Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kennosuke Ichikawa | -- | 1831 | 2023-03-15 09:45:19 | | | |
| 2 | Dean Liu | -3 word(s) | 1828 | 2023-03-21 02:23:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ichikawa, K.; Horiuchi, H. Primordial Germ Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/42219 (accessed on 01 March 2026).
Ichikawa K, Horiuchi H. Primordial Germ Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/42219. Accessed March 01, 2026.
Ichikawa, Kennosuke, Hiroyuki Horiuchi. "Primordial Germ Cells" Encyclopedia, https://encyclopedia.pub/entry/42219 (accessed March 01, 2026).
Ichikawa, K., & Horiuchi, H. (2023, March 15). Primordial Germ Cells. In Encyclopedia. https://encyclopedia.pub/entry/42219
Ichikawa, Kennosuke and Hiroyuki Horiuchi. "Primordial Germ Cells." Encyclopedia. Web. 15 March, 2023.
Copy Citation
Primordial germ cells (PGCs) are precursor cells of sperm and eggs. The fate decisions of chicken PGCs in terms of their development, integrity, and sex determination have unique features, thereby providing insights into evolutionary developmental biology.
avian species
chicken
primordial germ cells
1. Introduction
The chicken (Gallus gallus) is a valuable species in terms of protein resources. Additionally, chickens have been used as a model organism for amniotes to elucidate embryonic developmental mechanisms [1]. Furthermore, chickens have been used as avian models in various areas of biology, thereby contributing to the understanding of vertebrate evolution. Thus, research using chickens greatly enhances both industrial development and developmental and evolutionary biology.
Primordial germ cells (PGCs) are the precursor cells of sperm and eggs. PGCs are the only cell lineage that can transmit genetic information to the next generation. Thus, elucidating the fate decision of PGCs, namely the mechanisms by which they develop, maintain integrity, differentiate into gametes of optimal sexes, and control their self-renewal, is a valuable research subject. Avian PGCs have characteristic developmental features, such as migration into the gonads using the vascular system [2]. Chicken PGCs have also shown a unique sex determination mechanism in which PGC-intrinsic factors may occur in a cell-autonomous manner [3][4][5]. In addition, several studies have revealed the self-renewal mechanism of chicken PGCs, resulting in the establishment of stable culture protocols for chicken PGCs [6][7]. Currently, culturing PGCs is a fundamental technique to establish genome-edited chickens and conserve avian genetic resources so that objective offspring can be produced via germline chimeras transplanted from cultured PGCs and directly incubated [8][9] or incubated with an ex ovo culture system [10]. Therefore, studies on the fate decisions of avian PGCs have demonstrated their unique features and have aided the development of avian biotechnologies.
With the development of essential technologies such as genome editing and RNA sequencing (RNA-seq) analysis, studies on chicken PGCs have shown rapid advancement over the last ten years. For example, the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas) system [11] has been used not only to produce genome-edited chickens [12], but also to conduct functional analysis of PGC-intrinsic factors via application of this system to cultured PGCs [13]. Alternatively, RNA-seq technology enables us to predict the fate decision of chicken PGCs even at a single-cell resolution level [14][15].
2. Early Development of Chicken PGCs
2.1. Origin and Identification of Chicken PGCs
In vertebrates, PGC formation is generally classified into two models: preformation and epigenesis [16]. The preformation model has been observed in zebrafish (Danio rerio) [17] and anuran amphibians (Xenopus laevis) [18]. In this model, germplasm, a maternal factor, acts as a determinant of germ cell formation. The germplasm is composed of maternally inherited RNAs and proteins and is partially asymmetrically partitioned to cells during cleavage divisions and development. As a result, PGCs arise from the partitioned cells. This mechanism is conserved in several non-vertebrate species, including Drosophila melanogaster [19] and Caenorhabditis elegans [20]. In contrast, the epigenesis model has been shown in urodele amphibians (Ambystoma mexicanum) [21] and mammals (Mus musculus) [22]. In these species, PGCs are induced in somatic cells via “epigenetic” regulation during embryonic development; thus, the germplasm is absent.
