Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohamed Hassan Eisa | -- | 1813 | 2023-03-14 17:10:50 | | | |
| 2 | Rita Xu | -20 word(s) | 1793 | 2023-03-15 02:57:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kausar, A.; Ahmad, I.; Eisa, M.H.; Maaza, M. Graphene Nanocomposites in Space Sector. Encyclopedia. Available online: https://encyclopedia.pub/entry/42194 (accessed on 08 March 2026).
Kausar A, Ahmad I, Eisa MH, Maaza M. Graphene Nanocomposites in Space Sector. Encyclopedia. Available at: https://encyclopedia.pub/entry/42194. Accessed March 08, 2026.
Kausar, Ayesha, Ishaq Ahmad, M. H. Eisa, Malik Maaza. "Graphene Nanocomposites in Space Sector" Encyclopedia, https://encyclopedia.pub/entry/42194 (accessed March 08, 2026).
Kausar, A., Ahmad, I., Eisa, M.H., & Maaza, M. (2023, March 14). Graphene Nanocomposites in Space Sector. In Encyclopedia. https://encyclopedia.pub/entry/42194
Kausar, Ayesha, et al. "Graphene Nanocomposites in Space Sector." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Graphene is one of the most significant carbon nanomaterials, with a one-atom-thick two-dimensional nanostructure. Like other nanocarbons, graphene has been used as a polymer reinforcement.
graphene
epoxy
nanocomposite
fabrication
1. Introduction
Polymer composites/nanocomposites have found potential for aerospace-related technological and engineering areas [1]. In the aeronautical field, fiber-based composite structures were initially applied in space structures [2]. Later research focused on the significance of polymers and nanofiller/additive-based nanocomposites in this sector [3][4]. Accordingly, using nanofillers (instead of macrofillers) remarkably enhanced the durability, fatigue resistance, strength, and toughness properties of aeronautical materials [5]. Moreover, using lightweight nanocomposites in aerospace vehicles has advantages of reducing fuel consumption and improved performance compared to heavy metal space structures [6][7]. Most importantly, carbon nanoparticles such as graphene, carbon nanotubes, and nanodiamond nanofillers have been used to form aerospace nanocomposites [8]. Among carbon nanoparticles, graphene is recognized as an important carbon nanostructure [9]. It has carbon atoms arranged in a honeycomb lattice structure. A graphene nanosheet is one-atom-thick with exceptional structural and physical properties. Polymer/graphene nanocomposites offer fine processability, resilience, frivolity, mechanical stability, thermal conductivity, and electrical conductivity properties [10]. Owing to the remarkable features of graphene and polymer/graphene nanocomposites, their potential applications have been discovered for aerospace [11].
In essence, this advanced review focused on the design, features, and potential of graphene and derived nanomaterials for aerospace. Incidentally, various combinations of polymer/graphene and epoxy/graphene nanocomposites, related fabrication approaches, and possible utilizations were considered. Some previous literature reports were observed on the design and performance of polymer/graphene nanocomposites; however, the reported literature is not updated enough to portray the true current state of polymer/graphene materials and graphene-modified carbon fiber composites for aerospace [12]. Likewise, the previous literature does not depict the main progress in this field during recent years. In this regard, future developments in functional polymer/graphene nanocomposite are not possible for aerospace-related researchers without gaining prior knowledge of the recent relevant literature.
2. Graphene
Graphene is a two-dimensional nanosheet made up of sp2-hybridized carbon atoms [13]. Single-layer graphene was produced and recognized in 2004 by Andre Geim and Konstantin Novoselov [14], although it was theoretically explored by P. R. Wallace in 1947 and experimentally by Hanns-Peter Boehm and his coworkers in 1962. Graphene has been prepared through various approaches such as graphite exfoliation, graphite mechanical cleavage, chemical vapor deposition, laser techniques, and numerous other organic synthesis routes [15]. Graphene has revealed exceptional structural and physical features. First of all, graphene (a one-atom-thick nanosheet) is the thinnest known material [16]. Moreover, graphene has a high electron mobilization of ~200,000 cm2V−1s−1. It also shows a high thermal conductivity of ~3000–5000 W/mK [17]. The significantly high Young’s modulus of graphene has been observed as 1 TPa, i.e., 200 times stronger than steel [18]. Owing to van der Waals forces, graphene nanosheets possess a wrinkling tendency, and so can easily crumple [19]. To resolve the nanosheet wrinkling and dispersion issues, graphene has been modified to generate functional groups on the surface [20]. Consequently, graphene has been oxidized to form graphene oxide as a modified form with hydroxyl, epoxide, carbonyl, and carboxylic acid functionalities on the surface. Figure 1 portrays the general structures of graphene nanosheets, whereas Figure 2 displays the structures of graphene oxide. The majority of functional groups in graphene oxide have been found as epoxy and hydroxyl functionalities. Graphene has superior characteristics such as mechanical consistency, thermal stability, chemical stability, and electrical and thermal conductivity [21]. The properties of graphene have been further improved through incorporation in nanocomposites. Due to the unique structure and properties, graphene and graphene-derived nanomaterials have been applied in electronics [22][23][24], sensors [25], energy devices [26], membranes [27], etc. In addition, the impact of graphene has also been analyzed for aerospace purposes [28].
