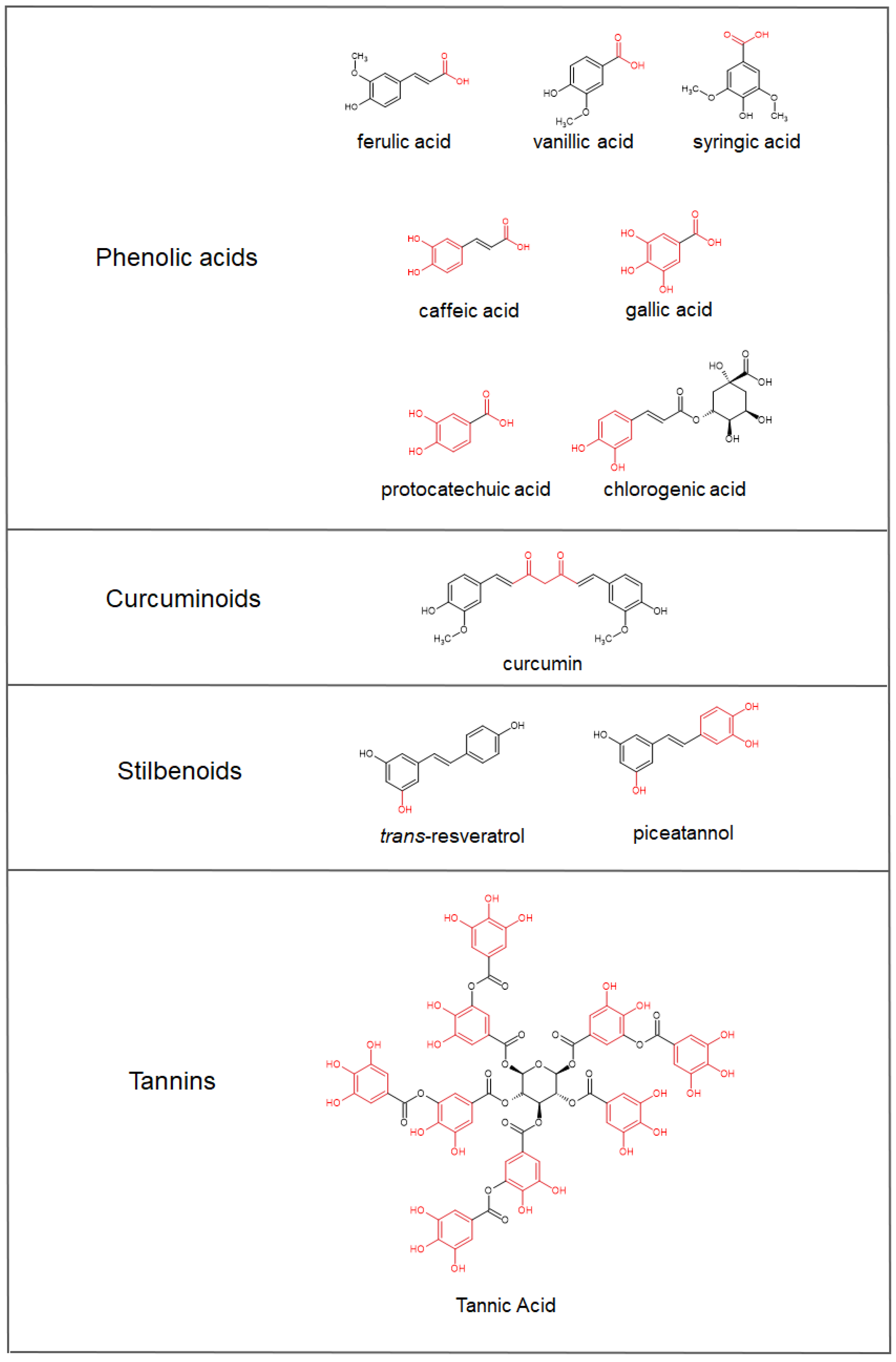
4. Polyphenols’ Bioavailability
| Polyphenol Compound—Food Source | Dosage | Cmax 1 of the Main Compounds in Plasma (tmax 2) | Main Compounds Excreted in Urine (Time) | Reference |
|---|---|---|---|---|
| Quercetin-4’-O-glucoside | 100 mg | 2.12 µg·mL−1 of quercetin-4’-O-glucoside (0.70 h) | NA 3 | [80] |
| Quercetin-3-O-rutinoside | 200 mg | 0.32 µg·mL−1 of quercetin-3-O-rutinoside (6.98 h) | NA | [80] |
| Fried onions (containing 275 µmol flavonols, principally quercetin-4’-glucoside and quercetin-3,4’-diglucoside) | 270 g | 665 nM of quercetin-3’-sulfate (0.75 h) 351 nM of quercetin-3-glucuronide (0.60 h) 63 nM of quercetin diglucuronide (0.80 h) |
274 nM quercetin diglucuronide (8–24 h) | [81] |
| Fresh blackcurrant (containing 897 mg total anthocyanins) | 100 g | Anthocyanins not detected |
339 µg total anthocyanins (48 h) 17.3 mg hippuric acid (0–24 h) |
[70] |
| Elderberry concentrate (containing 1.9 g anthocyanins and equivalent to 235 mL fresh juice) | 11 g | NA | 15.8 µg cyanidin-3-glucoside (1 h) 29.8 µg cyanidin-3-sambubioside (2 h) |
[82] |
| Homogenized raspberries (containing 292 µmol anthocyanins, 6.3 µmol ellagic acids, 251 µmol ellagitannins, and 2.5 µmol phenolic acids) | 300 g | 180 nmol·L−1 of 3’,4’-dihydroxyphenylaceticacid (6 h) 78 nmol·L−1 of 4’-Hydroxyhippuricacid (1 h) 47 nmol·L−1 of ferulic acid-4′-sulfate (1.5 h) 18 nmol·L−1 of ferulic acid-4′-O-glucuronide (1.5 h) 14 nmol of isoferulic acid-3’-O-glucuronide (1.5 h) |