Several studies have been conducted to determine the origins of avian PGCs. In particular, studies focusing on the vasa gene have strongly supported the preformation model for avian PGC formation. VASA is a germ-cell-specific RNA helicase that is localized in germ cells as a germplasm component across various species, such as X. laevis and D. melanogaster. Tsunekawa et al. demonstrated the expression patterns of the chicken vasa homolog (CVH) [23]. The CVH is localized in cleavage furrows and asymmetrically distributed to limited cells. Additionally, the CVH is colocalized with the mitochondrial cloud, corresponding to the germplasm feature in D. melanogaster. Recent functional analyses have shown that the CVH significantly contributes to germ cell development in both males and females [24][25]. These studies suggest that chickens possess germplasm-like features and follow the preformation model for PGC formation.
Previous studies targeting deletion in the azoospermia-like (DAZL) protein also support the preformation model in chickens. DAZL is a germ-cell-specific RNA-binding protein localized in the germplasm in some vertebrates [26][27]. In chickens, DAZL is also localized in cleavage furrows as well as the CVH and is specifically expressed in germ cells [28]. Additionally, knockdown of DAZL in chicken PGCs causes apoptosis and aberrant expression patterns of germ-cell-characteristic genes, albeit under culture conditions [28]. Recently, whole transcriptome analysis predicted that chicken DAZL was co-expressed with its potential interacting genes according to zygotic genome activation, suggesting its central role in germ-cell specification [29].
Therefore, nowadays, avian germ cells are thought to be specified by the preformation model; however, the possibility that avian PGCs are formed via epigenetic regulation cannot be excluded [30]. Thus, while several studies have supported the preformation model in avian PGC formation, the origin of PGCs remains unknown.
2.2. Migration of Chicken PGCs into Embryonic Gonads
A chick embryo in an egg incubates for 0 h, corresponding to Eyal-Giladi and Kochav (EG & K) stage X, and consists of approximately 60,000 cells [31]. At this embryonic stage, approximately 30 CVH-positive cells, namely the origin of PGCs, are scattered at the center of the area pellucida in the blastoderm (Figure 1) [23]. The CVH-positive cells then start to migrate into the germinal crescent, an anterior extraembryonic region. At Hamburger–Hamilton (HH) stage 4 (chick embryos in eggs after 18–19 h of incubation), PGCs accumulated at a high density in the germinal crescent (Figure 1) [32]. Previously, it was thought that PGCs passively translocate into the germinal crescent via the morphogenetic movement of hypoblasts [33]. However, Kang et al. demonstrated that chick fibroblasts exogenously transplanted into the subgerminal cavity of the recipient could not settle in the germinal crescent, whereas transplanted PGCs could [34]. This indicates that PGC-intrinsic factors are also related to this migration. Recently, Huss et al. revealed that quail (Coturnix japonica) PGCs contribute to the extracellular matrix in the germinal crescent [35]. Although the molecular mechanism of PGC migration remains unclear, these previous studies showed their “active” role.
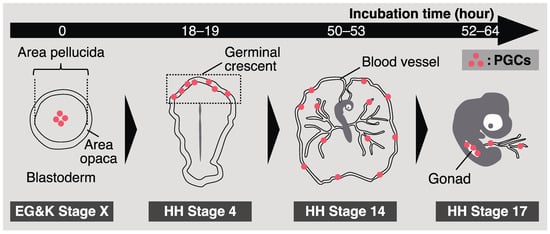
Figure 1. A schematic illustration of chicken primordial germ cell (PGC) development.
After the settlement of PGCs into the germinal crescent, PGCs begin to migrate to the gonads. In many vertebrates, such as mice and zebrafish, PGCs migrate into gonads via “amoeboid migration” [36]. However, in birds (and reptiles), PGCs use the vascular system to migrate into the gonads. Several studies have attempted to elucidate this unique migration system in avian PGCs.
Around HH stage 6 (chick embryos in eggs incubated for 23–25 h), aggregations of endothelial cell progenitors and blood cell progenitors, called blood islands, appear in the extraembryonic mesoderm [37]. Murai et al. demonstrated that more than 60% of quail PGCs in an embryo were enveloped by differentiating endothelial cells forming blood islands in the germinal crescent [38]. Then, the PGCs flowed along with the heartbeat at HH stage 12 (after 48–49 h of incubation). This indicates that most avian PGCs were passively translocated into the vascular system.