Figure 1. Graphene and graphene oxide.
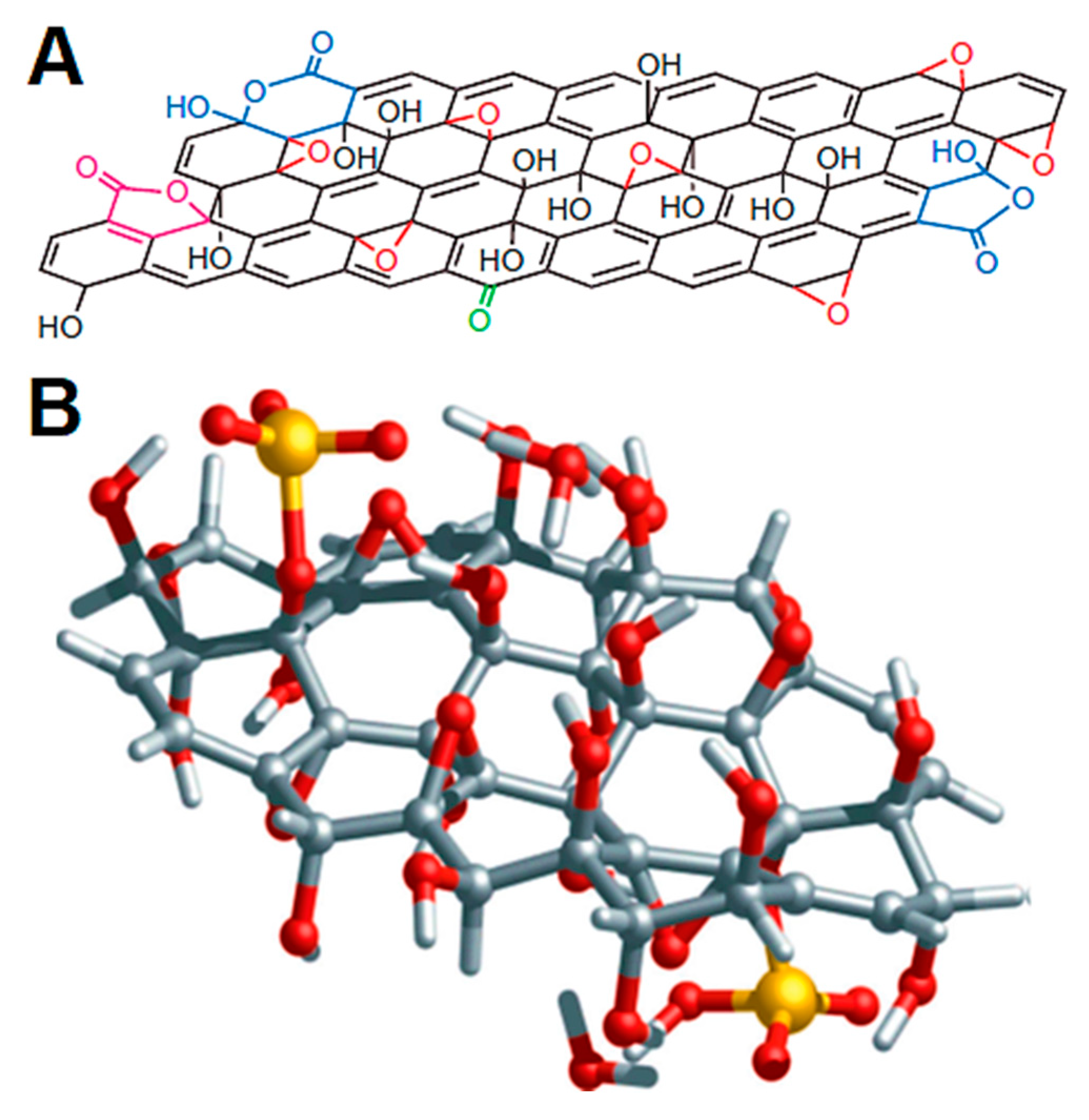
Figure 2. (A) Structural model of graphene oxide, taking into account five- and six-membered lactol rings (blue), ester of a tertiary alcohol (purple), hydroxyl (black), epoxy (red), and ketone (green) functionalities. The relative ratios are likely to be 115 (hydroxyl and epoxy): 3 (lactol O–C–O): 63 (graphitic sp2 carbon): 10 (lactol/ester/acid carbonyl): 9 (ketone carbonyl). The model here only shows the chemical connectivity, and not the steric orientation, of these functionalities and (B) Structure of graphene oxide with epoxy and hydroxyl as dominant functional groups.
