Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jose Rivera | -- | 1481 | 2023-03-14 12:28:25 | | | |
| 2 | Conner Chen | Meta information modification | 1481 | 2023-03-16 04:37:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marín-Quílez, A.; Díaz-Ajenjo, L.; Di Buduo, C.A.; Zamora-Cánovas, A.; Lozano, M.L.; Benito, R.; González-Porras, J.R.; Balduini, A.; Rivera, J.; Bastida, J.M. Role of Glycosylation in Thrombopoiesis and Platelet Clearance. Encyclopedia. Available online: https://encyclopedia.pub/entry/42178 (accessed on 11 March 2026).
Marín-Quílez A, Díaz-Ajenjo L, Di Buduo CA, Zamora-Cánovas A, Lozano ML, Benito R, et al. Role of Glycosylation in Thrombopoiesis and Platelet Clearance. Encyclopedia. Available at: https://encyclopedia.pub/entry/42178. Accessed March 11, 2026.
Marín-Quílez, Ana, Lorena Díaz-Ajenjo, Christian A. Di Buduo, Ana Zamora-Cánovas, María Luisa Lozano, Rocío Benito, José Ramón González-Porras, Alessandra Balduini, José Rivera, José María Bastida. "Role of Glycosylation in Thrombopoiesis and Platelet Clearance" Encyclopedia, https://encyclopedia.pub/entry/42178 (accessed March 11, 2026).
Marín-Quílez, A., Díaz-Ajenjo, L., Di Buduo, C.A., Zamora-Cánovas, A., Lozano, M.L., Benito, R., González-Porras, J.R., Balduini, A., Rivera, J., & Bastida, J.M. (2023, March 14). Role of Glycosylation in Thrombopoiesis and Platelet Clearance. In Encyclopedia. https://encyclopedia.pub/entry/42178
Marín-Quílez, Ana, et al. "Role of Glycosylation in Thrombopoiesis and Platelet Clearance." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Protein glycosylation, including sialylation, involves complex and frequent post-translational modifications, which play a critical role in different biological processes. The conjugation of carbohydrate residues to specific molecules and receptors is critical for normal hematopoiesis, as it favors the proliferation and clearance of hematopoietic precursors. Through this mechanism, the circulating platelet count is controlled by the appropriate platelet production by megakaryocytes, and the kinetics of platelet clearance.
glycosylation
inherited thrombocytopenia
inherited platelet disorder
1. Introduction
Glycosylation is a key process by which carbohydrates or saccharides bind to proteins, lipids, and other biomolecules. It is a highly prevalent, conserved, and complex post-translational alteration [1]. Glycosylation influences a wide range of cellular processes, including control of protein secretion and degradation, cell signaling, adhesion and migration, host–pathogen interactions, or immune defense including both innate and acquired immunity [2][3][4][5].
Glycosylation is a highly modular process, whereby carbohydrate building blocks are repeatedly linked and assembled in varying lengths and branches. It is an unplanned process that gives rise to a wide and diverse repertoire of functional molecules [6] and plays a crucial role in the correct folding of proteins, their stability, and the formation of mature and functional proteins [7].
The presentation of glycans on cell surfaces is governed by more than 200 glycosyltransferases, sugar–nucleotide synthesis, and transport proteins, mainly located in the endoplasmic reticulum and Golgi apparatus [8][9]. The glycoconjugate forms are generally based on nine monosaccharides (Figure 1A). The glycan residues can be conjugated to asparagine (N-glycan) or serine/threonine (O-glycan) residues to form the glycoproteins. Two N-acetylglucosamine (GlcNAc) and three mannose (Man) residues usually constitute the core of N-glycans, which are generally highly branched. It can distinguish high mannose, hybrid, and complex N-linked glycans [10][11]. In contrast, O-glycans are linked with N-acetylgalactosamine (GalNAc) and are, in general, less branched than N-glycans (Figure 1B). Between the N- and O-branches, traces of galactose (Gal), GalNAc, GlcNAc, fucose (Fuc) and the final sialic acid (Sial) can be detected (Figure 1B). Glycosphingolipids are conjugated at the plasma membrane, whereas glycosaminoglycans are mainly composed of an initial xylose (Xyl) followed by glucuronic acid (GlcA) and GlcNAc or GalNAc branches (Figure 1B) [10].
