
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgio Ciprandi | -- | 1851 | 2023-03-15 10:15:04 | | | |
| 2 | Jessie Wu | Meta information modification | 1851 | 2023-03-16 03:36:51 | | | | |
| 3 | Jessie Wu | + 2 word(s) | 1853 | 2023-03-16 03:40:11 | | |
Video Upload Options
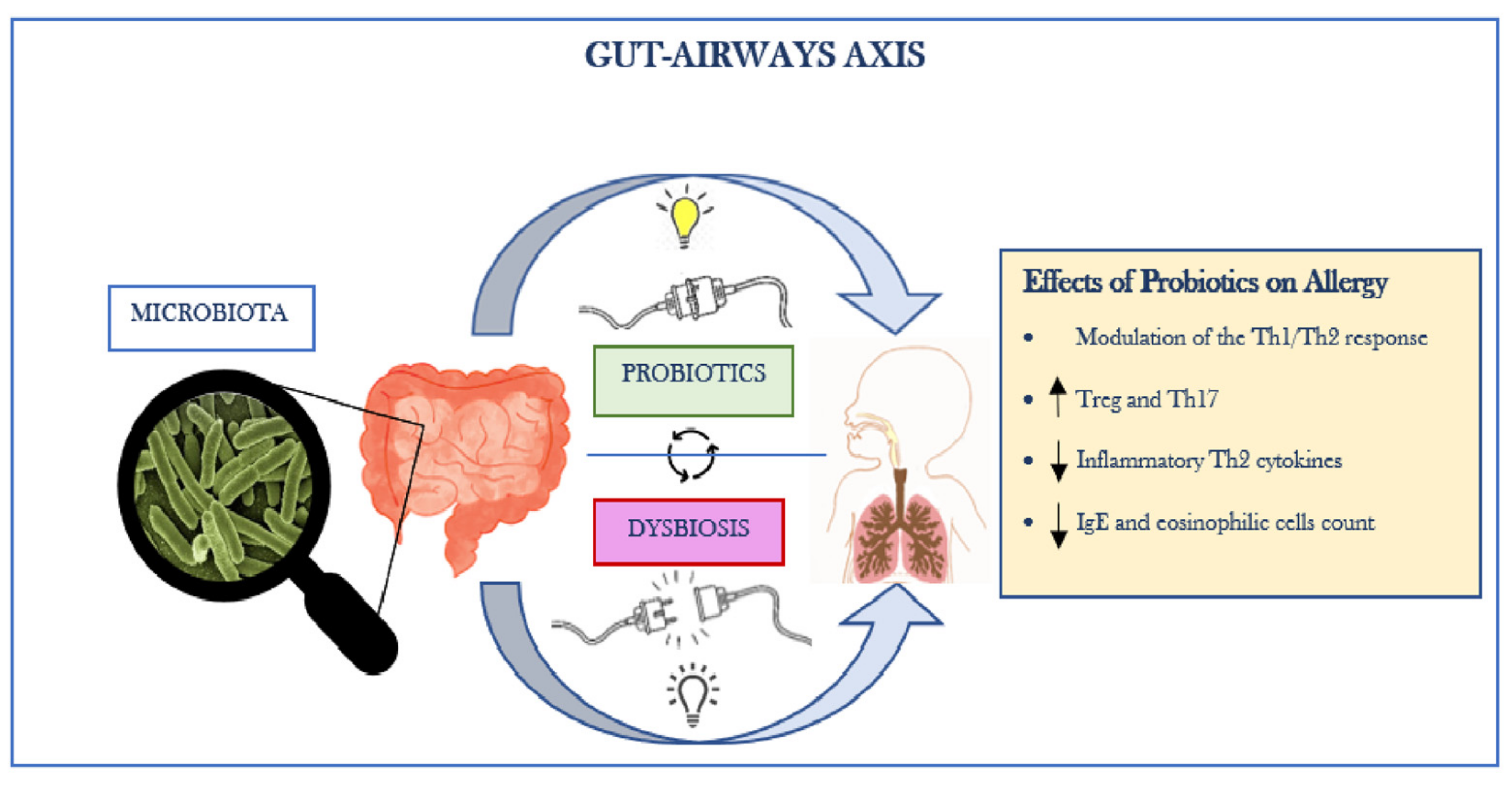
Allergic rhinitis (AR) is a respiratory disease caused by an IgE-mediated inflammatory process mediated by one or more antigens (allergens) against which the subject is sensitized. The most common symptoms are rhinorrhea, sneezing, itching, nasal obstruction, and frequent conjunctivitis. However, drugs used to treat AR may accompany adverse side effects (e.g., dry mouth, drowsiness, dizziness related to anti-H1 drugs). The use of probiotics as an additional option is increasing globally. The consumption of probiotics is expected to modulate immune responses in AR patients, reduce the damage caused by inflammation, and restore a balanced gut microbiota. Gut microbiota is known to function as immunomodulator, barrier, and protective tool against infections. It is constituted of more than a trillion microorganisms reunited in a complex and dynamic ecosystem, regulating the immune system and systemic physiology.