3. Composite/Nanocomposite Materials Regarding Aerospace
For technical and engineering industries, polymeric composites/nanocomposites have revealed several design and property advantages [31]. Before the application of composites, pristine polymers were used in aerospace structural parts [32][33]. Later, the development of composites and nanocomposites was focused on for aerospace purposes [34]. Compared with unfilled polymers, composite/nanocomposite materials showed better thermal and mechanical properties. In this regard, a number of thermoplastics, thermosets, and rubbery matrices have been applied in the space sector [35][36]. Composites/nanocomposites have the advantage of being lightweight in aerospace, relative to metal-based structures [37]. Using low-density materials also decreased the fuel ingestion of the spacecrafts [38][39]. Moreover, these materials have high strength, modulus, toughness, thermal stability, friction resistance, fatigue performance, and shear resistance characteristics [40][41][42]. Consequently, the structural durability and working life of aerospace structures have been improved [43][44][45]. Multifunctional composites/nanocomposites have been found to be valuable in increasing the capability of space structures in bearing shocks and jerks [46][47] and lightning strikes [48], in radiation shielding [49], and as high-temperature stable engine components [50]. In aerospace nanocomposites, carbon nanoparticles (carbon nanotubes, carbon nanofibers, etc.) and inorganic nanoparticles (metal, metal oxide, etc.) have been used as reinforcements [51][52]. In particular, widely used polymer/carbon nanotube nanocomposites have revealed reasonably high heat stability, flame defiance, thermal conductivity, strength, and mechanical stability for aerospace structures [53][54][55]. Nevertheless, research on aerospace-related composites/nanocomposites is continuously growing, in search of new high-performance structures [56].
4. Graphene in Polymeric Nanocomposites for Space Relevance
Graphene and related nanofillers have effectively increased the electrical conductivity, thermal conductivity, and mechanical features of nanocomposites [57][58][59]. For aerospace applications, nanocomposites have been investigated for physical property improvements and morphological profiles [60]. Various thermosetting and thermoplastic polymers have been used to form graphene-derived nanocomposites for aerospace structures [61]. Among thermosetting polymers, epoxy resins have been extensively used in aerospace-related applications [62]. Graphene and related nanofillers have been reinforced in epoxy matrices to enhance the essential conducting, thermal, and mechanical properties [63][64][65]. According to studies, up to 20 wt.% graphene nanofiller contents may increase the high thermal conductivity, toughness, and fatigue resistance properties of epoxy nanocomposites [66][67][68]. Moreover, graphene oxide nanofiller also improves the tensile strength, fatigue resistance, toughness, heat stability, and tribological properties of epoxy matrices [69][70]. Most importantly, epoxy nanocomposites with graphene and related nanofillers have been used in structural components, adhesives, coatings, etc., for aerospace vehicles [71].
Thermoplastic polymers such as polyethylene, polypropylene, poly(methyl methacrylate), poly(vinyl alcohol), and others have been applied in space applications [72]. Suner and coworkers [73] fabricated polyethylene and graphene-oxide-derived nanocomposites for enhanced mechanical stability. The addition of 0.5 wt.% nanofiller significantly improved the mechanical characteristics of the nanocomposites due to matrix–nanofiller compatibility [74]. Song et al. [75] reported on the polypropylene/graphene nanocomposites fabricated using the solution casting and melt method. The conditions and processes involved in the preparation of polypropylene/graphene nanocomposites are displayed in Figure 3. Graphene oxide was obtained from graphite flakes using the Hummers method [76]. Then, graphene oxide was processed through a solution as well as melt routes to form the nanocomposites. The mechanical properties of the polypropylene/graphene nanocomposites are displayed in Table 1. The inclusion of up to 1 wt.% nanofiller content was found to enhance the yield strength and tensile strength of the nanocomposites due to the mechanical interlocking of the polymer chains with the graphene nanostructure. Moreover, the enhanced mechanical properties were attributed to better graphene dispersion and load transfer between the matrix and the nanofiller [77]. Moreover, the increasing graphene loading was found to improve the crystallinity of the nanocomposite due to orderly nanofiller dispersion and interaction with the polymer matrix [78]. Nanocomposites have been found to be valuable for aerospace applications.
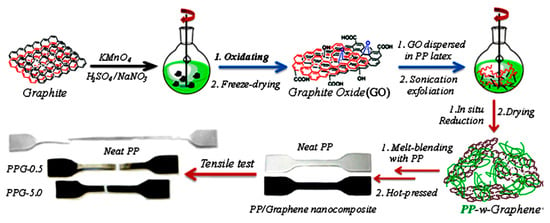
Figure 3. Schematic of polypropylene/graphene nanocomposite fabrication. PP = polypropylene; G = graphene; PPG = polypropylene/graphene.