Once avian PGCs are translocated into the blood vessels, they start circulating. The concentration of chicken PGCs in the bloodstream reaches a peak at HH stage 14 (after 50–53 h of incubation), and these settle in gonads from HH stage 15 (after 50–55 h of incubation) to HH stage 17 (after 52–64 h of incubation) (Figure 1) [39][40]. Recent studies have demonstrated the molecular mechanisms involved in gonadal migration of avian PGCs. The role of the interaction between chemotactic molecular stromal cell-derived factor 1 (SDF1) and its receptor C-X-C chemokine receptor type 4 (CXCR4) is a well-known system that directs PGCs to the gonads in vertebrates, whose PGCs utilize amoeboid migration [41][42][43][44][45]. In chickens, the expression of CXCR4 has also been observed in PGCs [46], and PGCs are attracted to ectopically expressed SDF1s [47]. Furthermore, the transplantation of CXCR4 knockout (KO) PGCs into recipient embryos resulted in a reduction in their capacity to migrate into gonads, suggesting a critical role of the CXCR4–SDF1 interaction in this migration [13]. In contrast, recent studies have proposed other factors for gonadal migration of avian PGCs. Saito et al. showed that circulating avian PGCs were stiffer than blood cells; thus, PGCs were efficiently occluded at the vascular plexus near presumptive gonads, resulting in their homing to developing gonads [48]. Huang et al. proposed that platelet-derived growth factor signaling could be involved in the migration of avian PGCs into gonads using RNA-seq analysis [49]. These molecular studies have advanced the understanding of how avian PGCs migrate to gonads. Notably, previous research focusing on the migration of avian PGCs has been conducted mainly using chickens and quails. Thus, whether the molecular mechanism of this migration is conserved across avian species remains unknown.
3. Integrity of Chicken PGCs
3.1. Epigenetic Regulation
Epigenetic regulation is essential for PGCs to maintain their properties for germinal transmission, namely, establishing their integrity. In mice, PGCs undergo genome-wide epigenetic reprogramming between embryonic day (E) 8.5 and E13.5, and these embryonic stages correspond to the migration and colonization of PGCs to the gonads [50]. Epigenetic reprogramming is required to establish germ cell specification and erase somatic epigenetic memory [51]. The importance of epigenetic modifications in the establishment of PGC integrity has also been observed across species [52].
Several studies have been conducted to reveal epigenetic modifications of chicken PGCs. Yu et al. demonstrated that chicken PGCs undergo global DNA demethylation via ten-eleven translocation 1 during HH stage 21 (after 3.5 d of incubation) to HH stage 28 (after 5.5 d of incubation) [53]. These embryonic stages correspond to states in which chicken PGCs migrate to the gonads and form colonies within the gonads. Rengaraj et al. investigated the expression patterns of the DNA methyltransferase (DNMT) families DNMT1, DNMT 3 α (DNMT3A), and DNMT 3 β (DNMT3B) in chicken PGCs during embryogenesis and suggested that DNMT3B-dependent de novo DNA methylation occurred after PGCs settled into the gonads [54]. Jang et al. showed the characteristic DNA methylation patterns of gonadal PGCs by comparing them with those of chicken embryonic fibroblasts [55]. Although the understanding of DNA methylation in PGCs is not yet complete, these analyses are beginning to elucidate the underlying mechanism.
Furthermore, several studies have been reported concerning the elucidation of histone modification in avian PGCs. In mice, after the transient loss of histone modifications during epigenetic reprogramming, PGCs regain both histone H3 lysine 9 (H3K9) and H3K27 trimethylation (me3) around E12.5 [56]. However, histone modification of chicken PGCs was based on H3K9me3 rather than H3K27me3, suggesting the existence of avian-specific epigenetic regulation in PGCs [57]. For other modifications, H3K4me2 activates signaling pathways essential for avian PGC formation, such as bone morphogenetic protein 4 (BMP4) signaling [58]. Additionally, H3K9 acetylation (H3K9ac) contributes to maintaining the integrity of avian PGCs via the regulation of NANOG, a key transcription factor for germ cell development [59] (described below). These analyses have revealed histone modifications in avian PGCs, including avian-specific H3K9me3-dominant gene expression regulation.
3.2. Key Molecules for the Integrity of Avian PGC
Key molecules for the integrity of PGCs, including transcription factors, are well-conserved in vertebrates. Recently, several researchers conducted functional analyses of these molecules in avian PGCs. Interestingly, these key molecules exhibited bird-like features. Researchers describe the functions and characteristics of the molecules involved in the integrity of chicken PGCs, along with their differences from those in other model organisms.
References
- Sheng, G. Defining epithelial-mesenchymal transitions in animal development. Development 2021, 148, dev198036.