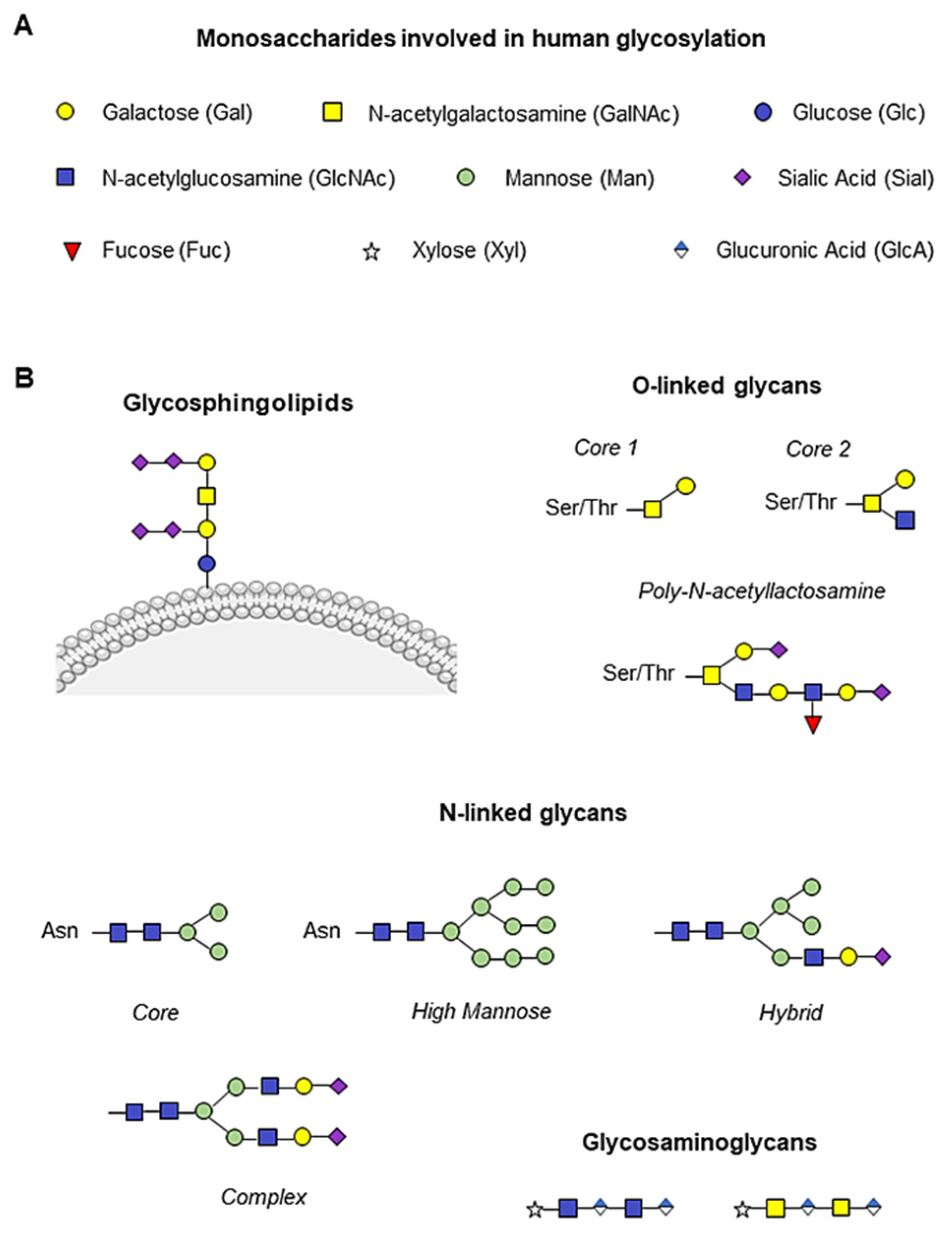
Figure 1. Human monosaccharides and their glycoconjugate forms. (A) Representation of the nine monosaccharides involved in human glycosylation. (B) Schematic illustration of glycan structures. Addition of N-acetylgalactosamine (GalNAc, yellow square) to serine/threonine (Ser/Thr) residues initiates O-glycan synthesis, while two N-acetylglucosamine (GlcNAc, blue square) and three mannose (Man, green circle) constitute the N-glycan core, and the glycosylation branches are formed by galactose molecules (Gal, yellow circle), GalNAc, GlcNAc, fucose (Fuc, red triangle) and the final sialic acid (Sial, purple rhombus). Glycosphingolipids are conjugated to the plasma membrane, whereas glycosaminoglycans are mainly composed of an initial xylose (Xyl, white star) followed by glucuronic acid (GlcA, blue and white rhombus) and GlcNAc or GalNAc.
More than half of the proteins in human cells and 50–70% of serum proteins are glycosylated [12]. Platelets express highly glycosylated proteins on their surface, which are involved in platelet hemostasis and function, as well as in their interaction with other cells [13]. To maintain a normal circulating platelet count between 150–400 × 109/L, about 1011 of them are cleared daily, highlighting the importance of the balance between production and removal of these cells. Glycosyltransferases and synthesis and transport proteins are involved in both processes, and their dysregulation leads to variations in platelet counts and/or functional alterations [14].
2. Role of Glycosylation in Thrombopoiesis and Platelet Clearance