Table 1. Mechanical properties of polypropylene/graphene oxide nanocomposites with various loadings. PP = polypropylene; YS = yield strength; TS = tensile strength; %YS = percent increment of yield strength.
| Sample | YS (MPa) | TS (MPa) | YS (%) |
|---|---|---|---|
| Neat PP | 22 ± 0.8 | 24 ± 0.8 | - |
| PP + 0.1 wt.% nanofiller | 30 ± 1.1 | 33 ± 1.4 | 36 |
| PPG + 0.5 wt.% nanofiller | 36 ± 1.2 | 36 ± 1.5 | 50 |
| PP + 1.0 wt.% nanofiller | 38 ± 1.7 | 37 ± 1.6 | 75 |
Huang and coresearchers [79] produced flame-retardant poly(methyl methacrylate) nanocomposites for aerospace. An in situ method was used to develop the nanocomposites [80]. Graphene and layered double hydroxide were added as intumescent flame retardants in the poly(methyl methacrylate) matrix to enhance the flame retardancy of the matrix. The poly(methyl methacrylate) matrix with the 1 wt.% graphene and 5 wt.% layered hydroxide considerably enhanced the nonflammability properties through improving the limiting oxygen index. Xu et al. [81] developed poly(vinyl alcohol)/graphene oxide nanocomposites via the vacuum filtration procedure. As compared to the Young’s modulus of the neat poly(vinyl alcohol) matrix (2.1 MPa), the addition of 3 wt.% graphene oxide increased the property up to 4.8 GPa (by 128%). Moreover, including 3 wt.% nanofiller boosted the tensile strength by 110 MPa (70%), as compared to the neat polymer (65 MPa). The increase in the mechanical properties was credited to the interfacial interactions in the matrix–nanofiller, leading to relevance in aerospace. Hence, different polymers reinforced with graphene nanofillers have enhanced mechanical, thermal, and conducting profiles and are found to be useful as aerospace materials.
In the aerospace industry, high-performance matrices such as poly(ether ether ketone) (PEEK) [82][83], poly(ether ketone ketone) (PEKK) [84][85], polyetherimide [86], and polysulfone [87] have been found to be desirable due to high mechanical, thermal, and electrical properties. Puértolas et al. [88] fabricated PEEK/graphene nanocomposites using solvent-free melt-blending and injection-molding techniques. The 1–10 wt.% graphene nanofiller was included in PEEK matrix. The inclusion of graphene caused a 60% improvement in the hardness of the material. The coefficient of friction and the wear factor were decreased by 38% and 83%, respectively. Thus, graphene was found to be an important nanofiller to enhance the surface hardness and tribological properties of the PEEK matrix, desirable for aerospace uses. Alvaredo et al. [89] introduced graphene nanoplatelets in PEEK using the melt-blending technique. The 1–10 wt.% nanofiller was included in the PEEK matrix. The inclusion of graphene nanoplatelets enhanced the complex viscosity and rheological behavior of the nanocomposites and they were found to be effective for the space sector. Wang et al. [90] produced PEKK/graphene nanomaterials with enhanced electrical conductivity and mechanical performance, suitable for high-tech industrial relevance. Sun et al. [91] fabricated high-performance polyetherimide/graphene oxide nanocomposites. The inclusion of graphene oxide nanofiller improved the tensile strength and temperature-dependent tensile behaviors of the polyetherimide/graphene oxide nanocomposites. These materials have fine suitability for the space sector. Ionita et al. [92] developed polysulfone/graphene oxide nanocomposites using the phase inversion technique. The inclusion of 0.25–2 wt.% nanofiller was studied. The homogeneous nanofiller dispersion considerably improved the thermal stability of the nanocomposites. Janire Peña-Bahamonde et al. [93] also prepared polysulfone/graphene oxide nanocomposites through the solvent-free extrusion–injection technique. The addition of 3 wt.% nanofiller upsurged the dispersion, rheological properties, and toughness of the nanocomposites. These materials can be feasible candidates for aerospace applications.
References
- Lebedev, O.V.; Kurkin, T.S.; Golubev, E.K.; Vasiliev, A.L.; Gatin, A.K.; Goncharuk, G.P.; Ozerin, A.N. Detonation Synthesis Nanodiamond Soot as a Promising Filler for Polymer Composites. C 2022, 8, 69.
- Padmanaban, D.B.; Mohan, L.; Giri, P.; Bera, P.; Anandan, C.; Barshilia, H.C. Effect of Molybdenum Content on Mechanical and Tribological Properties of Diamond-Like Carbon Coatings over Titanium β-21S Alloy. C 2020, 7, 1.
- Gbordzoe, S.; Adusei, P.K.; Chauhan, D.; Alvarez, N.T.; Haase, M.R.; Mansari, K.; Kanakaraj, S.N.; Hsieh, Y.-Y.; Shanov, V. A Simple Two-Step Process for Producing Strong and Aligned Carbon Nanotube-Polymer Composites. C 2019, 5, 35.
- Susi, B.T.; Tu, J.F. Digital Synthesis of Realistically Clustered Carbon Nanotubes. C 2022, 8, 34.
- Driscoll, A.J.; Johnson, P.A. Numerical modeling of analyte diffusion and adsorption behavior in microparticle and nanoparticle based biosensors. Chem. Eng. Sci. 2018, 184, 141–148.
