Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masahiro Ayano | -- | 2557 | 2023-03-14 12:49:21 | | | |
| 2 | Lindsay Dong | -21 word(s) | 2536 | 2023-03-15 09:03:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ayano, M.; Horiuchi, T. Complement as a Biomarker for Systemic Lupus Erythematosus. Encyclopedia. Available online: https://encyclopedia.pub/entry/42179 (accessed on 07 February 2026).
Ayano M, Horiuchi T. Complement as a Biomarker for Systemic Lupus Erythematosus. Encyclopedia. Available at: https://encyclopedia.pub/entry/42179. Accessed February 07, 2026.
Ayano, Masahiro, Takahiko Horiuchi. "Complement as a Biomarker for Systemic Lupus Erythematosus" Encyclopedia, https://encyclopedia.pub/entry/42179 (accessed February 07, 2026).
Ayano, M., & Horiuchi, T. (2023, March 14). Complement as a Biomarker for Systemic Lupus Erythematosus. In Encyclopedia. https://encyclopedia.pub/entry/42179
Ayano, Masahiro and Takahiko Horiuchi. "Complement as a Biomarker for Systemic Lupus Erythematosus." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Systemic lupus erythematosus (SLE) is a disease of immune complex deposition; therefore, complement plays a vital role in the pathogenesis of SLE. In general, complement levels in blood and complement deposition in histological tests are used for the management of SLE. Thus, the evaluation of complement status can be useful in the diagnosis of SLE, assessment of disease activity, and prediction of treatment response and prognosis. In addition, novel complement biomarkers, such as split products and cell-bound complement activation products, are considered to be more sensitive than traditional complement markers, such as serum C3 and C4 levels and total complement activity (CH50), which become more widely used.
systemic lupus erythematosus
lupus nephritis
complement
1. Introduction
Systemic lupus erythematosus (SLE) is a typical systemic autoimmune disease characterized by the production of a variety of autoantibodies and the formation of immune complexes, with a chronic disease course and diverse organ involvement [1][2][3]. In 2014, besides the enhancement of SLE drugs, the treat-to-target concept was proposed for SLE [4], as previously proposed for rheumatoid arthritis. In addition, the basic framework of treatment strategies for SLE was established. In a treat-to-target strategy, treatment aiming at remission or where remission cannot be reached, the lowest possible disease activity must be conducted after periodically assessing overall SLE disease activity [4]. Furthermore, this strategy aims to ensure long-term survival, prevent organ damage, and optimize health-related quality of life by controlling disease activity and minimizing comorbidities and drug toxicity [4]. Therefore, these treatment strategies require strict management based on disease activity monitoring. Biomarkers can be used in this management, but they have not yet been established because SLE is associated with various organs with diverse immunological abnormalities [5][6][7].
SLE is a disease of immune complex deposition; thus, complements play a vital role in the pathogenesis of SLE [8][9][10]. In SLE, complements have a biphasic nature, which is known as the “lupus paradox [11].” That is, complement activation via immune complexes deposited in tissues causes tissue damage, whereas congenital deficiencies of the early components of complement activation pathways, such as C1q and C4, which are involved in the processing of apoptotic cells, frequently lead to the development of SLE [12]. In SLE, complement tests are usually abnormal, indicating complement-mediated pathologies. In general, complement levels in blood and complement deposition in histological tests are used for the management of SLE. Moreover, complement biomarkers can be useful in various settings, including the diagnosis of SLE, assessment of disease activity, and prediction of treatment response and prognosis.