19.9 nmol cyanidin-3-O-glucoside (0–48 h) 6.4 nmol 4-Hydroxybenzoic acid (0–48 h) 6.5 nmol ferulic acid-4-sulfate (0–48 h) 16.1 nmol 4-hydroxyhippuricacid (0–48 h) 239 nmol hippuric acid (0–48 h) |
[83] |
| Evelor 500 mg tablets (containing trans-resveratrol) | 500 g | 71.18 mg·mL−1 of trans-resveratrol (1.339 h) 1516.014 mg·mL−1 of sulfated resveratrol 4083.900 mg·mL−1 of glucuronated resveratrol |
NA | [84] |
| Coffee beverage, containing various doses of chlorogenic acid: 412 µmol (A) 635 µmol (B) 795 µmol (C) |
200 mL | 808 µmol of total chlorogenic acid derivatives (0.5–6 h) (A) 1242 µmol of total chlorogenic acid derivatives (0.5–6 h) (B) 1164 µmol of total chlorogenic acid derivatives (0.5–6 h) (C) |
100.7 µmol of total chlorogenic acid derivatives (0–24 h) (A) 160.0 µmol of total chlorogenic acid derivatives (0–24 h) (B) 125.2 µmol of total chlorogenic acid derivatives (0–24 h) (C) |
[85] |
| Curcuminoids as native powder, micronized powder, or liquid micelles (containing 410 mg curcumin, 80 mg demethoxycurcumin, and 10 mg bis-demethoxycurcumin) |
500 mg | 7.1 nmol curcumin (7.5 h) (native powder) 41.6 nmol curcumin (8.8 h) (micronized powder) 3228 nmol curcumin (1.1 h) (liquid micelles) |
5.1 nmol 4 curcumin (0–24 h) (native powder) 70.6 nmol 4 curcumin (0–24 h) (micronized powder) 753 nmol 4 curcumin (0–24 h) (liquid micelles) |
[86] |
1 Cmax: maximal plasma concentration. 2 tmax: time to reach Cmax. 3 NA: not available. 4 data expressed as nmol·g−1 creatinine.