- Rambabu, P.; Prasad, N.E.; Kutumbarao, V.; Wanhill, R. Aluminium alloys for aerospace applications. In Aerospace Materials and material Technologies; Springer: Singapore, 2017; pp. 29–52.
- Gorbatikh, L.; Wardle, B.L.; Lomov, S.V. Hierarchical lightweight composite materials for structural applications. Mrs Bull. 2016, 41, 678–682.
- Bilisik, K.; Akter, M. Graphene nanocomposites: A review on processes, properties, and applications. J. Ind. Text. 2022, 51, 3718S–3766S.
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 15, 1012.
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A review on the production methods and applications of graphene-based materials. Nanomaterials 2021, 11, 2414.
- Kausar, A. Aerospace applications of polymer/carbonaceous nanofiller nanocomposites: Mechanical, thermal, nonflammability, and physical aspects. In Polymeric Nanocomposites with Carbonaceous Nanofillers for Aerospace Applications; Elsevier: Amsterdam, The Netherlands, 2022; p. 85.
- Liu, Y.; Du, H.; Liu, L.; Leng, J. Shape memory polymers and their composites in aerospace applications: A review. Smart Mater. Struct. 2014, 23, 023001.
- Gao, Y.; Zhang, Y.; Chen, P.; Li, Y.; Liu, M.; Gao, T.; Ma, D.; Chen, Y.; Cheng, Z.; Qiu, X. Toward single-layer uniform hexagonal boron nitride–graphene patchworks with zigzag linking edges. Nano Lett. 2013, 13, 3439–3443.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Wei, C.; Negishi, R.; Ogawa, Y.; Akabori, M.; Taniyasu, Y.; Kobayashi, Y. Turbostratic multilayer graphene synthesis on CVD graphene template toward improving electrical performance. Jpn. J. Appl. Phys. 2019, 58, SIIB04.
- Narayanam, P.K.; Botcha, V.D.; Ghosh, M.; Major, S.S. Growth and Photocatalytic Behaviour of Transparent Reduced GO-ZnO Nanocomposite Sheets. Nanotechnology 2019, 30, 485601.
- Shen, X.J.; Zeng, X.L.; Dang, C.Y. Graphene Composites. Handb. Graphene 2019, 1, 1–25.
- Zandiatashbar, A.; Lee, G.-H.; An, S.J.; Lee, S.; Mathew, N.; Terrones, M.; Hayashi, T.; Picu, C.R.; Hone, J.; Koratkar, N. Effect of defects on the intrinsic strength and stiffness of graphene. Nat. Commun. 2014, 5, 3186.
- Zhou, Q.; Xia, G.; Du, M.; Lu, Y.; Xu, H. Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water. Appl. Surf. Sci. 2019, 483, 52–59.
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228.
- Lee, H.; Lee, K.S. Interlayer Distance Controlled Graphene, Supercapacitor and Method of Producing the Same. U.S. Patent 10,214,422, 26 February 2019.
- Han, J.T.; Jang, J.I.; Cho, J.Y.; Hwang, J.Y.; Woo, J.S.; Jeong, H.J.; Jeong, S.Y.; Seo, S.H.; Lee, G.-W. Synthesis of nanobelt-like 1-dimensional silver/nanocarbon hybrid materials for flexible and wearable electronics. Sci. Rep. 2017, 7, 4931.
- Schwierz, F. Graphene transistors: Status, prospects, and problems. Proc. IEEE 2013, 101, 1567–1584.
- Marconcini, P.; Macucci, M. Transport Simulation of Graphene Devices with a Generic Potential in the Presence of an Orthogonal Magnetic Field. Nanomaterials 2022, 12, 1087.
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for biology and medicine: Sensing, imaging, and drug delivery. Chem. Rev. 2019, 119, 9559–9656.
- Tang, C.; Titirici, M.-M.; Zhang, Q. A review of nanocarbons in energy electrocatalysis: Multifunctional substrates and highly active sites. J. Energy Chem. 2017, 26, 1077–1093.
- Bao, Q.; Zhang, H.; Yang, J.x.; Wang, S.; Tang, D.Y.; Jose, R.; Ramakrishna, S.; Lim, C.T.; Loh, K.P. Graphene–polymer nanofiber membrane for ultrafast photonics. Adv. Funct. Mater. 2010, 20, 782–791.
- Wazalwar, R.; Sahu, M. Novel applications of graphene in the aerospace industry. In Novel Applications of Carbon Based Nano-Materials; CRC Press: Boca Raton, FL, USA, 2022; pp. 180–198.
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408.
- Eigler, S.; Dotzer, C.; Hof, F.; Bauer, W.; Hirsch, A. Sulfur species in graphene oxide. Chem. Eur. J. 2013, 19, 9490–9496.
- Bailey, E.J.; Winey, K.I. Dynamics of polymer segments, polymer chains, and nanoparticles in polymer nanocomposite melts: A review. Prog. Polym. Sci. 2020, 105, 101242.
