Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Natacha Entz-Werle | -- | 1451 | 2023-03-15 17:41:43 | | | |
| 2 | Conner Chen | Meta information modification | 1451 | 2023-03-16 07:03:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lhermitte, B.; Wolf, T.; Chenard, M.P.; Coca, A.; Todeschi, J.; Proust, F.; Hirsch, E.; Schott, R.; Noel, G.; Guerin, E.; et al. Glial Tumor Types Are Associated to BRAF Mutations. Encyclopedia. Available online: https://encyclopedia.pub/entry/42239 (accessed on 08 February 2026).
Lhermitte B, Wolf T, Chenard MP, Coca A, Todeschi J, Proust F, et al. Glial Tumor Types Are Associated to BRAF Mutations. Encyclopedia. Available at: https://encyclopedia.pub/entry/42239. Accessed February 08, 2026.
Lhermitte, Benoit, Thibaut Wolf, Marie Pierre Chenard, Andres Coca, Julien Todeschi, François Proust, Edouard Hirsch, Roland Schott, Georges Noel, Eric Guerin, et al. "Glial Tumor Types Are Associated to BRAF Mutations" Encyclopedia, https://encyclopedia.pub/entry/42239 (accessed February 08, 2026).
Lhermitte, B., Wolf, T., Chenard, M.P., Coca, A., Todeschi, J., Proust, F., Hirsch, E., Schott, R., Noel, G., Guerin, E., Reita, D., Chammas, A., Salmon, A., Martin, S., Dontenwill, M., & Entz-Werlé, N. (2023, March 15). Glial Tumor Types Are Associated to BRAF Mutations. In Encyclopedia. https://encyclopedia.pub/entry/42239
Lhermitte, Benoit, et al. "Glial Tumor Types Are Associated to BRAF Mutations." Encyclopedia. Web. 15 March, 2023.
Copy Citation
Drugs targeting activating BRAF mutations have transformed the prognosis and treatment of MAPK-pathway-induced cancers. In neuro-oncology, the better knowledge of the MAPK pathway’s involvement in gliomagenesis offers hope in a subset of brain cancers where conventional therapies have produced disappointing results. The temptation to use BRAF inhibitors alone or in combination in cerebral mutant tumors is high and is providing survival benefit in trials.
MAPK-induced gliomas
BRAF p.V600E mutation
oncogene-induced senescence
1. Introduction
In the last few decades, glioma stratification for patient diagnosis and management has impressively evolved [1]. The greater understanding of their biology has led to a new histo-molecular classification, going beyond tumor morphology, and subsequently improved accurate diagnostic procedures and targeted treatments [1][2]. In the heterogeneous group of gliomas, the deregulation of the MAPK pathway is frequently evidenced in pediatric low-grade gliomas (PLGGs), but also in rarer adult and pediatric high-grade gliomas (HGGs). Transducing the signal from the cell membrane to the nucleus, this molecular signaling encompasses proteins, whose paired genes are frequently mutated or fused in gliomas. In fact, the physiological activation of the MAPK pathway results from the ligand-dependent stimulation of tyrosine-kinase transmembrane receptors (TKR), which belong mostly to the HER (such as EGFR, FGFR or PDGFR families). The receptor homo- or heterodimerization leads then to downstream cascade phosphorylation and activation, involving RAS, RAF kinase, MEK1/2 and, finally, ERK. Activated ERK proteins translocate to the nucleus, where they phosphorylate and regulate various transcription factors, promoting changes in gene expression. This signal transduction contributes to the regulation of normal cellular processes [3], such as proliferation, differentiation, survival, or senescence [4][5]. In gliomagenesis, the MAPK pathway balances cellular pro-tumoral (an increased proliferation and a prolonged cell survival) and anti-tumoral (cell differentiation and a senescence induction) effects. The dual role of MAPK deregulation is inducing tumors that are mostly low-grade gliomas and tend to stay that way unless other genetic alterations occur [6]. MAPK pathway dysregulation is driven by gene fusions or mutations arising in all genes of the cascade, as described in Figure 1 [1]. The two main actionable therapeutic targets are MEK and BRAF activations, providing alternative therapeutic strategies in the case of unsuccessful standard chemotherapies. The recent advances in genomic and transcriptomic fields have supplied larger information about their specific abnormalities in gliomas. Nevertheless, little is known about the associated biomarkers involved potentially in gliomagenesis modifications or acceleration and therapeutic resistances along the patient journey with BRAF-altered gliomas.