References
- Scarano, A.; Chieppa, M.; Santino, A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 2018, 7, 98.
- Scarano, A.; Chieppa, M.; Santino, A. Plant polyphenols-biofortified foods as a novel tool for the prevention of human gut diseases. Antioxidants 2020, 9, 1225.
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246.
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142.
- Speer, H.; D’Cunha, N.M.; Botek, M.; McKune, A.J.; Sergi, D.; Georgousopoulou, E.; Mellor, D.D.; Naumovski, N. The effects of dietary polyphenols on circulating cardiovascular disease biomarkers and iron status: A systematic review. Nutr. Metab. Insight 2019, 12, 1–12.
- Koch, W. Dietary Polyphenols-Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039.
- De Santis, S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2021, 61, 1804–1826.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
- Delvecchio, F.R.; Vadrucci, E.; Cavalcanti, E.; De Santis, S.; Kunde, D.; Vacca, M.; Myers, J.; Allen, F.; Bianco, G.; Huang, A.Y.; et al. Polyphenol administration impairs T-cell proliferation by imprinting a distinct dendritic cell maturational profile. Eur. J. Immunol. 2015, 45, 2638–2649.
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Daheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728.
- Patra, S.; Pradha, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554.
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711.
- Hare, D.; Ayton, S.; Bush, A.; Lei, P. A delicate balance: Iron metabolism and diseases of the brain. Front. Aging Neurosci. 2013, 5, 34.
- Fan, F.S. Iron deficiency anemia due to excessive green tea drinking. Clin. Case Rep. 2016, 4, 1053–1056.
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A review on iron chelators in treatment of iron overload syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247.
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189.
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624.
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028.
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750.
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell Longev. 2016, 2016, 7432797.
- de Graft-Johnson, J.; Nowak, D. Effect of selected plant phenolics on Fe2+-EDTA-H2O2 system mediated deoxyribose oxidation: Molecular structure-derived relationships of anti- and pro-oxidant actions. Molecules 2017, 22, 59.
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin: Effects on lipid peroxidation, hydroxyl radical generation and bleomycin-dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865.
- Makácová, K.; Mladěnka, P.; Filipský, T.; Říha, M.; Jahodár, L.; Trejtnar, F.; Bovicelli, P.; Proietti Silvestri, I.; Hrdina, R.; Saso, L. Iron reduction potentiates hydroxyl radical formation only in flavonols. Food Chem. 2012, 135, 2584–2592.
- Yen, G.C.; Duh, P.D.; Tsai, H.L.; Huang, S.L. Pro-oxidative properties of flavonoids in human lymphocytes. Biosci. Biotechnol. Biochem. 2003, 67, 1215–1222.
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flvaonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584.
- Hodnick, W.F.; Kung, F.S.; Bohmont, C.W.; Pardini, R.S. Inhibition of mitochondrial respiration and production of toxic oxygen radicals by flavonoids. A structure-activity study. Biochem. Pharmacol. 1986, 35, 2345–2357.
- Procházková, D.; Boušová, I.; Wilhemová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523.
- Puppo, A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry 1992, 31, 85–88.
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870.
- Inoue, S.; Ito, K.; Yamamoto, K.; Kawanishi, S. Caffeic acid causes metal-dependent damage to cellular and isolated DNA through H2O2 formation. Carcinogenesis 1992, 13, 1497–1502.
- Dalvi, L.T.; Moreira, D.C.; Andrade Jr, R.; Ginani, J.; Alonso, A.; Hermes-Lima, M. Ellagic acid inhibits iron-mediated free radical formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 910–917.
- Bradshaw, P.C. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients 2019, 11, 504.