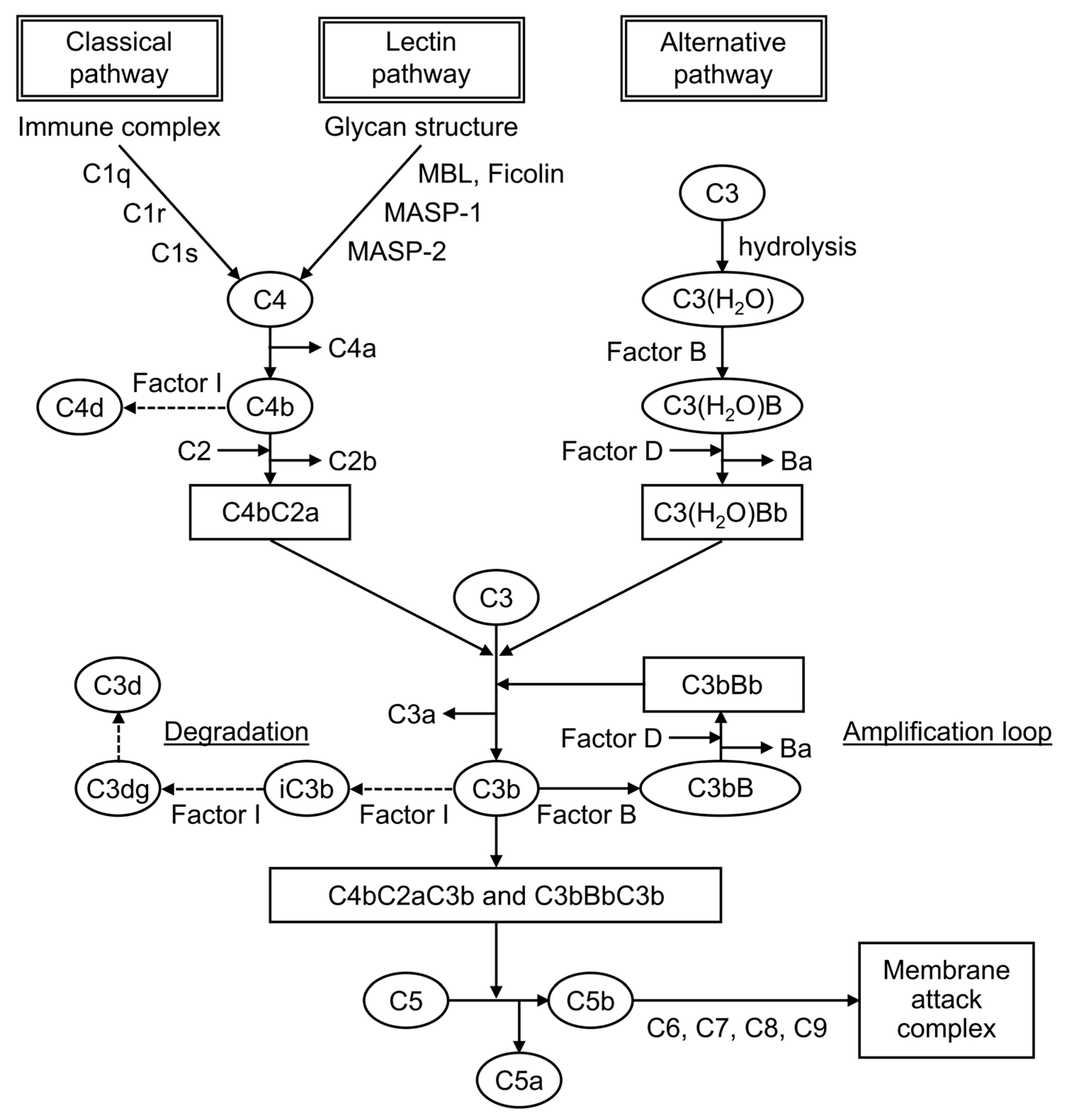
2. Complement
Complement is formed from more than 30 components found in blood and cell membranes, including plasma proteins, receptors on cell membranes, and regulatory factors. As shown in Figure 1, complement activation occurs through a series of chain reactions, which is known as a cascade. C3 activation is the most critical event. The complement response can be divided into two pathways: the “complement activation pathway” until C3 is degraded and the “late complement pathway” until the subsequent formation of the membrane attack complex. The complement activation pathway is divided into three: the classical pathway, the lectin pathway, and the alternative pathway. After C3 activation, the cascade to its deposition on a target as C3b and the formation of membrane attack complexes below C5 are common.

Figure 1. The complement cascade.