2. BRAF Mutations
The more frequent established aberrations of the MAPK pathway encountered in gliomas are related to the RAF serine-threonine kinases. Three isoforms of RAF kinases exist and are named A-RAF, B-RAF, and C-RAF. Commonly, they bear the same general structure consisting of an N-terminal regulatory domain that physiologically inhibits the C-terminal kinase domain. This latter domain is activated when RAS binds to the RAF N-terminal end. The predominance of the BRAF-altered tumors is subsequent to B-RAF’s specific role in downstream MEK activation, unlike A-RAF and C-RAF. In addition, B-Raf protein possesses only two kinase activation sites, whereas the two other isoforms have four sites. These protein characteristics explain how it might be easier to dysregulate B-RAF with single point mutations or specific fusions than in the other two protein isoforms [4][6].
In low-grade gliomas (LGGs), two main types of BRAF alterations are described and considered as main drivers. First, a cytogenetic abnormality led to the loss of the N-terminal regulator domain of B-RAF, whereas the C-terminal kinase domain is retained, resulting in a constitutive activation of B-RAF independently from RAS activation. This molecular aberration is a tandem duplication on chromosome 7q34 involving BRAF and a centromeric gene, namely KIAA1549 or, rarely, FAM131B. Other transcripts are more and more frequently being described [1][7]. The fused tumors are specifically and mostly pilocytic astrocytomas (PAs) [8]. The second way to activate the MAPK pathway in gliomas is a BRAF point mutation in its C-terminal domain, consisting generally of a substitution of a valine (V) by a glutamic acid (E) at amino acid 600. The BRAF p.V600E mutation leads to a constant phosphorylation of the threonine in position 599 and the serine in position 602. Subsequently, the B-Raf mutated protein permanently activates MEK and ERK, independently from RAS stimulation [9]. This mutation is a class I BRAF alteration. In contrast to BRAF duplications, BRAFv600e mutation is significantly associated with both low- and high-grade glial histopathologies [1][8][9][10].
In the LGG subtypes, pathological activation of the MAPK pathway may rarely result in mutations or gene fusions occurring in downstream effectors including ROS1, ALK, KRAS, MAP2K1 or NF1 [10], as described in Figure 1. Extremely rare fusions are described with the RAF1 gene in PA [8].
In MAPK-activated HGGs, beyond the BRAFv600 mutants, abnormalities can be observed in TKRs and in KRAS or with induced proliferation throughout CDKN2A deletion. HGGs exhibit other BRAF mutations extremely rarely, considered as class II and III mutations [11][12]. Mostly, A-RAF and C-RAF/RAF1 are overexpressed in HGGs, leading to a more aggressive cell phenotype and a worse patient outcome, but no mutations have so far been diagnosed for those RAF isoforms [13][14]. RAF1 was also known as a fusion partner of ATG7 [8][14][15].
3. Specific Glial Tumor Types Are Associated to BRAF Mutations
In 2016, the WHO (World Health Organization) classification of central nervous tumors became a more complex histo-molecular classification based on molecular markers specifically paired to histological diagnoses. The more recent WHO 2021 classification now includes low- and high-grade entities strictly linked to MAPK pathway activation, listed in Figure 1 [1][2][16].
Roughly, three types are part of the MAPK pathway activate brain tumors: (1) MAPK-pathway-altered diffuse pediatric LGGs; (2) circumscribed astrocytic gliomas, comprising the PAs, the high-grade astrocytoma with piloïd features (HGAP), and the pleomorphic xanthoastrocytomas (PXA); and (3) indolent epileptogenic lesions in the glioneuronal and neuronal categories with the gangliogliomas (GGLs), dysembryoplastic neuroepithelial tumors (DNET), multinodular and vacuolating neuronal tumors (MVNT), and diffuse leptomeningeal glioneuronal tumors (DLGNT).
In the diffuse LGGs, the pediatric-type gliomas are histologically indistinguishable from adult forms, apart from their molecular abnormalities and intra-cerebral locations. They mostly bear a BRAF p.V600E alteration [8][17][18] that might be considered as a specific molecular initiator of gliomagenesis in pediatrics. In fact, adult studies estimate the prevalence of all BRAF mutations at less than 1%, whereas in children and adolescents, rates reach 8 to more than 30% of the cases.
In the group of circumscribed astrocytic gliomas, the more frequent alteration is a fusion involving BRAF gene and, most frequently, a KIAA1549 partner, especially in the frequent PAs and in the rare HGAP entity [1][7][19][20]. PXAs, diagnosed in both pediatric and young-adult settings, frequently behave indolently, and are considered as WHO grade 2 tumors. The cases where mitotic activity is higher (5 mitoses per 10 high-power fields) are defined as grade 3 anaplastic gliomas. Most of them carry a BRAF p.V600E mutation combined with a homozygous CDKN2A deletion (e.g., 65% of the cases). Extremely rarely, PXA are characterized by a RAF1 or C-RAF fusion [15].