- Marì, M.; de Gregorio, E.; de Dios, E.; Roca-Agujetas, V.; Cucarull, B.; Tutusaus, A.; Morales, A.; Colell, A. Mitochondrial glutathione: Recent insights and role in disease. Antioxidants 2020, 9, 909.
- Zhitkovich, A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526.
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100.
- Romano, G.; Del Coco, L.; Milano, F.; Durante, M.; Palombieri, S.; Sestili, F.; Visioni, A.; Abderrazek, J.; Fanizzi, F.P.; Laddomada, B. Phytochemical Profiling and Untargeted Metabolite Fingerprinting of the MEDWHEALTH Wheat, Barley and Lentil Whole-Meal Flours. Foods 2022, 11, 4070.
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50.
- Henle, E.S.; Han, Z.; Tang, N.; Rai, P.; Luo, Y.; Linn, S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reaction has possible biological implications. J. Biol. Chem. 1999, 274, 962–971.
- Andjelković, M.; Camp, J.V.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 2006, 98, 23–31.
- Braakhuis, A. Evidence on the health benefits of supplemental propolis. Nutrients 2019, 11, 2705.
- Wang, X.; Li, Y.; Han, L.; Li, J.; Liu, C.; Sun, C. Role of flavonoids in the treatment of iron overload. Front. Cell Dev. Biol. 2021, 9, 685364.
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877.
- Borges, A.; Abreu, A.; Dias, C.; Saavedra, M.; Borges, F.; Simões, M. New perspectives on the use of phytochemicals as an emergent strategy to control bacterial infections including biofilms. Molecules 2016, 21, 877.
- Khalili, M.; Ebrahimzadeh, M.; Kosaryan, M. In vivo iron-chelating activity and phenolic profiles of the Angel’s wings mushroom, Pleurotus porrigens (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 847–856.
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111.
- Galano, A.; Marquez, M.F.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918.
- Alfei, S.; Marengo, B.; Zuccari, G. Oxidative stress, antioxidant capabilities and bioavailability: Ellagic acid or urolithins? Antioxidants 2020, 9, 707.
- Messner, D.J.; Sivam, G.; Kowdley, K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009, 29, 63–72.
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150.
- Jiao, Y.; Wilkinson IV, J.; Pietsch, E.C.; Buss, J.L.; Wan, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160.
- Jiao, Y.; Wilkinson IV, J.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino Jr, R.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469.
- Karlíčková, J.; Macáková, K.; Říha, M.; Teixeira Pinheiro, L.M.; Filipský, T.; Horňasová, V.; Hrdina, R.; Mladěnka, P. Isoflavones reduce copper with minimal impact on iron in vitro. Oxid. Med. Cell Longev. 2015, 2015, 437381.
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098.
- Lakey-Beitia, J.; Burillo, A.M.; La Penna, G.; Hedge, M.L.; Rao, K.S. Polyphenols as potential metal chelation compounds against Alzeimer’s disease. J. Alzheimer’s Dis. 2021, 82, S335–S357.
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. BioMetals 2000, 13, 77–83.
- Perron, N.R.; Hodges, J.N.; Jenkins, M.; Brumaghim, J.L. Predicting how polyphenol antioxidants prevent DNA damage by binding to iron. Inorg. Chem. 2008, 47, 6153–6161.
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A review on coordination properties of Al(III) and Fe(III) toward natural antioxidant molecules: Experimental and theoretical insights. Molecules 2021, 26, 2603.
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168.
- Fiuza, S.M.; Van Besien, E.; Milhazes, N.; Borges, F.; Marques, M.P.M. Conformational analysis of a trihydroxylated derivative of cinnamic acid—A combined Raman spectroscopy and ab initio study. J. Mol. Struct. 2004, 693, 103–118.
- Mladěnka, P.; Macáková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701.
- McGhie, T.K.; Walton, M.C. the bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007, 51, 702–713.