The factors that trigger the complement activation pathway are distinct, and they mediate different complement molecules. In the classical pathway, when an antibody binds to an antigen to form an immune complex, C1q binds to this immune complex and immediately activates C1r, which activates C1s. Then, this activated C1s activates C4 and then C2, leading to form C4bC2a, which is a C3-converting enzyme that activates C3. When C3 is activated, it is cleaved into fragments C3a and C3b, and C3b associates with C4bC2a to form C4bC2aC3b, a C5-converting enzyme that activates C5. It mediates the cleavage of the α-chain of C3b, leading to the formation of iC3b, followed by its further degradation into C3dg and C3d. In the lectin pathway, when mannose-binding lectin (MBL) and ficolin recognize and bind to the characteristic glycan structure of the pathogen cell wall, MBL-associated serine protease, a serine protease that is bound to MBL, is activated.
3. Complement Tests
In routine clinical practice, serum C3 and C4 levels and CH50 are usually measured in blood samples, and complement deposition is identified by fluorescent antibody assay of tissues.
C3 and C4 are proteins of the complement cascade. In general, C3 and C4 are measured in serum, but samples such as urine, pleural fluid, and spinal fluid can also be used. In SLE, hypocomplementemia, decreased levels either of C3 and C4 or both occurs as an immunological abnormality. C3 is included in the common cascade after the convergence of the three pathways. By contrast, C4 is included in the classical and lectin pathways. Therefore, low levels of both proteins indicate the activation of these two pathways, whereas low C3 and normal C4 indicate the activation of the alternative pathway.
Plasma C4 levels are strongly influenced by and correlated with C4 gene copy numbers [13][14][15][16]. Each copy of the C4 gene encodes for one of two isotypes, acidic C4A or basic C4B. Congenital deficiency or a low copy number of the C4A gene has been associated with the development of SLE [17]. By contrast, a low C4B gene copy number did not show this association, and a medium to high C4B copy number was associated with thrombosis and hypertension in patients with pediatric SLE [13]. The profiles of C4 and C3 protein levels are different in each patient with SLE depending on the C4 gene copy number; therefore, there is a group of patients with low C4 levels due to low C4 gene copy number and without reflecting disease activity [14]. If only C4 shows persistently low levels, then the possibility of a low copy number of the C4 genes should be considered in the evaluation [14][15][16].
CH50 measures the hemolytic activity of serum samples against sensitized sheep erythrocytes. It is indexed by its ability to form membrane attack complexes via the classical pathway, and it reflects immune complex formation. It helps in complement-screening tests because it simultaneously measures all the complement activities from C1 to C9. In active SLE, CH50 levels are low because of complement consumption caused by the increased activation of the classical pathway. If they are extremely low, then the possibility of a congenital defect is considered. In the case of low CH50 levels with normal C3 and C4 levels, it is important to exclude artificial in vitro complement activation (cold activation) after the blood sample is taken.
Complements are also used in the immunological testing of tissues. In SLE, reactions to C1q, C3, and C4 are observed in the renal glomeruli and at the dermo–epidermal border of the skin by fluorescent antibody assay. It is useful in evaluating affected organs and in the differentiation of other diseases.
Other novel biomarkers used include C1q, which is essential for the activation of the classical pathway; split product, which is a degraded product of complement; and cell-bound complement activation product (CB-CAP), a cell surface binding of C4d.
4. Traditional Complement Markers in SLE Diagnosis
4.1. SLE Diagnosis
The diagnosis of SLE has no gold standard; thus, it is based on classification criteria that reflect the variety of immunological abnormalities and diverse organ involvement characteristics of SLE. Although the SLE classification criteria have been revised in recent years, the currently used 2019 European League Against Rheumatism (EULAR) and American College of Rheumatology (ACR) classification criteria for SLE emphasizes immunological abnormalities and nephritis [18]. Consequently, complement, which was not included in the 1997 ACR classification criteria, was added to the list of immune domains in the current classification criteria, along with SLE-specific and antiphospholipid antibodies [18][19][20]. Hypocomplementemia is defined as a decrease in C3, C4, or CH50 based on the 2012 Systemic Lupus International Collaborating Clinics classification criteria, or low C3 and/or C4 based on the 2019 EULAR/ACR classification criteria [18][20]. A decrease in both C3 and C4, which suggests the involvement of the classical pathway, was considered as a more important finding, which was given a higher weight. Regarding the validation studies of the 2019 EULAR/ACR classification criteria, the frequency of hypocomplementemia (low C3 or C4) at diagnosis of SLE is 50%–89%, which is not commonly observed in all cases [21][22][23].