The third group encompassing the spectrum of epileptogenic tumors with the most frequent diagnosis is ganglioglioma (GGL). This brain neoplasm is a WHO grade 1 glioneuronal tumor, typically arising in the temporal lobe of children and young adults and following mostly an indolent course. This tumor has nevertheless the rare possibility of anaplastic transformation in grade 3 cancers. Those grade 1 and 3 forms harbor genetic alterations responsible for MAPK pathway activation, where BRAF p.V600E is evidenced in 10 to 60% cases. Rarely, other SNVs in BRAF are described [21][22]. Other epileptogenic indolent tumors are polymorphous low-grade neuroepithelial tumors of the young (PLNTY), DNET, and MVNT, which usually exhibit abnormalities in the MAPK pathway corresponding notably to BRAF mutations.
The high-grade MAPK-pathway-induced gliomas are not considered as a real category in the 2021 WHO classification, but they clearly overlap with the previously described grade 3 PXA or GGL and are mostly enriched in BRAF p.V600E mutated forms [2][22]. The global frequency of this mutation in HGGs is estimated to be 1 to 3% [21][22][23]. A distinct but moving entity is the epithelioid variant of glioblastoma, which highly overlaps with the PXA entity in young adults but presents a better prognosis. In older adults, this variant bears a poor prognosis, as with the IDH-wild-type glioblastomas (GBMs). All epithelioid morphologies seem to be linked with a higher frequency of BRAF p.V600E mutation [24].
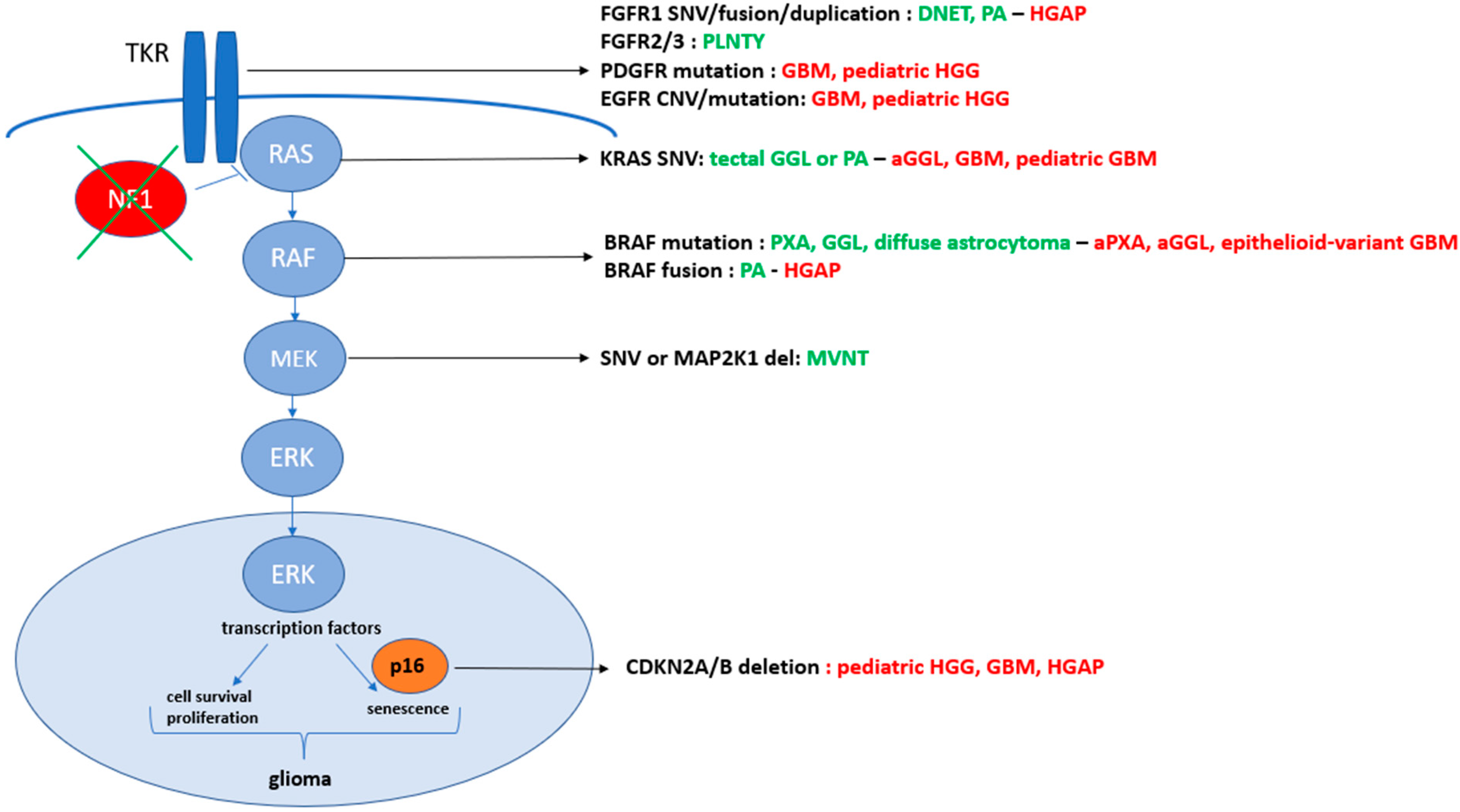
Figure 1. Summary of the molecular abnormalities in the MAPK pathway driving low- and high-grade gliomas. The low-grade entities are listed in green with their more frequent paired molecular aberrations, whereas the high-grade gliomas (HGGs) are described in red and LGG in greed. The alterations are listed in black color. DNET, dysembryoplastic neuroepithelial; PA, pilocytic astrocytoma; HGAP, high-grade astrocytoma with piloïd features; PLNTY, polymorphous low-grade neuroepithelial tumor of the young; GBM, glioblastoma; HGG, high-grade glioma; GGL, ganglioglioma; aGGL, anaplastic GGL; PXA, pleomorphic xanthoastrocytomas; aPXA, anaplastic PXA; MVNT, multinodular and vacuolating neuronal tumors.
References