1. Gut–Lung Axis
2. Probiotic Food
3. Probiotics and Allergic Rhinitis: Evidence and Challenges
4. The Role of Probiotics in the Prevention of Allergy during Pregnancy
References
- De Benedictis, F.; del Giudice, M.; Severini, S.; Bonifazi, F. Rhinitis, sinusitis and asthma: One linked airway disease. Paediatr. Respir. Rev. 2001, 2, 358–364.
- Klain, A.; Indolfi, C.; Dinardo, G.; Licari, A.; Cardinale, F.; Caffarelli, C.; Manti, S.; Ricci, G.; Pingitore, G.; Tosca, M.; et al. United Airway Disease. Acta Bio Medica Atenei Parm. 2021, 92 (Suppl 7), 2021526.
- Probiotics in Food Health and Nutritional Properties and Guidelines for Evaluation FAO Food and Nutrition Paper. Available online: https://books.google.rs/books/about/Probiotics_in_Food.html?id=kNxxQgAACAAJ&redir_esc=y (accessed on 13 December 2022).
- Hajavi, J.; Esmaeili, S.; Varasteh, A.; Vazini, H.; Atabati, H.; Mardani, F.; Momtazi-Borojeni, A.A.; Hashemi, M.; Sankian, M.; Sahebkar, A. The immunomodulatory role of probiotics in allergy therapy. J. Cell. Physiol. 2018, 234, 2386–2398.
- Liu, P.; Hu, T.; Kang, C.; Liu, J.; Zhang, J.; Ran, H.; Zeng, X.; Qiu, S. Research Advances in the Treatment of Allergic Rhinitis by Probiotics. J. Asthma Allergy 2022, 15, 1413–1428.
- Farahmandi, K.; Mohr, A.E.; McFarland, L.V. Effects of Probiotics on Allergic Rhinitis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Am. J. Rhinol. Allergy 2022, 36, 440–450.
- Luo, C.; Peng, S.; Li, M.; Ao, X.; Liu, Z. The Efficacy and Safety of Probiotics for Allergic Rhinitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 848279.
- Luoto, R.; Ruuskanen, O.; Waris, M.; Kalliomäki, M.; Salminen, S.; Isolauri, E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2014, 133, 405–413.
- Waki, N.; Matsumoto, M.; Fukui, Y.; Suganuma, H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: An open-label pilot study. Lett. Appl. Microbiol. 2014, 59, 565–571.
- Namba, K.; Hatano, M.; Yaeshima, T.; Takase, M.; Suzuki, K. Effects of Bifidobacterium longum BB536 Administration on Influenza Infection, Influenza Vaccine Antibody Titer, and Cell-Mediated Immunity in the Elderly. Biosci. Biotechnol. Biochem. 2010, 74, 939–945.
- Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples from a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95.
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001.
- Liu, Y.; Kuang, D.; Li, D.; Yang, J.; Yan, J.; Xia, Y.; Zhang, F.; Cao, H. Roles of the gut microbiota in severe SARS-CoV-2 infection. Cytokine Growth Factor Rev. 2022, 63, 98–107.
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471.
- Terpou, A.; Papadaki, A.; Lappa, I.; Kachrimanidou, V.; Bosnea, L.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591.
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691.
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102.
- Sağiroğlu, A.; Özdemir, N.; Çon, A.H. Multifunctional Potentials of Lactic Acid Bacterial Isolates from Turkish Traditional Fermented Foods. Lett. Appl. Microbiol. 2023, 76, 1–14.
- Butler, M.I.; Bastiaanssen, T.F.S.; Long-Smith, C.; Berding, K.; Morkl, S.; Cusack, A.-M.; Strain, C.; Busca, K.; Porteous-Allen, P.; Claesson, M.J.; et al. Recipe for a Healthy Gut: Intake of Unpasteurised Milk Is Associated with Increased Lactobacillus Abundance in the Human Gut Microbiome. Nutrients 2020, 12, 1468.
- Jeon, H.-Y.; Kim, K.-S.; Kim, S. Effects of yogurt containing probiotics on respiratory virus infections: Influenza H1N1 and SARS-CoV-2. J. Dairy Sci. 2023, 106, 1549–1561.