Thrombopoietin (TPO) is a hematopoietic growth factor essential for thrombopoiesis that is produced predominantly by the liver [15]. Binding of TPO to the c-Mpl receptor (encoded by MPL) on platelets and megakaryocytes (MKs) activates a cascade of signaling molecules driving MK development and platelet formation [16]. The plasma concentration of TPO correlates inversely with platelet number, and circulating levels are determined as a function of its binding to platelets and MK, leading to its internalization and degradation along with the c-Mpl receptor [17]. Decreased platelet turnover rate or reduced platelet number results in increased levels of free TPO, which induces a compensatory response dependent on bone marrow MK concentration to increase platelet production [18].
N-linked and O-linked glycans play an essential role in the stability of major MK and platelet surface glycoproteins, including the GPIb-IX-V complex, GPIIb-IIIa (integrin αIIbβ3) and GPVI. Alteration of their glycosylation negatively influences glycoprotein functions, leading to abnormal morphology, defective platelet activation and excessive bleeding [19]. In addition, platelet GPIbα is responsible for the maintenance of steady-state hepatic TPO production [20]. It has been described that the absence of GPIbα in the MK membrane leads to reduced thrombopoiesis due to aberrant membrane development during MK maturation, impaired formation of the membrane demarcation system (DMS), and disruption of the microtubule cytoskeleton, as described in Bernard Soulier syndrome (BSS) [21]. In addition, one group has recently described a defect in GPIbα glycosylation that affects thrombopoiesis and actin cytoskeleton remodeling [22]. These findings highlight the essential role of protein glycosylation during megakaryopoiesis and thrombopoiesis.