- Swift, C.H. Origin and early history of the primordial germ-cells in the chick. Am. J. Anat. 1914, 15, 483–516.
- Ichikawa, K.; Ezaki, R.; Furusawa, S.; Horiuchi, H. Comparison of sex determination mechanism of germ cells between birds and fish: Cloning and expression analyses of chicken forkhead box L3-like gene. Dev. Dyn. 2019, 248, 826–836.
- Soler, L.; Alves, S.; Brionne, A.; Jacques, A.; Guérin, V.; Cherif-Feildel, M.; Combes-Soia, L.; Fouchécourt, S.; Thélie, A.; Blesbois, E.; et al. Protein expression reveals a molecular sexual identity of avian primordial germ cells at pre-gonadal stages. Sci. Rep. 2021, 11, 19236.
- Ichikawa, K.; Nakamura, Y.; Bono, H.; Ezaki, R.; Matsuzaki, M.; Horiuchi, H. Prediction of sex-determination mechanisms in avian primordial germ cells using RNA-seq analysis. Sci. Rep. 2022, 12, 13528.
- Van de Lavoir, M.C.; Diamond, J.H.; Leighton, P.A.; Mather-Love, C.; Heyer, B.S.; Bradshaw, R.; Kerchner, A.; Hooi, L.T.; Gessaro, T.M.; Swanberg, S.E.; et al. Germline transmission of genetically modified primordial germ cells. Nature 2006, 441, 766–769.
- Whyte, J.; Glover, J.D.; Woodcock, M.; Brzeszczynska, J.; Taylor, L.; Sherman, A.; Kaiser, P.; McGrew, M.J. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 2015, 5, 1171–1182.
- Lázár, B.; Molnár, M.; Sztán, N.; Végi, B.; Drobnyák, Á.; Tóth, R.; Tokodyné, S.N.; McGrew, M.J.; Gócza, E.; Patakiné, V.E. Successful cryopreservation and regeneration of a partridge colored Hungarian native chicken breed using primordial germ cells. Poult. Sci. 2021, 100, 101207.
- Boes, J.; Boettcher, P.; Honkatukia, M. (Eds.) Innovations in Cryoconservation of Animal Genetic Resources—Practical Guide. In FAO Animal Production and Health Guidelines; FAO: Rome, Italy, 2023; No. 33.
- Perry, M.M. A complete culture system for the chick embryo. Nature 1988, 331, 70–72.
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821.
- Oishi, I.; Yoshii, K.; Miyahara, D.; Kagami, H.; Tagami, T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016, 6, 23980.
- Lee, J.H.; Park, J.W.; Kim, S.W.; Park, J.; Park, T.S. C-X-C chemokine receptor type 4 (CXCR4) is a key receptor for chicken primordial germ cell migration. J. Reprod. Dev. 2017, 63, 555–562.
- Rengaraj, D.; Cha, D.G.; Lee, H.J.; Lee, K.Y.; Choi, Y.H.; Jung, K.M.; Kim, Y.M.; Choi, H.J.; Choi, H.J.; Yoo, E.; et al. Dissecting chicken germ cell dynamics by combining a germ cell tracing transgenic chicken model with single-cell RNA sequencing. Comput. Struct. Biotechnol. J. 2022, 20, 1654–1669.
- Rengaraj, D.; Cha, D.G.; Park, K.J.; Lee, K.Y.; Woo, S.J.; Han, J.Y. Finer resolution analysis of transcriptional programming during the active migration of chicken primordial germ cells. Comput. Struct. Biotechnol. J. 2022, 20, 5911–5924.
- Extavour, C.G.; Akam, M. Mechanisms of germ cell specification across the metazoans: Epigenesis and preformation. Development 2003, 130, 5869–5884.
- Yoon, C.; Kawakami, K.; Hopkins, N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 1997, 124, 3157–3165.
- Houston, D.W.; Zhang, J.; Maines, J.Z.; Wasserman, S.A.; King, M.L. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 1998, 125, 171–180.
- Ephrussi, A.; Lehmann, R. Induction of germ cell formation by oskar. Nature 1992, 358, 387–392.
- Seydoux, G.; Strome, S. Launching the germline in Caenorhabditis elegans: Regulation of gene expression in early germ cells. Development 1999, 126, 3275–3283.
