Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisete Galego | -- | 1355 | 2023-03-14 11:27:40 | | | |
| 2 | Catherine Yang | Meta information modification | 1355 | 2023-03-15 02:32:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Da Silva, A.A.; Galego, L.; Arraiano, C.M. BolA Function and Regulation. Encyclopedia. Available online: https://encyclopedia.pub/entry/42171 (accessed on 07 February 2026).
Da Silva AA, Galego L, Arraiano CM. BolA Function and Regulation. Encyclopedia. Available at: https://encyclopedia.pub/entry/42171. Accessed February 07, 2026.
Da Silva, Ana Alves, Lisete Galego, Cecília Maria Arraiano. "BolA Function and Regulation" Encyclopedia, https://encyclopedia.pub/entry/42171 (accessed February 07, 2026).
Da Silva, A.A., Galego, L., & Arraiano, C.M. (2023, March 14). BolA Function and Regulation. In Encyclopedia. https://encyclopedia.pub/entry/42171
Da Silva, Ana Alves, et al. "BolA Function and Regulation." Encyclopedia. Web. 14 March, 2023.
Copy Citation
The BolA-like protein family is widespread among prokaryotes and eukaryotes. BolA was originally described in E. coli as a gene induced in the stationary phase and in stress conditions. The BolA overexpression makes cells spherical. It was characterized as a transcription factor modulating cellular processes such as cell permeability, biofilm production, motility, and flagella assembly. BolA is important in the switch between motile and sedentary lifestyles having connections with the signaling molecule c-di-GMP. BolA was considered a virulence factor in pathogens such as Salmonella Typhimurium and Klebsiella pneumoniae and it promotes bacterial survival when facing stresses due to host defenses.
bolA
BolA-like proteins
phosphorylation
biofilm
flagella
1. The Role of BolA in E. coli Survival
BolA was discovered in E. coli in the 1980s and its name is due to its ability to produce osmotically stable spherical cells when overexpressed. It was shown to be involved in switching the cells between elongation and septation systems [1] during the cell division cycle, and the expression of bolA was shown to be growth-rate regulated, being induced during the transition into stationary phase [2][3][4]. BolA overexpression was responsible for spherical morphology in rod-shaped E. coli cells. Later, it was shown that BolA could also be induced in the exponential phase of growth, in response to several stresses [5][6][7]. BolA-like proteins constitute a widely conserved family of proteins widespread among prokaryotes and eukaryotes [1][8]. Phylogenetic analyses allowed to group BolA proteins into four subfamilies: BolA1-like (present in both