Desialylated and/or senescent platelets increase TPO production. Loss of the final sialic acid is responsible for platelet clearance, as exposure of the penultimate Gal residue is recognized by hepatic Ashwell-Morell receptors (AMR), whereas exposure of the GlcNAc residue is recognized by resident hepatic macrophages (Kupffer cells), via the αMβ2 integrin. Consequently, AMR activation drives hepatic TPO mRNA expression through Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) signaling, triggering a feedback mechanism to increase TPO levels and promote platelet formation [23][24] (Figure 2A). AMR preferentially binds to complex branched glycans, suggesting that N-glycans are the main site of ligand recognition [25]. However, desialylation of O-glycans on GPIbα is known to favor receptor signaling and surface expression of neuraminidase which, by desialylating platelet N-glycans, would allow AMR-mediated clearance. On the other hand, Kupffer cells also play an important role in the clearance of aged platelets and during immune-mediated thrombocytopenia [20][26].
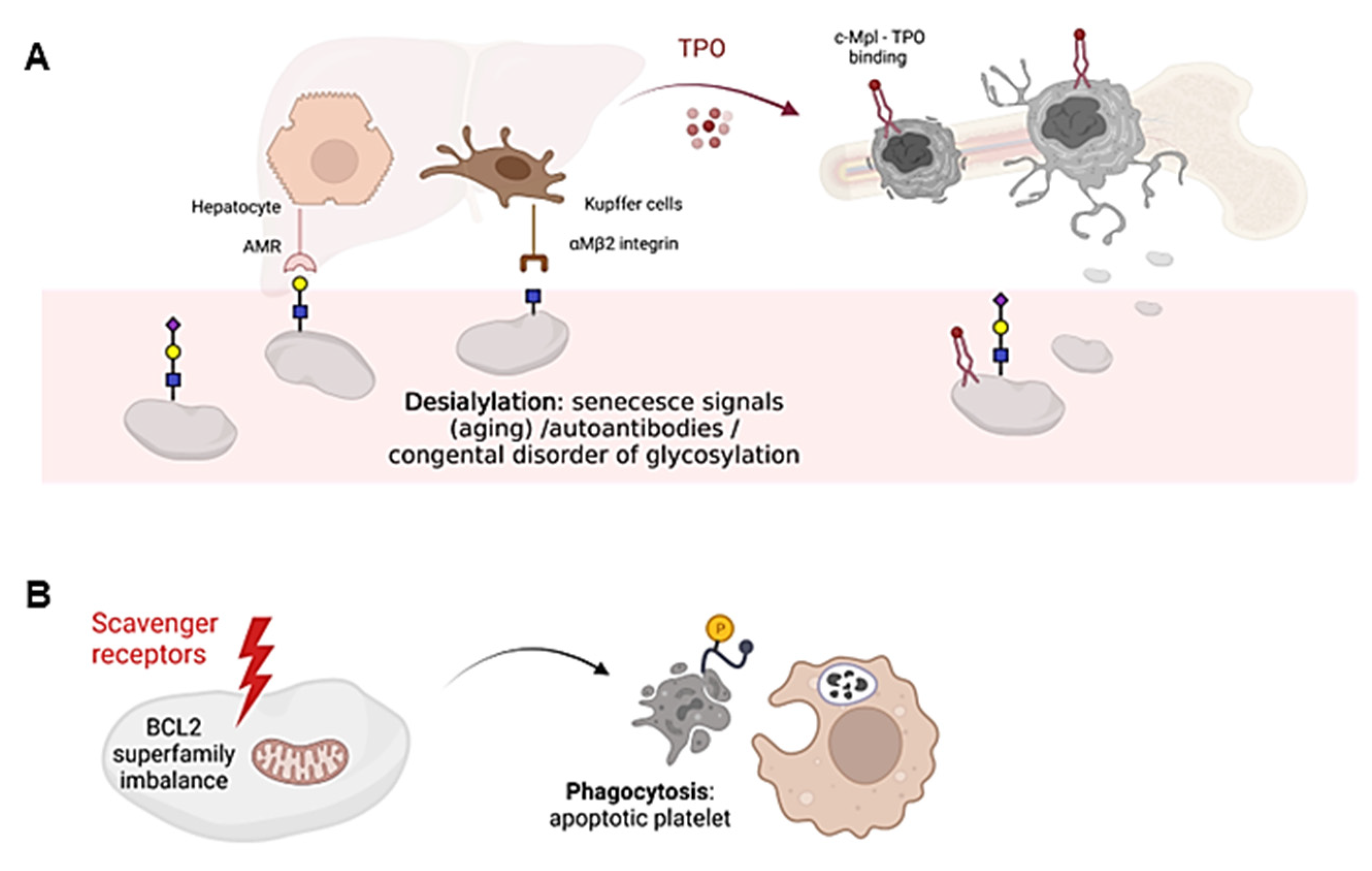
Figure 2. Schematic representation of platelet clearance mechanisms. (A) Platelet desialylation can be triggered by senescence signals in “aged” platelets, by the presence of autoantibodies (especially in autoimmune diseases) or by congenital alterations in genes involved in glycosylation. Desialylated platelets are recognized by hepatic AMR to regulate hepatic TPO production and thrombopoiesis, or by Kupffer cells. Bone marrow MKs produce and release young platelets containing sialic acid into the bloodstream. Young platelets maximally internalize TPO through Mpl receptors. (B) Platelet survival is regulated by the interaction between pro-survival and pro-apoptotic members of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway. Up-regulation of pro-apoptotic signals mediates platelet clearance through scavenger receptors. Cells undergoing apoptosis modify the redistribution of phosphatidylserine (PS, yellow circle) from the inner to the outer lamella of the plasma membrane, which serves as a molecular signal for clearance by phagocytes. The figure was created Biorender.com (accessed on 13 February 2023).