- Brożek, J.L.; Bousquet, J.; Baena-Cagnani, C.E.; Bonini, S.; Canonica, G.W.; Casale, T.B.; van Wijk, R.G.; Ohta, K.; Zuberbier, T.; Schünemann, H.J. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 Revision. J. Allergy Clin. Immunol. 2010, 126, 466–476.
- Steiner, N.C.; Lorentz, A. Probiotic Potential of Lactobacillus Species in Allergic Rhinitis. Int. Arch. Allergy Immunol. 2021, 182, 807–818.
- Yamashita, M.; Matsumoto, K.; Matsumoto, N.; Kobatake, E.; Kabuki, T. Anti-allergic effect of Lactobacillus helveticus SBT2171 on murine model of pollen allergy. Funct. Foods Health Dis. 2019, 9, 166.
- Peng, G.-C.; Hsu, C.-H. The efficacy and safety of heat-killed Lactobacillus paracasei for treatment of perennial allergic rhinitis induced by house-dust mite. Pediatr. Allergy Immunol. 2005, 16, 433–438.
- Haghighat, L.; Crum-Cianflone, N.F. The potential risks of probiotics among HIV-infected persons: Bacteraemia due to Lactobacillus acidophilus and review of the literature. Int. J. STD AIDS 2016, 27, 1223–1230.
- Joshi, S.; Udani, S.; Sen, S.; Kirolikar, S.; Shetty, A. Bacillus Clausii Septicemia in a Pediatric Patient After Treatment with Probiotics. Pediatr. Infect. Dis. J. 2019, 38, e228–e230.
- Vahabnezhad, E.; Mochon, A.B.; Wozniak, L.; Ziring, D.A. Lactobacillus Bacteremia Associated with Probiotic Use in a Pediatric Patient With Ulcerative Colitis. J. Clin. Gastroenterol. 2013, 47, 437–439.
- Fiocchi, A.; Pawankar, R.; Cuello-Garcia, C.; Ahn, K.; Al-Hammadi, S.; Agarwal, A.; Beyer, K.; Burks, W.; Canonica, G.W.; Ebisawa, M.; et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ. J. 2015, 8, 1–4.
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host Remodeling of the Gut Microbiome and Metabolic Changes during Pregnancy. Cell 2012, 150, 470–480.
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253.
- Kuperman, A.A.; Koren, O. Antibiotic use during pregnancy: How bad is it? BMC Med. 2016, 14, 1–7.
- Gomez de Agüero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302.
- Neuman, H.; Koren, O. The Pregnancy Microbiome. Nestle Nutr. Inst. Workshop Ser. 2017, 88, 1–9.
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.-A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; Veyver, I.V.D.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466.
- Lundelin, K.; Poussa, T.; Salminen, S.; Isolauri, E. Long-term safety and efficacy of perinatal probiotic intervention: Evidence from a follow-up study of four randomized, double-blind, placebo-controlled trials. Pediatr. Allergy Immunol. 2016, 28, 170–175.
- Zuccotti, G.V.; Meneghin, F.; Aceti, A.; Barone, G.; Callegari, M.L.; Di Mauro, A.; Fantini, M.P.; Gori, D.; Indrio, F.; Maggio, L.; et al. Probiotics for prevention of atopic diseases in infants: Systematic review and meta-analysis. Allergy 2015, 70, 1356–1371.
- Bertelsen, R.J.; Brantsæter, A.L.; Magnus, M.C.; Haugen, M.; Myhre, R.; Jacobsson, B.; Longnecker, M.P.; Meltzer, H.M.; London, S.J. Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J. Allergy Clin. Immunol. 2013, 133, 165–171.e8.
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260.
- Arango, L.F.G.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics and Pregnancy. Curr. Diabetes Rep. 2014, 15, 1–9.
- Sohn, K.; Underwood, M.A. Prenatal and postnatal administration of prebiotics and probiotics. Semin. Fetal Neonatal Med. 2017, 22, 284–289.
- Boyle, R.J.; Ismail, I.H.; Kivivuori, S.; Licciardi, P.V.; Robins-Browne, R.M.; Mah, L.-J.; Axelrad, C.; Moore, S.; Donath, S.; Carlin, J.B.; et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: A randomized controlled trial. Allergy 2010, 66, 509–516.
- Simpson, M.R.; Dotterud, C.K.; Storrø, O.; Johnsen, R.; Øien, T. Perinatal probiotic supplementation in the prevention of allergy related disease: 6 year follow up of a randomised controlled trial. BMC Dermatol. 2015, 15, 1–8.