prokaryotes and eukaryotes), BolA2-like and BolA3-like (found in eukaryotes) and BolA4-like (present only in photosynthetic organisms) [1][9][10][11]. A diversity of phenotypes has been linked to this protein family, although the molecular mechanisms that mediate BolA cellular effects are not yet well understood. Often, organisms encode several BolA members, performing different functions within a species and across the species. A phylogenetic tree for bolA was constructed based on the sequences of bolA1 genes from different species (Figure 1). The sequences of each gene were obtained from the NCBI database [12]. The phylogenetic reconstruction was created in MEGA software v11.0.11 [13].
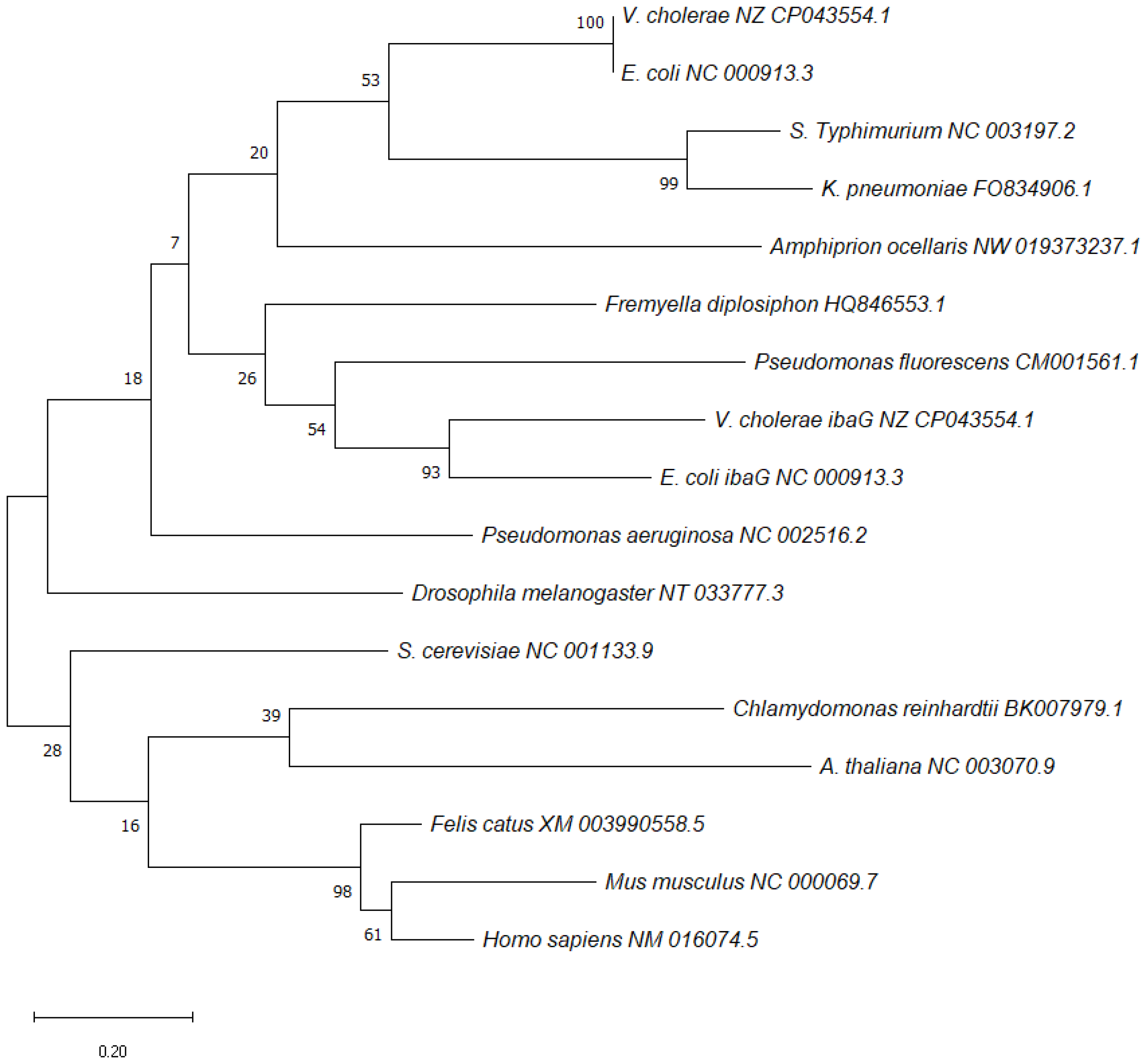
Figure 1. Maximum-likelihood phylogenetic reconstructions of bolA1 using MEGA software v11.0.11 [13]. Distance estimation was obtained by Tamura–Nei model. Numbers at the nodes represent bootstrap values (%) based on 1000 replicates. Gene sequences were aligned using MUSCLE and the trees were reconstructed using default settings. The sequences of each gene were obtained from NCBI database [12] and the GenBank accession numbers are written next to each species.
In E. coli, BolA is a small protein (≈12 kDa) [14] which is induced at the stationary phase of growth and by several stresses. It has been linked to membrane permeability, motility, cell morphology and biofilm development [1][4][5][6][7][15]. In 1988, Aldea and colleagues discovered that BolA is a FtsZ-dependent morphogene, and its overexpression made E. coli rod-shaped cells become spherical. This gave origin to the name of the gene: bolA (meaning ball) [1]. The mechanism by which BolA affects cell morphology is mediated by different factors. For instance, BolA binds to the promoter region of mreBCD decreasing the expression of the actin-like mreB [16]), and upregulates the genes dacA and dacC which codify the two main d-d-carboxypeptidases (respectively Penicillin-binding protein PBP5 and PBP6), regulating peptidoglycan biosynthesis [1][15][17]. It was also shown that BolA controlled the transcription of ampC (AmpC), a class C beta-lactamase, thus connecting for the first-time penicillin-binding proteins (PBPs) and beta-lactamases at the level of gene regulation [15].
The overexpression of BolA has an influence in the outer membrane permeability of E. coli. High levels of BolA have been shown to increase the ratio of OmpC/OmpF porins, turning the cell less permeable, and conferring protection from unfavorable environments [5]. Overexpression of BolA could even confer protection from detergents and from the antibiotic Vancomycin.