4.2. Diagnosis and Prediction of Organ Involvement
After the diagnosis of SLE, proper evaluation of the affected organs is important to provide optimal treatment. SLE presents with a variety of organ involvement, and it may be classified into several subgroups based on the organs affected and immunological abnormalities. Studies investigating the relationship between complements and organ involvement have shown the association of hypocomplementemia with nephritis, hematologic disorders such as autoimmune hemolytic anemia and thrombocytopenia, skin rash, and arthritis [24][25][26].
The measurement of complements is also useful in appropriately ruling out other diseases to confirm whether organ involvement is caused by SLE. In a report in which renal biopsies were performed on 48 patients with SLE without abnormal urinalysis and renal impairment, 36 patients had lupus nephritis (class I or II, 72%; class III or IV, 17%; and type V, 11%), and hypocomplementemia (low titers of CH50 and C3) and a high titer of anti-Sm antibodies were identified as predictive factors for silent lupus nephritis. Complements measured in samples other than serum are used to differentiate other diseases [27].
5. Traditional Complement Markers in the Assessment of SLE Activity
In a treat-to-target strategy, SLE treatment is aimed at remission or, at least, low disease activity after regular evaluation of the overall disease activity [4]. Therefore, disease activity assessment indicators and treatment goals must be established.
In general, the assessment of disease activity in SLE is based on a combination of the type and severity of organ involvement and immunological abnormalities. Complements, along with anti-dsDNA antibody titers, are used in routine clinical practice as a biomarker for immunological abnormalities in SLE, and hypocomplementemia reflects the activity of SLE. The systemic lupus erythematosus disease activity index (SLEDAI), the validated activity index, frequently used for assessing global SLE activity, includes the presence of low complements, that is the decrease in CH50, C3, or C4 in qualitative assessment [28]. On the contrary, the British Isles Lupus Assessment Group index, which evaluates disease activity by each organ-based system, does not include immunological abnormalities [29].
A composite definition is also used for low disease activity and remission, which are treatment targets of SLE. Although no criteria have been established, the lupus low disease activity state (LLDAS) is used as a general low disease activity criterion, and the definitions of remission in SLE (DORIS) is used as a remission criterion [30][31]. These composite indices combine the disease activity measured using the SLEDAI, the exclusion of any current disease activity not included in the SLEDAI, physician global assessment, and medication use, including glucocorticoid dosage. The definition of LLDAS allows for a SLEDAI-2K score of 4 or less. LLDAS may also be met, although hypocomplementemia and elevated anti-dsDNA antibodies remain unchanged. On the contrary, DORIS initially proposed complete remission, which requires no serological activities [32].
6. Traditional Complement Markers in Predicting SLE Treatment Response
In recent years, biologic drugs such as belimumab, an anti-B-cell activating factor (BAFF) monoclonal antibody, and anifrolumab, an anti-interferon-α receptor monoclonal antibody, have become available for SLE. Compared with glucocorticoids and conventional immunosuppressive drugs, which act on a relatively broad range of immune cells, biologic drugs have a clear therapeutic target, and the mechanism of action of the drug may predict treatment response. Belimumab is known to be more effective at high BAFF levels and anifrolumab at high interferon signatures [33][34]. However, these markers cannot be measured in routine clinical practice. Thus, markers predicting response to biologic drugs must be established within routine clinical practice.
7. Traditional Complement Markers in Predicting SLE Prognosis
The prevention of flares is an important goal in the management of SLE. Early detection of signs of flares through careful monitoring of the global and organ-specific disease activity is essential, but understanding the risk factors that predict flares is also important. The 2019 update of the EULAR recommendations for the management of SLE identifies persistent serological activity (low complement and/or high anti-dsDNA antibodies) as risk factors, along with younger age at disease onset, no use of antimalarials, and persistent generalized disease activity [35].