- Scarano, A.; Chieppa, M.; Santino, A. 2022. Microbiota as a metabolic organ processing dietary polyphenols. In Comprehensive Gut Microbiota; Glibetic, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 20–26.
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 95215.
- Hussain, M.B.; Hassan, S.; Waheed, M.; Javed, A.; Farooq, M.A.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; InTechOpen: London, UK, 2019.
- Lomozová, Z.; Catapano, M.C.; Hrubša, M.; Karlíčkova, J.; Macáková, K.; Kučera, R.; Mladěnka, P. Chelation of iron and copper by quercetin B-ring methyl metabolites, isorhamnetin and tamarixetin, and their effect on metal-based Fenton chemistry. J. Agric. Food Chem. 2021, 69, 5926–5937.
- Saito, A.; Sugisawa, A.; Umegaki, K.; Sunagawa, H. Protective effects of quercetin and its metabolites on H2O2-induced chromosomal damage to WIL2-NS cells. Biosci. Biotechnol. Biochem. 2004, 68, 271–276.
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468.
- Catalkava, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Capanoglu, E. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133.
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives. Food Front. 2020, 1, 420–434.
- Holland, W.; Brett, G.M.; Radreau, P.; Saha, S.; Teucher, B.; Bennet, R.; Kroon, P. Processing blackcurrants dramatically reduces the content and does not enhance urinary yield of anthocyanins in human subjects. Food Chem. 2008, 108, 869–878.
- Li, H.; Li, M.; Fu, J.; Ao, H.; Wang, W.; Wang, X. Enhancement of oral bioavailability of quercetin by metabolic inhibitory nanosuspensions compared to conventional nanosuspensions. Drug Deliv. 2021, 28, 1226–1236.
- Kasikci, M.B.; Bagdatlioglu, N. Bioavailability of quercetin. Curr. Res. Nutr. Food Sci. 2016, 4, 146–151.
- Crespy, V.; Morand, C.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999, 277, G120–G126.
- Matsukawa, N.; Matsumoto, M.; Hara, H. High biliary excretion levels of quercetin metabolites after administration of a quercetin glycoside in conscious bile duct cannulated rats. Biosci. Biotechnol. Biochem. 2009, 73, 1863–1865.
- Lee, J.H.; Oh, J.-H.; Lee, Y.-J. Biliary excretion of curcumin is mediated by multidrug resistance-associated protein 2. Biol. Pharm. Bull. 2012, 35, 777–780.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent development in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18.
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE 2012, 7, e29647.
- Shen, C.; Chen, R.; Qian, Z.; Meng, X.; Hu, T.; Li, Y.; Chen, Z.; Huang, C.; Hu, C.; Li, J. Intestinal absorption mechanisms of MTBH, a novel hesperetin derivative, in Caco-2 cells, and potential involvement of monocarboxylate transporter 1 and multidrug resistance protein 2. Eur. J. Pharm. Sci. 2015, 78, 214–224.
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Merillon, J.-M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005, 53, 798–803.
- Graefe, E.U.; Witting, J.; Pharm, D.; Mueller, S.; Riethling, A.-K.; Uehleke, B.; Drewelow, B.; Pforte, H.; Jacobasch, G.; Derendorf, H. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001, 41, 492–499.
- Mullen, W.; Edwards, C.A.; Crozier, A. Absorption, excretion and metabolite profiling of methyl-, glucuronyl-, glucosyl- and sulpho-conjugates of quercetin in human plasma and urine after ingestion of onions. Br. J. Nutr. 2006, 96, 107–116.
- Mülleder, U.; Murkovic, M.; Pfannhauser, W. Urinary excretion of cyanidin glycosides. J. Biochem. Biophys. Methods 2002, 53, 61–66.
- Ludwig, I.A.; Mena, P.; Calani, L.; Borges, G.; Pereira-Caro, G.; Bresciani, L.; Del Rio, D.; Lean, M.E.; Crozier, A. New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic. Biol. Med. 2015, 89, 758–769.
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170.
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737.
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527.