BolA is controlled at transcriptional, post-transcriptional and post-translational levels. Transcription of bolA can start at two different promoters. P2 is a constitutive promoter that is under the control of σ70 and is detectable in low amounts during all stages of growth. P1 is located 80 nt downstream, is under the control of σS and is expressed in stationary phase or under stress conditions [4][6]. For instance, in the face of stress conditions such as heat shock and acidic stress, bolA1p mRNA levels are increased [6]. Heat shock induction is almost immediate while the acidic stress is associated with a more gradual induction of bolA mRNA. In response to carbon starvation and osmotic shock bolA1p is highly induced and the level of its expression can largely exceed the ones reached in the stationary phase. These stresses make cells change their morphology to a rounder shape similar to those cells in which BolA is overexpressed in the stationary phase. On the other hand, oxidative stress leads to a moderate increase in mRNA bolA1p levels inhibiting growth and viability [6]. H-NS, a histone-like protein, was found to negatively regulate bolA expression in vivo and to interact with both bolA1p and bolA2p regions in vitro [18]. OmpR, in its phosphorylated form (phospho-OmpR), binds to the OmpR-binding region of bolA1, repressing its transcription [19]. Endoribonuclease RNase III acts as a post-transcriptional modulator of bolA expression under carbon-starvation conditions [20]. RNase III positively regulates bolA1p mRNA levels and stabilities. RNase III is furthermore shown to be necessary for the normal expression of σS, ensuring normal levels of rpoS mRNA and σS protein under glucose starvation. Accordingly, under this stress, bolA transcript is increased and is more stable. This shows that bolA transcriptional and post-transcriptional controls are consonant to achieve the global regulation of the expression of this gene [5]. In 1997, Cao and Sarkar discovered that poly (A)-polymerase was able to directly regulate mRNA levels of both bolA and rpoS, and bolA transcript could be polyadenylated at its 3′end [20][21].
2. The Role of BolA in Virulence
BolA could be involved in different pathways directly related to bacterial virulence [22]. Salmonella enterica serovar Typhimurium (S. Typhimurium) is a pathogen that makes use of several virulence factors in order to overcome host defenses surviving inside host cells [23][24]. In order to unravel the role of BolA protein in the virulence of S. Typhimurium, the greater wax moth Galleria mellonella, was used as the infection model. G. mellonella has been extensively used as a model organism for a wide range of bacterial species including S. Typhimurium. BolA proved to be a determinant factor in the virulence capacity of S. Typhimurium and in its ability to survive and overcome host defenses. It conferred resistance to acidic and oxidative stress promoting its survival under harsh conditions [25]. When cells were infected with S. Typhimurium the wild-type bacteria could survive and multiply but the number of bacteria inside each cell was substantially reduced in the S. Typhimurium bolA deletion mutant.
To further explore the role of BolA in virulence, S. Typhimurium metabolism was investigated. Using 1H-NMR metabolomics, the metabolic differences between strains expressing different levels of BolA in a minimal virulence-inducing medium (LPM medium) were accessed. The strain overexpressing BolA revealed increased levels of acetate, valine, alanine, NAD+, succinate, coenzyme A, glutathione, and putrescine. These metabolites are implicated in pathways related to stress resistance and virulence. This suggests that BolA has an important role in metabolic regulation and that potentiates the virulence of S. Typhimurium [26].
Recently, BolA has also been identified as a virulence factor in Klebsiella pneumoniae [27] K. pneumonia bolA deletant mutants are less resistant to bile and oxidative stresses than wild-type cells. BolA is required for maintaining a proper cell morphology in the stationary phase of growth. In a Galleria melonella infection model, the larvae infected with bolA deletant K. pneumoniae, survived 53% more than the larvae infected with wild-type strain. BolA promoted the adhesion of K. pneumoniae to human cancer epithelial cells and significantly decreased the bacterial ability to colonize the liver, spleen, lung and kidney organs in a mouse model. Additionally, the formation of liver abscesses was not observed in mice infected with bolA deletant K. pneumoniae. BolA positively regulated siderophores production and biofilm formation as well as metabolites related to stress response and virulence (agmatine, cadaverine, guanosine, flavin adenine dinucleotide [FAD] and d-biotin). According to the authors, the downregulation of these metabolites may be the factor leading to the loss of virulence and stress resistance of the ΔbolA strain of K. pneumoniae [27].
References
- Aldea, M.; Hernández-Chico, C.; de la Campa, A.G.; Kushner, S.R.; Vicente, M. Identification; cloning; and expression of bolA; an ftsZ-dependent morphogene of Escherichia coli. J. Bacteriol. 1988, 170, 5169–5176.
- Lange, R.; Hengge-Aronis, R. Growth phase-regulated expression of bolA and morphology of stationary-phase Escherichia coli cells are controlled by the novel sigma factor sigma S. J. Bacteriol. 1991, 173, 4474–4481.
- Lange, R.; Hengge-Aronis, R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 1991, 5, 49–59.