In routine clinical practice, the assessment of the disease activity often considers the fluctuation in complement levels and anti-dsDNA antibody titers, but many clinical studies have reported the presence of hypocomplementemia at a single point in time, such as at the achievement of remission or at the beginning of observation. Gensous et al. and Kostopoulou et al. conducted a systematic literature review on whether complement predicts SLE flares and reported that many studies have shown that hypocomplementemia (low serum C3 and C4 levels) is a predictor of flares [36][37].
8. Novel Complement Biomarkers in SLE Management
8.1. C1q
C1q is essential for activating the classical pathway, and a decrease in C1q levels, as with C3 and C4 levels, indicates high disease activity in SLE. Although the utility of C1q deposition in renal tissue and anti-C1q antibodies in the management of SLE is well established [38], serum C1q can also be a useful biomarker. Serum C1q levels were decreased in patients with SLE compared with healthy controls, and they were associated with disease activity as measured by SLEDAI [39]. Serum C1q levels were also decreased in patients with lupus nephritis compared with healthy controls and patients with SLE uncomplicated by nephritis, indicating the activity of nephritis and the histological activity score of renal tissue [39][40][41].
8.2. Split Products
Split products are the cleavage fragments of the complement components. Known cleavage fragments of C3 include iC3b, C3dg, and C3d, and C4d is a cleavage fragment of C4 (Figure 1). The split products are only produced by complement activation, unaffected by increased production by the acute-phase response. Therefore, the assay is characterized by its ability to reflect complement activation in vivo more sensitively than the currently used C3 and C4.
iC3b is the breakdown product of C3b, a cleavage fragment of C3. Serum iC3b levels were elevated in patients with SLE compared with healthy controls, and the serum iC3b/C3 ratio was more sensitive to changes in disease activity than serum C3 or C4 levels, making it more useful for detecting active SLE and predicting flares [42].
C4d is a cleavage product of C4b produced from the degradation of C4. Plasma C4d levels were elevated in patients with SLE compared with healthy controls, and they were particularly high in patients with nephritis [43][44]. Plasma C4d levels correlated with C4d deposition in renal tissue, indicating nephritis activity, and they could be used in predicting flares and treatment response [44].
8.3. Cell-Bound Complement Activation Product (CB-CAP)
C4d is not only present in plasma, but it is also bound to the surface of blood cells. It is known as CB-CAP, which binds to erythrocytes, platelets, and B cells. C4d on each cell surface is measured using a flow cytometer. In 2004, erythrocyte C4d was measured and reported to be a biomarker with better sensitivity and specificity than traditional complement markers for the diagnosis of SLE [45].
References
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update in the Diagnosis and Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25.
- Pan, L.; Lu, M.P.; Wang, J.H.; Xu, M.; Yang, S.R. Immunological Pathogenesis and Treatment of Systemic Lupus Erythematosus. World J. Pediatr. 2020, 16, 19–30.
- Dörner, T.; Furie, R. Novel Paradigms in Systemic Lupus Erythematosus. Lancet 2019, 393, 2344–2358.
- van Vollenhoven, R.F.; Mosca, M.; Bertsias, G.; Isenberg, D.; Kuhn, A.; Lerstrøm, K.; Aringer, M.; Bootsma, H.; Boumpas, D.; Bruce, I.N.; et al. Treat-to-Target in Systemic Lupus Erythematosus: Recommendations from an International Task Force. Ann. Rheum. Dis. 2014, 73, 958–967.
- Capecchi, R.; Puxeddu, I.; Pratesi, F.; Migliorini, P. New Biomarkers in SLE: From Bench to Bedside. Rheumatology 2020, 59, V12–V18.
- González, L.A.; Ugarte-Gil, M.F.; Alarcón, G.S. Systemic Lupus Erythematosus: The Search for the Ideal Biomarker. Lupus 2021, 30, 181–203.
- Yu, H.; Nagafuchi, Y.; Fujio, K. Clinical and Immunological Biomarkers for Systemic Lupus Erythematosus. Biomolecules 2021, 11, 928.