Platelet clearance by aging (senescence) induces signals including loss of sialic acid mediated by up-regulation of platelet sialidases Neu1 and Neu3, which are expressed in the granular and plasma membrane compartments, respectively [27]. Neu1 and Neu3 usually impact sialic acid binding on GPIbα, leading to its degradation [27]. In addition, antibody-mediated platelet destruction occurs via Fc receptors on primarily splenic macrophages and it is frequent in primary immune thrombopenia (ITP) [28]. In this disease, circulating autoantibodies with specificity for membrane glycoproteins, such as GPIIb-IIIa or GPIbα, can bind to platelets, thus triggering platelet desialylation by secretion of active Neu1, and additionally favoring their clearance by cytotoxic CD8 T lymphocytes [29][30].
Platelet survival also depends on the interplay between antiapoptotic and proapoptotic factors of the Bcl-2 family, which are critical regulators of the intrinsic apoptotic pathway [31] (Figure 2B). However, it is still unclear whether Bcl-2 family members alter the sialic acid content on the surface of platelets. Platelet loss of function and death is governed by unclear mechanisms that share some similarity to those used by nucleated cells for programmed cell death [32]. In addition, platelets express certain components of the extrinsic pathway of apoptosis, including caspase 8, but the limited data available to date do not support their critical role in regulating platelet lifespan [33]. The consequences of platelet death include the formation of a new platelet–platelet interaction that occurs between nonviable platelets, and the shedding of the collagen receptor GPVI and GPIbα. Both processes appear to be regulated by metalloproteinase activity [34]. Although it is unclear how senescent platelets are removed from circulation, many cells undergoing apoptosis shift the redistribution of phosphatidylserine (PS) from the inner to the outer lamella of the plasma membrane, which serves as a molecular signal for removal by phagocytes [35] (Figure 2B). Overall, it remains to be elucidated whether loss of sialic acid triggers the intrinsic apoptotic machinery in platelets during the clearance mechanisms that regulate platelet counts.
Thousands of enzymes regulated by glycosylation processes are involved in platelet formation and clearance. Alterations in any of them could result in an imbalance between the two processes and consequently impact platelet counts. Until relatively recently, a very limited number of molecular variants had been described in only few genes that were related to IT [36].
References
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489.