- Zhao, Q.; Qi, H.J.; Xie, T. Recent progress in shape memory polymer: New behavior, enabling materials, and mechanistic understanding. Prog. Polym. Sci. 2015, 49, 79–120.
- Pilate, F.; Toncheva, A.; Dubois, P.; Raquez, J.-M. Shape-memory polymers for multiple applications in the materials world. Eur. Polym. J. 2016, 80, 268–294.
- Ghori, S.W.; Siakeng, R.; Rasheed, M.; Saba, N.; Jawaid, M. The role of advanced polymer materials in aerospace. In Sustainable Composites for Aerospace Applications; Jawaid, M., Thariq, M., Eds.; Woodhead Publishing, Elsevier: Amsterdam, The Netherlands, 2018; pp. 19–34.
- Ramli, N.; Norkhairunnisa, M.; Ando, Y.; Abdan, K.; Leman, Z. Advanced Polymer Composite for Aerospace Engineering Applications. In Advanced Composites in Aerospace Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–21.
- Zeyrek, B.; Aydogan, B.; Dilekcan, E. Review of Thermoplastic Composites in Aerospace Industry. Int. J. Eng. Tech. Inf. 2022, 3, 1–6.
- Norkhairunnisa, M.; Chai Hua, T.; Sapuan, S.; Ilyas, R. Evolution of Aerospace Composite Materials. In Advanced Composites in Aerospace Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 367–385.
- Pervaiz, M.; Panthapulakkal, S.; Sain, M.; Tjong, J. Emerging trends in automotive lightweighting through novel composite materials. Mater. Sci. Appl. 2016, 7, 26.
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, present and future prospective of global carbon fibre composite developments and applications. Compos. Part B Eng. 2022, 250, 110463.
- McIlhagger, A.; Archer, E.; McIlhagger, R. Manufacturing processes for composite materials and components for aerospace applications. In Polymer Composites in the Aerospace Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 59–81.
- Long, V.T.; Tung, H.V. Thermomechanical Nonlinear Buckling of Pressurized Shear Deformable FGM Cylindrical Shells Including Porosities and Elastically Restrained Edges. J. Aerosp. Eng. 2021, 34, 04021011.
- Weber, T.A.; Ruff-Stahl, H.-J.K. Advances in composite manufacturing of helicopter parts. Int. J. Aviat. Aeronaut. Aerosp. 2017, 4, 5.
- Tiwari, K.A.; Raisutis, R.; Samaitis, V. Hybrid signal processing technique to improve the defect estimation in ultrasonic non-destructive testing of composite structures. Sensors 2017, 17, 2858.
- Duodu, E.; Gu, J.; Shang, Z.; Ding, W.; Tang, S. Damage induced by high-velocity impact on composite structures using finite element simulation. Iran. J. Sci. Technol. Trans. Mech. Eng. 2017, 41, 97–107.
- Rana, S.; Fangueiro, R. Advanced Composite Materials for Aerospace Engineering: Processing, Properties and Applications; Woodhead Publishing: Sawston, UK, 2016.
- Wang, Z.; Zulifqar, A.; Hu, H. Auxetic composites in aerospace engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 213–240.
- Carey, J.; Melenka, G.; Hunt, A.; Cheung, B.; Ivey, M.; Ayranci, C. Braided composites in aerospace engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 175–212.
- Kamiyama, S.; Hirano, Y.; Okada, T.; Ogasawara, T. Lightning strike damage behavior of carbon fiber reinforced epoxy, bismaleimide, and polyetheretherketone composites. Compos. Sci. Technol. 2018, 161, 107–114.
- Adam, T.J.; Liao, G.; Petersen, J.; Geier, S.; Finke, B.; Wierach, P.; Kwade, A.; Wiedemann, M. Multifunctional composites for future energy storage in aerospace structures. Energies 2018, 11, 335.
- Padture, N.P. Advanced structural ceramics in aerospace propulsion. Nat. Mater. 2016, 15, 804.
- Ghoshal, S. Polymer/carbon nanotubes (CNT) nanocomposites processing using additive manufacturing (three-dimensional printing) technique: An overview. Fibers 2017, 5, 40.
- Safaei, B.; Moradi-Dastjerdi, R.; Behdinan, K.; Chu, F. Critical buckling temperature and force in porous sandwich plates with CNT-reinforced nanocomposite layers. Aerosp. Sci. Technol. 2019, 91, 175–185.
- Rafique, I.; Kausar, A.; Anwar, Z.; Muhammad, B. Exploration of epoxy resins, hardening systems, and epoxy/carbon nanotube composite designed for high performance materials: A review. Polym.-Plast. Technol. Eng. 2016, 55, 312–333.
- Ji, C.; Yan, C.; Wang, Y.; Xiong, S.; Zhou, F.; Li, Y.; Sun, R.; Wong, C.-P. Thermal conductivity enhancement of CNT/MoS2/graphene− epoxy nanocomposites based on structural synergistic effects and interpenetrating network. Compos. Part B Eng. 2019, 163, 363–370.