- Weinstein, A.; Alexander, R.V.; Zack, D.J. A Review of Complement Activation in SLE. Curr. Rheumatol. Rep. 2021, 23, 4–11.
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The Complement System in Systemic Lupus Erythematosus: An Update. Ann. Rheum. Dis. 2014, 73, 1601–1606.
- Sharma, M.; Vignesh, P.; Tiewsoh, K.; Rawat, A. Revisiting the Complement System in Systemic Lupus Erythematosus. Expert Rev. Clin. Immunol. 2020, 16, 397–408.
- Carroll, M.C. The Lupus Paradox. Nat. Genet. 1998, 19, 3–4.
- Macedo, A.C.L.; Isaac, L. Systemic Lupus Erythematosus and Deficiencies of Early Components of the Complement Classical Pathway. Front. Immunol. 2016, 7, 55.
- Mulvihill, E.; Ardoin, S.; Thompson, S.D.; Zhou, B.; Yu, G.R.; King, E.; Singer, N.; Levy, D.M.; Brunner, H.; Wu, Y.L.; et al. Elevated Serum Complement Levels and Higher Gene Copy Number of Complement C4B Are Associated with Hypertension and Effective Response to Statin Therapy in Childhood-Onset Systemic Lupus Erythematosus (SLE). Lupus Sci. Med. 2019, 6, e000333.
- Wu, Y.L.; Higgins, G.C.; Rennebohm, R.M.; Chung, E.K.; Yang, Y.; Zhou, B.; Nagaraja, H.N.; Birmingham, D.J.; Rovin, B.H.; Hebert, L.A.; et al. Three distinct profiles of serum complement C4 proteins in pediatric systemic lupus erythematosus (SLE) patients: Tight associations of complement C4 and C3 protein levels in SLE but not in healthy subjects. Adv. Exp. Med. Biol. 2006, 586, 227–247.
- Margery-Muir, A.A.; Bundell, C.; Wetherall, J.D.; Whidborne, R.; Martinez, P.; Groth, D.M. Insights on the Relationship between Complement Component C4 Serum Concentrations and C4 Gene Copy Numbers in a Western Australian Systemic Lupus Erythematosus Cohort. Lupus 2018, 27, 1687–1696.
- Lundtoft, C.; Pucholt, P.; Martin, M.; Bianchi, M.; Lundström, E.; Eloranta, M.L.; Sandling, J.K.; Sjöwall, C.; Jönsen, A.; Gunnarsson, I.; et al. Complement C4 Copy Number Variation Is Linked to SSA/Ro and SSB/La Autoantibodies in Systemic Inflammatory Autoimmune Diseases. Arthritis Rheumatol. 2022, 74, 1440–1450.
- Savelli, S.L.; Roubey, R.A.S.; Kitzmiller, K.J.; Zhou, D.; Nagaraja, H.N.; Mulvihill, E.; Barbar-Smiley, F.; Ardoin, S.P.; Wu, Y.L.; Yu, C.Y. Opposite Profiles of Complement in Antiphospholipid Syndrome (APS) and Systemic Lupus Erythematosus (SLE) among Patients with Antiphospholipid Antibodies (APL). Front. Immunol. 2019, 10, 885.
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412.
- Hochberg, M.C. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997, 40, 1725.
- Petri, M.; Orbai, A.M.; Alarcõn, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and Validation of the Systemic Lupus International Collaborating Clinics Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheum. 2012, 64, 2677–2686.
- Pons-Estel, G.J.; Ugarte-Gil, M.F.; Harvey, G.B.; Wojdyla, D.; Quintana, R.; Saurit, V.; Soriano, E.R.; Bonfa, E.; Massardo, L.; Cardiel, M.; et al. Applying the 2019 EULAR/ACR Lupus Criteria to Patients from an Established Cohort: A Latin American Perspective. RMD Open 2020, 6, e001097.
- Johnson, S.R.; Brinks, R.; Costenbader, K.H.; Daikh, D.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. Performance of the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in Early Disease, across Sexes and Ethnicities. Ann. Rheum. Dis. 2020, 79, 1333–1339.