- Johnson, A.D.; Bachvarova, R.F.; Drum, M.; Masi, T. Expression of axolotl DAZL RNA, a marker of germ plasm: Widespread maternal RNA and onset of expression in germ cells approaching the gonad. Dev. Biol. 2001, 234, 402–415.
- Tam, P.P.; Zhou, S.X. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 1996, 178, 124–132.
- Tsunekawa, N.; Naito, M.; Sakai, Y.; Nishida, T.; Noce, T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000, 127, 2741–2750.
- Taylor, L.; Carlson, D.F.; Nandi, S.; Sherman, A.; Fahrenkrug, S.C.; McGrew, M.J. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 2017, 144, 928–934.
- Aduma, N.; Izumi, H.; Mizushima, S.; Kuroiwa, A. Knockdown of DEAD-box helicase 4 (DDX4) decreases the number of germ cells in male and female chicken embryonic gonads. Reprod. Fertil. Dev. 2019, 31, 847–854.
- Maegawa, S.; Yasuda, K.; Inoue, K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 1999, 81, 223–226.
- Houston, D.W.; King, M.L. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 2000, 127, 447–456.
- Lee, H.C.; Choi, H.J.; Lee, H.G.; Lim, J.M.; Ono, T.; Han, J.Y. DAZL expression explains origin and central formation of primordial germ cells in chickens. Stem Cells Dev. 2016, 25, 68–79.
- Rengaraj, D.; Won, S.; Han, J.W.; Yoo, D.; Kim, H.; Han, J.Y. Whole-transcriptome sequencing-based analysis of DAZL and its interacting genes during germ cells specification and zygotic genome activation in chickens. Int. J. Mol. Sci. 2020, 21, 8170.
- Zhou, S.; Li, T.; Zhang, M.; Chen, C.; Gao, X.; Zhang, C.; Hu, C.; Zuo, Q.; Chen, G.; Li, B. Epigenetic modification cooperates with Zeb1 transcription factor to regulate Bmp4 to promote chicken PGCs formation. Gene 2021, 794, 145760.
- Eyal-Giladi, H.; Kochav, S. From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick: I. Dev. Biol. 1976, 49, 321–337.
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92.
- Ginsburg, M.; Eyal-Giladi, H. Primordial germ cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development 1987, 101, 209–219.
- Kang, K.S.; Lee, H.C.; Kim, H.J.; Lee, H.G.; Kim, Y.M.; Lee, H.J.; Park, Y.H.; Yang, S.Y.; Rengaraj, D.; Park, T.S.; et al. Spatial and temporal action of chicken primordial germ cells during initial migration. Reproduction 2015, 149, 179–187.
- Huss, D.J.; Saias, S.; Hamamah, S.; Singh, J.M.; Wang, J.; Dave, M.; Kim, J.; Eberwine, J.; Lansford, R. Avian primordial germ cells contribute to and interact with the extracellular matrix during early migration. Front. Cell Dev. Biol. 2019, 7, 35.
- Grimaldi, C.; Raz, E. Germ cell migration-Evolutionary issues and current understanding. Semin. Cell Dev. Biol. 2020, 100, 152–159.
- Sheng, G. Primitive and definitive erythropoiesis in the yolk sac: A bird’s eye view. Int. J. Dev. Biol. 2010, 54, 1033–1043.
- Murai, H.; Shibuya, M.; Kishita, R.; Sunase, C.; Tamura, K.; Saito, D. Envelopment by endothelial cells initiates translocation of avian primordial germ cell into vascular tissue. Dev. Dyn. 2021, 250, 1410–1419.
- Tajima, A.; Hayashi, H.; Kamizumi, A.; Ogura, J.; Kuwana, T.; Chikamune, T. Study on the concentration of circulating primordial germ cells (cPGCs) in early chick embryos. J. Exp. Zool. 1999, 284, 759–764.
- Nakamura, Y.; Yamamoto, Y.; Usui, F.; Mushika, T.; Ono, T.; Setioko, A.R.; Takeda, K.; Nirasawa, K.; Kagami, H.; Tagami, T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 2007, 86, 2182–2193.
- Doitsidou, M.; Reichman-Fried, M.; Stebler, J.; Köprunner, M.; Dörries, J.; Meyer, D.; Esguerra, C.V.; Leung, T.; Raz, E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 2002, 111, 647–659.
- Knaut, H.; Werz, C.; Geisler, R.; Nüsslein-Volhard, C.; Tübingen 2000 Screen Consortium. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature 2003, 421, 279–282.