- Deng, Y.; Wang, Z.V.; Tao, C.; Gao, N.; Holland, W.L.; Ferdous, A.; Repa, J.J.; Liang, G.; Ye, J.; Lehrman, M.A.; et al. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. J. Clin. Investig. 2013, 123, 455–468.
- Janik, M.E.; Lityńska, A.; Vereecken, P. Cell migration-The role of integrin glycosylation. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 545–555.
- Moran, A.P.; Gupta, A.; Joshi, L. Sweet-talk: Role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 2011, 60, 1412–1425.
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the immune system. Science 2001, 291, 2370–2376.
- Kanie, Y.; Kanie, O. Addressing the glycan complexity by using mass spectrometry: In the pursuit of decoding glycologic. Biochem. Compd. 2017, 5, 3.
- Jayaprakash, N.G.; Surolia, A. Role of glycosylation in nucleating protein folding and stability. Biochem. J. 2017, 474, 2333–2347.
- Gupta, R.; Leon, F.; Thompson, C.M.; Nimmakayala, R.; Karmakar, S.; Nallasamy, P.; Chugh, S.; Prajapati, D.R.; Rachagani, S.; Kumar, S.; et al. Global analysis of human glycosyltransferases reveals novel targets for pancreatic cancer pathogenesis. Br. J. Cancer 2020, 122, 1661–1672.
- Handford, M.; Rodriguez-Furlán, C.; Orellana, A. Nucleotide-sugar transporters: Structure, function and roles in vivo. Braz. J. Med. Biol. Res. 2006, 39, 1149–1158.
- Lee-Sundlov, M.M.; Stowell, S.R.; Hoffmeister, K.M. Multifaceted role of glycosylation in transfusion medicine, platelets, and red blood cells. J. Thromb. Haemost. 2020, 18, 1535–1547.
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta—Mol. Cell Res. 2013, 1833, 2430–2437.
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta—Gen. Subj. 1999, 1473, 4–8.
- Wandall, H.H.; Rumjantseva, V.; Sørensen, A.L.T.; Patel-Hett, S.; Josefsson, E.C.; Bennett, E.P.; Italiano, J.E.; Clausen, H.; Hartwig, J.H.; Hoffmeister, K.M. The origin and function of platelet glycosyltransferases. Blood 2012, 120, 626–635.
- Ramírez-López, A.; Román, M.T.Á.; Manzano, E.M.; Acuña, P.; Arias-Salgado, E.G.; Salces, M.M.; Pollmar, M.I.R.; Yuste, V.J.; Sanz, R.J.; Barcenilla, S.G.; et al. The importance of platelet glycoside residues in the haemostasis of patients with immune thrombocytopaenia. J. Clin. Med. 2021, 10, 1661.
- Hitchcock, I.S.; Kaushansky, K. Thrombopoietin from beginning to end. Br. J. Haematol. 2014, 165, 259–268.
- Deutsch, V.R.; Tomer, A. Megakaryocyte development and platelet production. Br. J. Haematol. 2006, 134, 453–466.
- Hitchcock, I.S.; Chen, M.M.; King, J.R.; Kaushansky, K. YRRL motifs in the cytoplasmic domain of the thrombopoietin receptor regulate receptor internalization and degradation. Blood 2008, 112, 2222–2231.
- Deutsch, V.R.; Tomer, A. Advances in megakaryocytopoiesis and thrombopoiesis: From bench to bedside. Br. J. Haematol. 2013, 161, 778–793.
- Wang, Y.; Jobe, S.M.; Ding, X.; Choo, H.; Archer, D.R.; Mi, R.; Ju, T.; Cummings, R.D. Platelet biogenesis and functions require correct protein O-glycosylation. Proc. Natl. Acad. Sci. USA 2012, 109, 16143–16148.
- Karakas, D.; Xu, M.; Ni, H. GPIbα is the driving force of hepatic thrombopoietin generation. Res. Pract. Thromb. Haemost. 2021, 5, e12506.