- Chung, Y.K.; Ho, L.Y.; Lee, C.; To, C.H.; Mok, C.C. Validation of the 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in ANA-Positive Chinese Patients. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221100300.
- Reynolds, J.A.; McCarthy, E.M.; Haque, S.; Ngamjanyaporn, P.; Sergeant, J.C.; Lee, E.; Lee, E.; Kilfeather, S.A.; Parker, B.; Bruce, I.N. Cytokine Profiling in Active and Quiescent SLE Reveals Distinct Patient Subpopulations. Arthritis Res. Ther. 2018, 9, 173.
- Iwasaki, T.; Doi, H.; Tsuji, H.; Tabuchi, Y.; Hashimoto, M.; Kitagori, K.; Akizuki, S.; Murakami, K.; Nakashima, R.; Yoshifuji, H.; et al. Phenotypic Landscape of Systemic Lupus Erythematosus: An Analysis of the Kyoto Lupus Cohort. Mod. Rheumatol. 2022, 32, 571–576.
- Jung, J.Y.; Lee, H.Y.; Lee, E.; Kim, H.A.; Yoon, D.; Suh, C.H. Three Clinical Clusters Identified through Hierarchical Cluster Analysis Using Initial Laboratory Findings in Korean Patients with Systemic Lupus Erythematosus. J. Clin. Med. 2022, 11, 2406.
- Ishizaki, J.; Saito, K.; Nawata, M.; Mizuno, Y.; Tokunaga, M.; Sawamukai, N.; Tamura, M.; Hirata, S.; Yamaoka, K.; Hasegawa, H.; et al. Low Complements and High Titre of Anti-Sm Antibody as Predictors of Histopathologically Proven Silent Lupus Nephritis without Abnormal Urinalysis in Patients with Systemic Lupus Erythematosus. Rheumatology 2015, 54, 405–412.
- Touma, Z.; Urowitz, M.B.; Gladman, D.D. Systemic Lupus Erythematosus Disease Activity Index 2000 Responder Index-50 Website. J. Rheumatol. 2013, 40, 733.
- Isenberg, D.A.; Rahman, A.; Allen, E.; Farewell, V.; Akil, M.; Bruce, I.N.; D’Cruz, D.; Griffiths, B.; Khamashta, M.; Maddison, P.; et al. BILAG 2004. Development and Initial Validation of an Updated Version of the British Isles Lupus Assessment Group’s Disease Activity Index for Patients with Systemic Lupus Erythematosus. Rheumatology 2005, 44, 902–906.
- Franklyn, K.; Lau, C.S.; Navarra, S.V.; Louthrenoo, W.; Lateef, A.; Hamijoyo, L.; Wahono, C.S.; Chen, S.L.; Jin, O.; Morton, S.; et al. Definition and Initial Validation of a Lupus Low Disease Activity State (LLDAS). Ann. Rheum. Dis. 2016, 75, 1615–1621.
- van Vollenhoven, R.F.; Bertsias, G.; Doria, A.; Isenberg, D.; Morand, E.; Petri, M.A.; Pons-Estel, B.A.; Rahman, A.; Ugarte-Gil, M.F.; Voskuyl, A.; et al. 2021 DORIS Definition of Remission in SLE: Final Recommendations from an International Task Force. Lupus Sci. Med. 2021, 8, e000538.
- van Vollenhoven, R.F.; Voskuyl, A.; Bertsias, G.; Aranow, C.; Aringer, M.; Arnaud, L.; Askanase, A.; Balážová, P.; Bonfa, E.; Bootsma, H.; et al. A Framework for Remission in SLE: Consensus Findings from a Large International Task Force on Definitions of Remission in SLE (DORIS). Ann. Rheum. Dis. 2017, 76, 554–561.
- Wilkinson, C.; Henderson, R.B.; Jones-Leone, A.R.; Flint, S.M.; Lennon, M.; Levy, R.A.; Ji, B.; Bass, D.L.; Roth, D. The Role of Baseline BLyS Levels and Type 1 Interferon-Inducible Gene Signature Status in Determining Belimumab Response in Systemic Lupus Erythematosus: A Post Hoc Meta-Analysis. Arthritis Res. Ther. 2020, 22, 1–11.