- Wang, F.; Zhang, K.; Liang, W.; Wang, Z.; Tay, T.E.; Lu, S.; Yang, B. Epoxy/ X nanocomposite: Improved quasi-static, dynamic fracture toughness, and conductive functionalities by non-ionic surfactant treatment. Polym. Test. 2020, 81, 106256.
- Leng, J.; Lau, A.K.-T. Multifunctional Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2010.
- Cao, M.; Xiong, D.B.; Yang, L.; Li, S.; Xie, Y.; Guo, Q.; Li, Z.; Adams, H.; Gu, J.; Fan, T. Ultrahigh electrical conductivity of graphene embedded in metals. Adv. Funct. Mater. 2019, 29, 1806792.
- Kausar, A. Shape memory polymer/graphene nanocomposites: State-of-the-art. e-Polym. 2022, 22, 165–181.
- Garg, K.K.; Pandey, S.; Kumar, A.; Rana, A.; Sahoo, N.G.; Singh, R.K. Graphene nanosheets derived from waste plastic for cost-effective thermoelectric applications. Results Mater. 2022, 13, 100260.
- Evgin, T.; Turgut, A.; Hamaoui, G.; Spitalsky, Z.; Horny, N.; Micusik, M.; Chirtoc, M.; Sarikanat, M.; Omastova, M. Size effects of graphene nanoplatelets on the properties of high-density polyethylene nanocomposites: Morphological, thermal, electrical, and mechanical characterization. Beilstein J. Nanotechnol. 2020, 11, 167–179.
- Sing, S.L.; Yeong, W.Y. Emerging Materials for Additive Manufacturing. Materials 2022, 16, 127.
- Ahmadi, Z. Epoxy in nanotechnology: A short review. Prog. Org. Coat. 2019, 132, 445–448.
- Ahmad, H.; Laini, V.V.; Qian, T.Z.; Jelani, R.M.; Rosli, F.A.; Kamaruzaman, S. Efficient removal of lead from aqueous using hybrid graphite nanoflakes/mesoporous silica nanoparticles, amine functionalized mesoporous silica and graphite nanoflakes as adsorbents. Malays. J. Anal. Sci. 2020, 24, 236–246.
- Nie, Q.; Wei, X.; Qin, X.; Huang, Y.; Chen, G.; Yang, L.; Wang, B.; Tang, W. Microstructure and properties of graphite nanoflakes/Cu matrix composites fabricated by pressureless sintering and subsequent thermo-mechanical treatment. Diam. Relat. Mater. 2020, 108, 107948.
- Dimic-Misic, K.; Buffiere, J.; Imani, M.; Nieminen, K.; Sixta, H.; Gane, P. Improved stabilisation of graphite nanoflake dispersions using hydrothermally-produced nanocellulose. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125668.
- Eayal Awwad, K.; Yousif, B.; Fallahnezhad, K.; Saleh, K.; Zeng, X. Influence of Graphene Nanoplatelets on Mechanical Properties and Adhesive Wear Performance of Epoxy-Based Composites. Friction 2020, 9, 856–875.
- Vincent, V.A.; Kailasanathan, C.; Shanmuganathan, V.; Kumar, J.S.P.; Prakash, V.A. Strength characterization of caryota urens fibre and aluminium 2024-T3 foil multi-stacking sequenced SiC-toughened epoxy structural composite. Biomass Convers. Biorefin. 2020, 12, 4009–4019.
- Jayabalakrishnan, D.; Saravanan, K.; Ravi, S.; Prabhu, P.; Maridurai, T.; Prakash, V.A. Fabrication and characterization of acrylonitrile butadiene rubber and stitched E-glass fibre tailored Nano-silica epoxy resin composite. Silicon 2020, 13, 2509–2517.
- Qiu, J.; Wang, S. Enhancing polymer performance through graphene sheets. J. Appl. Polym. Sci. 2011, 119, 3670–3674.
- Kausar, A. Nanodiamond Reinforced Polymer Nanocomposite: Prospective Corrosion Protection Materials. In Phenomena and Theories in Corrosion Science, Methods of Prevention; Andras, G., Ed.; NOVA Science Publishers Inc.: Hauppauge, NY, USA, 2019; p. 181.
- Xue, G.; Zhang, B.; Sun, M.; Zhang, X.; Li, J.; Wang, L.; Song, C. Morphology, thermal and mechanical properties of epoxy adhesives containing well-dispersed graphene oxide. Int. J. Adhes. Adhes. 2019, 88, 11–18.
- Gopanna, A.; Rajan, K.P.; Thomas, S.P.; Chavali, M. Polyethylene and polypropylene matrix composites for biomedical applications. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–216.
- Suner, S.; Joffe, R.; Tipper, J.; Emami, N. Ultra high molecular weight polyethylene/graphene oxide nanocomposites: Thermal, mechanical and wettability characterisation. Compos. Part B Eng. 2015, 78, 185–191.