- WHO. WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; WHO classification of tumours series; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6, Available online: https://publications.iarc.fr/601 (accessed on 2 August 2021).
- De Blank, P.; Fouladi, M.; Huse, J.T. Molecular markers and targeted therapy in pediatric low-grade glioma. J. Neuro-Oncol. 2020, 150, 5–15.
- Ducreux, M.; Chamseddine, A.; Laurent-Puig, P.; Smolenschi, C.; Hollebecque, A.; Dartigues, P.; Samallin, E.; Boige, V.; Malka, D.; Gelli, M. Molecular targeted therapy of BRAF-mutant colorectal cancer. Ther. Adv. Med. Oncol. 2019, 11, 175883591985649.
- Maurer, G.; Tarkowski, B.; Baccarini, M. Raf kinases in cancer–roles and therapeutic opportunities. Oncogene 2011, 30, 3477–3488.
- Jacob, K.; Quang-Khuong, D.-A.; Jones, D.T.; Witt, H.; Lambert, S.; Albrecht, S.; Witt, O.; Vezina, C.; Shirinian, M.; Faury, D.; et al. Genetic Aberrations Leading to MAPK Pathway Activation Mediate Oncogene-Induced Senescence in Sporadic Pilocytic Astrocytomas. Clin. Cancer Res. 2011, 17, 4650–4660.
- Horbinski, C. To BRAF or Not to BRAF: Is That Even a Question Anymore? J. Neuropathol. Exp. Neurol. 2013, 72, 2–7.
- Lind, K.T.; Chatwin, H.V.; DeSisto, J.; Coleman, P.; Sanford, B.; Donson, A.M.; Davies, K.D.; Willard, N.; A Ewing, C.; Knox, A.J.; et al. Novel RAF Fusions in Pediatric Low-Grade Gliomas Demonstrate MAPK Pathway Activation. J. Neuropathol. Exp. Neurol. 2021, 80, 1099–1107.
- Ryall, S.; Zapotocky, M.; Fukuoka, K.; Nobre, L.; Stucklin, A.G.; Bennett, J.; Siddaway, R.; Li, C.; Pajovic, S.; Arnoldo, A.; et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 2020, 7, 569–583.e5.
- Garnett, M.J.; Marais, R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell 2004, 6, 313–319.
- Ellison, D.W.; Hawkins, C.; Jones, D.T.W.; Onar-Thomas, A.; Pfister, S.F.; Reifenberger, G.; Louis, D.N. cIMPACT-NOW update 4: Diffuse gliomas characterized by MYB, MYBL1, or FGFR1 alterations or BRAFV600E mutation. Acta Neuropathol. 2019, 137, 683–687.
- Khater, F.; Langlois, S.; Cassart, P.; Roy, A.-M.; Lajoie, M.; Healy, J.; Richer, C.; St-Onge, P.; Piché, N.; Perreault, S.; et al. Recurrent somatic BRAF insertion (p.V504_R506dup): A tumor marker and a potential therapeutic target in pilocytic astrocytoma. Oncogene 2018, 38, 2994–3002.