- Vital, E.M.; Merrill, J.T.; Morand, E.F.; Furie, R.A.; Bruce, I.N.; Tanaka, Y.; Manzi, S.; Kalunian, K.C.; Kalyani, R.N.; Streicher, K.; et al. Anifrolumab Efficacy and Safety by Type I Interferon Gene Signature and Clinical Subgroups in Patients with SLE: Post Hoc Analysis of Pooled Data from Two Phase III Trials. Ann. Rheum. Dis. 2022, 81, 951–961.
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745.
- Gensous, N.; Marti, A.; Barnetche, T.; Blanco, P.; Lazaro, E.; Seneschal, J.; Truchetet, M.E.; Duffau, P.; Richez, C. Predictive Biological Markers of Systemic Lupus Erythematosus Flares: A Systematic Literature Review. Arthritis Res. Ther. 2017, 19, 238.
- Kostopoulou, M.; Ugarte-Gil, M.F.; Pons-Estel, B.; van Vollenhoven, R.F.; Bertsias, G. The Association between Lupus Serology and Disease Outcomes: A Systematic Literature Review to Inform the Treat-to-Target Approach in Systemic Lupus Erythematosus. Lupus 2022, 31, 307–318.
- Trendelenburg, M. Autoantibodies against Complement Component C1q in Systemic Lupus Erythematosus. Clin. Transl. Immunol. 2021, 10, e1279.
- Sandholm, K.; Persson, B.; Skattum, L.; Eggertsen, G.; Nyman, D.; Gunnarsson, I.; Svenungson, E.; Nilsson, B.; Ekdahl, K.N. Evaluation of a Novel Immunoassay for Quantification of C1q for Clinical Diagnostic Use. Front. Immunol. 2019, 10, 7.
- Tan, Y.; Song, D.; Wu, L.H.; Yu, F.; Zhao, M.H. Serum Levels and Renal Deposition of C1q Complement Component and Its Antibodies Reflect Disease Activity of Lupus Nephritis. BMC Nephrol. 2013, 14, 63.
- Xu, B.; Zhang, Y.M.; Yang, Y.W.; Liu, Y.S.; Feng, J.F. Diagnostic Performance of Serum Cystatin C and Complement Component 1q in Lupus Nephritis. Arthritis Res. Ther. 2019, 21, 267.
- Kim, A.H.J.; Strand, V.; Sen, D.P.; Fu, Q.; Mathis, N.L.; Schmidt, M.J.; Bruchas, R.R.; Staten, N.R.; Olson, P.K.; Stiening, C.M.; et al. Association of Blood Concentrations of Complement Split Product IC3b and Serum C3 with Systemic Lupus Erythematosus Disease Activity. Arthritis Rheumatol. 2019, 71, 420–430.
- Martin, M.; Smolag, K.I.; Björk, A.; Gullstrand, B.; Okrój, M.; Leffler, J.; Jönsen, A.; Bengtsson, A.A.; Blom, A.M. Plasma C4d as Marker for Lupus Nephritis in Systemic Lupus Erythematosus. Arthritis Res. Ther. 2017, 19, 266.
- Martin, M.; Trattner, R.; Nilsson, S.C.; Björk, A.; Zickert, A.; Blom, A.M.; Gunnarsson, I. Plasma C4d Correlates with C4d Deposition in Kidneys and With Treatment Response in Lupus Nephritis Patients. Front. Immunol. 2020, 11, 582737.
- Manzi, S.; Navratil, J.S.; Ruffing, M.J.; Liu, C.C.; Danchenko, N.; Nilson, S.E.; Krishnaswami, S.; King, D.E.S.; Kao, A.H.; Ahearn, J.M. Measurement of Erythrocyte C4d and Complement Receptor 1 in Systemic Lupus Erythematosus. Arthritis Rheum. 2004, 50, 3596–3604.
More
Information
Subjects:
Rheumatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
719
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