- Bhawal, P.; Ganguly, S.; Das, T.K.; Mondal, S.; Choudhury, S.; Das, N. Superior electromagnetic interference shielding effectiveness and electro-mechanical properties of EMA-IRGO nanocomposites through the in-situ reduction of GO from melt blended EMA-GO composites. Compos. Part B Eng. 2018, 134, 46–60.
- Song, P.; Cao, Z.; Cai, Y.; Zhao, L.; Fang, Z.; Fu, S. Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties. Polymer 2011, 52, 4001–4010.
- Feicht, P.; Biskupek, J.; Gorelik, T.E.; Renner, J.; Halbig, C.E.; Maranska, M.; Puchtler, F.; Kaiser, U.; Eigler, S. Brodie’s or Hummers’ method: Oxidation conditions determine the structure of graphene oxide. Chem. Eur. J. 2019, 25, 8955–8959.
- Saha, M.; Tambe, P.; Pal, S. Thermodynamic approach to enhance the dispersion of graphene in epoxy matrix and its effect on mechanical and thermal properties of epoxy nanocomposites. Compos. Interfaces 2016, 23, 255–272.
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490.
- Huang, G.; Chen, S.; Song, P.; Lu, P.; Wu, C.; Liang, H. Combination effects of graphene and layered double hydroxides on intumescent flame-retardant poly (methyl methacrylate) nanocomposites. Appl. Clay Sci. 2014, 88, 78–85.
- Singho, N.D.; Lah, N.A.C.; Johan, M.R.; Ahmad, R. FTIR studies on silver-poly (methylmethacrylate) nanocomposites via in-situ polymerization technique. Int. J. Electrochem. Sci. 2012, 7, 5596–5603.
- Xu, Y.; Hong, W.; Bai, H.; Li, C.; Shi, G. Strong and ductile poly (vinyl alcohol)/graphene oxide composite films with a layered structure. Carbon 2009, 47, 3538–3543.
- Chen, B.; Berretta, S.; Evans, K.; Smith, K.; Ghita, O. A primary study into graphene/polyether ether ketone (PEEK) nanocomposite for laser sintering. Appl. Surf. Sci. 2018, 428, 1018–1028.
- Arif, M.; Alhashmi, H.; Varadarajan, K.; Koo, J.H.; Hart, A.; Kumar, S. Multifunctional performance of carbon nanotubes and graphene nanoplatelets reinforced PEEK composites enabled via FFF additive manufacturing. Compos. Part B Eng. 2020, 184, 107625.
- Xu, F.; Wu, X.; Wang, H.; Liu, H.; Ye, Z. Experimental research on moulding of graphene/PEKK composite powder by spark plasma sintering technology. Appl. Phys. A 2022, 128, 122.
- Cortes, L.Q.; Racagel, S.; Lonjon, A.; Dantras, E.; Lacabanne, C. Electrically conductive carbon fiber/PEKK/silver nanowires multifunctional composites. Compos. Sci. Technol. 2016, 137, 159–166.
- Shan, C.; Wang, L.; Han, D.; Li, F.; Zhang, Q.; Zhang, X.; Niu, L. Polyethyleneimine-functionalized graphene and its layer-by-layer assembly with Prussian blue. Thin Solid Film. 2013, 534, 572–576.
- Sali, S.; Mackey, H.R.; Abdala, A.A. Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 2019, 9, 769.
- Puértolas, J.; Castro, M.; Morris, J.; Ríos, R.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122.
- Alvaredo, Á.; Martín, M.I.; Castell, P.; Guzmán de Villoria, R.; Fernández-Blázquez, J.P. Non-isothermal crystallization behavior of PEEK/graphene nanoplatelets composites from melt and glass states. Polymers 2019, 11, 124.
- Wang, Q.; Jia, D.; Pei, X.; Wu, X.; Xu, F.; Wang, H.; Cao, M.; Chen, H. Investigation of electromagnetic pulse compaction on conducting graphene/PEKK composite powder. Materials 2021, 14, 636.
- Sun, Z.; Li, Y.-Q.; Huang, P.; Cao, H.-J.; Zeng, W.; Li, J.; Li, F.; Sun, B.-G.; Shi, H.-Q.; Zhou, Z.-l. Temperature-dependent mechanical properties of polyetherimide composites reinforced by graphene oxide-coated short carbon fibers. Compos. Struct. 2021, 270, 114075.
- Ionita, M.; Pandele, A.M.; Crica, L.; Pilan, L. Improving the thermal and mechanical properties of polysulfone by incorporation of graphene oxide. Compos. Part B Eng. 2014, 59, 133–139.
- Peña-Bahamonde, J.; San-Miguel, V.; Baselga, J.; Fernández-Blázquez, J.P.; Gedler, G.; Ozisik, R.; Cabanelas, J.C. Effect of polysulfone brush functionalization on thermo-mechanical properties of melt extruded graphene/polysulfone nanocomposites. Carbon 2019, 151, 84–93.
More
Information
Subjects:
Physics, Applied
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
804
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