- Pratt, D.; Camelo-Piragua, S.; McFadden, K.; Leung, D.; Mody, R.; Chinnaiyan, A.; Koschmann, C.; Venneti, S. BRAF activating mutations involving the beta3-alphaC loop in V600E-negative anaplastic pleomorphic xanthoastrocytoma. Acta Neuropathol. Commun. 2018, 6, 24.
- Hagemann, C.; Gloger, J.; Anacker, J.; Said, H.M.; Gerngras, S.; Kühnel, S.; Meyer, C.; Rapp, U.R.; Kämmerer, U.; Vordermark, D.; et al. RAF expression in human astrocytic tumors. Int. J. Mol. Med. 2009, 23, 17–31.
- Vaubel, R.; Zschernack, V.; Tran, Q.T.; Jenkins, S.; Caron, A.; Milosevic, D.; Smadbeck, J.; Vasmatzis, G.; Kandels, D.; Gnekow, A.; et al. Biology and grading of pleomorphic xanthoastrocytoma—What have we learned about it? Brain Pathol. 2021, 31, 20–32.
- Phillips, J.J.; Gong, H.; Chen, K.; Joseph, N.M.; van Ziffle, J.; Bastian, B.C.; Grenert, J.P.; Kline, C.N.; Mueller, S.; Banerjee, A.; et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019, 29, 85–96.
- Louis, D.N.; Wesseling, P.; Aldape, K.; Brat, D.J.; Capper, D.; Cree, I.A.; Eberhart, C.; Figarella-Branger, D.; Fouladi, M.; Fuller, G.N.; et al. cIMPACT-NOW update 6: New entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020, 30, 844–856.
- Penman, C.L.; Efaulkner, C.; Lowis, S.P.; Kurian, K.M. Current Understanding of BRAF Alterations in Diagnosis, Prognosis, and Therapeutic Targeting in Pediatric Low-Grade Gliomas. Front. Oncol. 2015, 5, 54.
- Kim, Y.-H.; Nonoguchi, N.; Paulus, W.; Brokinkel, B.; Keyvani, K.; Sure, U.; Wrede, K.; Mariani, L.; Giangaspero, F.; Tanaka, Y.; et al. Frequent BRAF Gain in Low-Grade Diffuse Gliomas with 1p/19q Loss. Brain Pathol. 2012, 22, 834–840.
- Reinhardt, A.; Stichel, D.; Schrimpf, D.; Sahm, F.; Korshunov, A.; Reuss, D.E.; Koelsche, C.; Huang, K.; Wefers, A.K.; Hovestadt, V.; et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018, 136, 273–291.
- Capper, D.; Rodriguez, F.J.; Varlet, P.; Jones, D.T.W. High-grade astrocytoma with piloid features. In WHO Classification of Tumours Editorial Board. Central Nervous System Tumours, 5th ed.; WHO classification of tumours series; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6.
- Pekmezci, M.; Villanueva-Meyer, J.E.; Goode, B.; Van Ziffle, J.; Onodera, C.; Grenert, J.P.; Bastian, B.C.; Chamyan, G.; Maher, O.M.; Khatib, Z.; et al. The genetic landscape of ganglioglioma. Acta Neuropathol. Commun. 2018, 6, 47.
- Mistry, M.; Zhukova, N.; Merico, D.; Rakopoulos, P.; Krishnatry, R.; Shago, M.; Stavropoulos, J.; Alon, N.; Pole, J.D.; Ray, P.N.; et al. BRAF Mutation and CDKN2A Deletion Define a Clinically Distinct Subgroup of Childhood Secondary High-Grade Glioma. J. Clin. Oncol. 2015, 33, 1015–1022.
- Andrews, L.J.; Thornton, Z.A.; Saincher, S.S.; Yao, I.Y.; Dawson, S.; McGuinness, L.A.; Jones, H.E.; Jefferies, S.; Jefferies, S.C.; Cheng, H.-Y.; et al. Prevalence of BRAFV600 in glioma and use of BRAF Inhibitors in patients with BRAFV600 mutation-positive glioma: Systematic review. Neuro-Oncol. 2022, 24, 528–540.
- McNulty, S.N.; Schwetye, K.E.; Ferguson, C.; Storer, C.E.; Ansstas, G.; Kim, A.H.; Gutmann, D.H.; Rubin, J.B.; Head, R.D.; Dahiya, S. BRAF mutations may identify a clinically distinct subset of glioblastoma. Sci. Rep. 2021, 11, 19999.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
16 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