- Molyneaux, K.A.; Zinszner, H.l.n.; Kunwar, P.S.; Schaible, K.; Stebler, J.; Sunshine, M.J.; O’Brien, W.; Raz, E.; Littman, D.; Wylie, C.; et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003, 130, 4279–4286.
- Takeuchi, T.; Tanigawa, Y.; Minamide, R.; Ikenishi, K.; Komiya, T. Analysis of SDF-1/CXCR4 signaling in primordial germ cell migration and survival or differentiation in Xenopus laevis. Mech. Dev. 2010, 127, 146–158.
- Amat-Fernandez, J.; Hammond, M.J.; Liang, D.; Wang, T.; Ventura, T.; Elizur, A.; Cummins, S.F. Molecular characterization of sdf1 and cxcr4 in the Mozambique tilapia, Oreochromis mossambicus. Anim. Reprod. Sci. 2017, 176, 51–63.
- Motono, M.; Ohashi, T.; Nishijima, K.; Iijima, S. Analysis of chicken primordial germ cells. Cytotechnology 2008, 57, 199–205.
- Stebler, J.; Spieler, D.; Slanchev, K.; Molyneaux, K.A.; Richter, U.; Cojocaru, V.; Tarabykin, V.; Wylie, C.; Kessel, M.; Raz, E. Primordial germ cell migration in the chick and mouse embryo: The role of the chemokine SDF-1/CXCL12. Dev. Biol. 2004, 272, 351–361.
- Saito, D.; Tadokoro, R.; Nagasaka, A.; Yoshino, D.; Teramoto, T.; Mizumoto, K.; Funamoto, K.; Kidokoro, H.; Miyata, T.; Tamura, K.; et al. Stiffness of primordial germ cells is required for their extravasation in avian embryos. iScience 2022, 25, 105629.
- Huang, X.; Meng, L.; Wang, S.; Man, Q.; Jiang, Y.; Zhu, G. Transcriptional dynamics of the circulating chicken primordial germ cells revealing key genes in cell adhesion and proliferation prior to gonad colonization. Mol. Reprod. Dev. 2022, 89, 214–226.
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 2012, 48, 849–862.
- Tang, W.W.C.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600.
- Strome, S.; Updike, D. Specifying and protecting germ cell fate. Nat. Rev. Mol. Cell Biol. 2015, 16, 406–416.
- Yu, M.; Li, D.; Cao, W.; Chen, X.; Du, W. Effects of ten–eleven translocation 1 (Tet1) on DNA methylation and gene expression in chicken primordial germ cells. Reprod. Fertil. Dev. 2019, 31, 509–520.
- Rengaraj, D.; Lee, B.R.; Lee, S.I.; Seo, H.W.; Han, J.Y. Expression patterns and miRNA regulation of DNA methyltransferases in chicken primordial germ cells. PLoS ONE 2011, 6, e19524.
- Jang, H.J.; Seo, H.W.; Lee, B.R.; Yoo, M.; Womack, J.E.; Han, J.Y. Gene expression and DNA methylation status of chicken primordial germ cells. Mol. Biotechnol. 2013, 54, 177–186.
- Hajkova, P.; Ancelin, K.; Waldmann, T.; Lacoste, N.; Lange, U.C.; Cesari, F.; Lee, C.; Almouzni, G.; Schneider, R.; Surani, M.A. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 2008, 452, 877–881.
- Kress, C.; Montillet, G.; Jean, C.; Fuet, A.; Pain, B. Chicken embryonic stem cells and primordial germ cells display different heterochromatic histone marks than their mammalian counterparts. Epigenetics Chromatin 2016, 9, 5.
- Zhang, C.; Zuo, Q.; Wang, M.; Chen, H.; He, N.; Jin, J.; Li, T.; Jiang, J.; Yuan, X.; Li, J.; et al. Narrow H3K4me2 is required for chicken PGC formation. J. Cell. Physiol. 2021, 236, 1391–1400.
- Jung, H.G.; Hwang, Y.S.; Park, Y.H.; Cho, H.Y.; Rengaraj, D.; Han, J.Y. Role of epigenetic regulation by the REST/CoREST/HDAC corepressor complex of moderate NANOG expression in chicken primordial germ cells. Stem Cells Dev. 2018, 27, 1215–1225.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
21 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