- Poujol, C.; Ware, J.; Nieswandt, B.; Nurden, A.T.; Nurden, P. Absence of GPIbα is responsible for aberrant membrane development during megakaryocyte maturation: Ultrastructural study using a transgenic model. Exp. Hematol. 2002, 30, 352–360.
- Marín-Quílez, A.; Di Buduo, C.A.; Díaz-Ajenjo, L.; Abbonante, V.; Vuelta, E.; Soprano, P.M.; Miguel-García, C.; Santos-Mínguez, S.; Serramito-Gómez, I.; Ruiz-Sala, P.; et al. Novel variants in GALE cause syndromic macrothrombocytopenia by disrupting glycosylation and thrombopoiesis. Blood 2023, 141, 406–421.
- Sørensen, A.L.; Rumjantseva, V.; Nayeb-Hashemi, S.; Clausen, H.; Hartwig, J.H.; Wandall, H.H.; Hoffmeister, K.M. Role of sialic acid for platelet life span: Exposure of β-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor-expressing liver macrophages and hepatocytes. Blood 2009, 114, 1645–1654.
- Hoffmeister, K.M.; Falet, H. Platelet clearance by the hepatic Ashwell-Morrell receptor: Mechanisms and biological significance. Thromb. Res. 2016, 141, S68–S72.
- Coombs, P.J.; Taylor, M.E.; Drickamer, K. Two categories of mammalian galactose-binding receptors distinguished by glycan array profiling. Glycobiology 2006, 16, 1C–7C.
- Wang, Y.; Chen, W.; Zhang, W.; Lee-sundlov, M.M.; Casari, C.; Berndt, M.C.; Lanza, F.; Bergmeier, W.; Hoffmeister, K.M.; Zhang, X.F.; et al. Desialylation of O-glycans on glycoprotein Ibα drives receptor signaling and platelet clearance. Haematologica 2021, 106, 220–229.
- Jansen, A.J.G.; Josefsson, E.C.; Rumjantseva, V.; Liu, Q.P.; Falet, H.; Bergmeier, W.; Cifuni, S.M.; Sackstein, R.; Von Andrian, U.H.; Wagner, D.D.; et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood 2012, 119, 1263–1273.
- Singh, A.; Uzun, G.; Bakchoul, T. Primary immune thrombocytopenia: Novel insights into pathophysiology and disease management. J. Clin. Med. 2021, 10, 789.
- Zheng, S.S.; Ahmadi, Z.; Leung, H.H.L.; Wong, R.; Yan, F.; Perdomo, J.S.; Chong, B.H. Antiplatelet antibody predicts platelet desialylation and apoptosis in immune thrombocytopenia. Haematologica 2022, 107, 2195–2205.
- Quach, M.E. GPIb-IX-V and platelet clearance. Platelets 2022, 33, 817–822.
- McArthur, K.; Chappaz, S.; Kile, B.T. Apoptosis in megakaryocytes and platelets: The life and death of a lineage. Blood 2018, 131, 605–610.
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951.
- Josefsson, E.C.; Burnett, D.L.; Lebois, M.; Debrincat, M.A.; White, M.J.; Henley, K.J.; Lane, R.M.; Moujalled, D.; Preston, S.P.; O’Reilly, L.A.; et al. Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat. Commun. 2014, 5, 3455.
- Hartley, P.S. Platelet senescence and death. Clin. Lab. 2007, 53, 157–166.
- Edward Quach, M.; Chen, W.; Li, R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018, 131, 1512–1521.
- Palma-Barqueros, V.; Revilla, N.; Sánchez, A.; Cánovas, A.Z.; Rodriguez-alén, A.; Marín-quílez, A.; González-porras, J.R.; Vicente, V.; Lozano, M.L.; Bastida, J.M.; et al. Inherited platelet disorders: An updated overview. Int. J. Mol. Sci. 2021, 22, 4521.
More
Information
Subjects:
Genetics & Heredity
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
16 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