- Aldea, M.; Garrido, T.; Hernández-Chico, C.; Vicente, M.; Kushner, S.R. Induction of a growth-phase-dependent promoter triggers transcription of bolA; an Escherichia coli morphogene. EMBO J. 1989, 8, 3923–3931.
- Freire, P.; Vieira, H.A.; Furtado, A.R.; de Pedro, M.A.; Arraiano, C.M. Effect of the morphogene bolA on the permeability of the Escherichia coli outer membrane. FEMS Microbiol. Lett. 2006, 260, 106–111.
- Santos, J.M.; Freire, P.; Vicente, M.; Arraiano, C.M. The stationary-phase morphogene bolA from Escherichia coli is induced by stress during early stages of growth. Mol. Microbiol. 1999, 32, 789–798.
- Vieira, H.A.; Freire, P.; Arraiano, C.M. Effect of Escherichia coli Morphogene bolA on Biofilms. Appl. Environ. Microbiol. 2004, 70, 5682–5684.
- Guinote, I.B.; Moreira, R.N.; Barahona, S.; Freire, P.; Vicente, M.; Arraiano, C.M. Breaking through the stress barrier: The role of BolA in Gram-negative survival. World J. Microbiol. Biotechnol. 2014, 30, 2559–2566.
- Qin, L.; Wang, M.; Zuo, J.; Feng, X.; Liang, X.; Wu, Z.; Ye, H. Cytosolic BolA Plays a Repressive Role in the Tolerance against Excess Iron and MV-Induced Oxidative Stress in Plants. PLoS ONE 2015, 10, e0124887.
- Rey, P.; Taupin-Broggini, M.; Couturier, J.; Vignols, F.; Rouhier, N. Is There a Role for Glutaredoxins and BOLAs in the Perception of the Cellular Iron Status in Plants? Front. Plant Sci. 2019, 10, 712.
- Kasai, T.; Inoue, M.; Koshiba, S.; Yabuki, T.; Aoki, M.; Nunokawa, E.; Seki, E.; Matsuda, T.; Matsuda, N.; Tomo, Y.; et al. Solution structure of a BolA-like protein from Mus musculus. Protein Sci. 2004, 13, 545–548.
- NCBI Resource Coordinators. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2016, 44, D7–D19.
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027.
- Galego, L.; Barahona, S.; Romão, C.; Arraiano, C.M. Phosphorylation status of BolA affects its role in transcription and biofilm development. FEBS J. 2021, 288, 961–979.
- Santos, J.M.; Lobo, M.; Matos, P.A.; de Pedro, M.A.; Arraiano, C.M. The gene bolA regulates dacA (PBP5); dacC (PBP6) and ampC (AmpC); promoting normal morphology in Escherichia coli. Mol. Microbiol. 2002, 45, 1729–1740.
- Freire, P.; Moreira, R.N.; Arraiano, C.M. BolA Inhibits Cell Elongation and Regulates MreB Expression Levels. J. Mol. Biol. 2009, 385, 1345–1351.
- Guinote, I.B.; Matos, R.G.; Freire, P.; Arraiano, C.M. BolA Affects Cell Growth; and Binds to the Promoters of Penicillin-Binding Proteins 5 and 6 and Regulates Their Expression. J. Microbiol. Biotechnol. 2011, 21, 243–251.
- Moreira, R.N.; Dressaire, C.; Domingues, S.; Arraiano, C.M. A new target for an old regulator: H-NS represses transcription of bolA morphogene by direct binding to both promoters. Biochem. Biophys. Res. Commun. 2011, 411, 50–55.
- Yamamoto, K. Negative regulation of the bolA1p of Escherichia coli K-12 by the transcription factor OmpR for osmolarity response genes. FEMS Microbiol. Lett. 2000, 186, 257–262.
- Freire, P.; Amaral, J.; Santos, J.; Arraiano, C. Adaptation to carbon starvation: RNase III ensures normal expression levels of bolA1p mRNA and σS. Biochimie 2006, 88, 341–346.
- Cao, G.; Sarkar, N. Stationary Phase-Specific mRNAs in Escherichia coli Are Polyadenylated. Biochem. Biophys. Res. Commun. 1997, 239, 46–50.
- Dressaire, C.; Moreira, R.N.; Barahona, S.; Alves de Matos, A.P.; Arraiano, C.M. BolA Is a Transcriptional Switch That Turns off Motility and Turns on Biofilm Development. mBio 2015, 6, e02352-14.
- Lhocine, N.; Arena, E.T.; Bomme, P.; Ubelmann, F.; Prévost, M.-C.; Robine, S.; Sansonetti, P.J. Apical Invasion of Intestinal Epithelial Cells by Salmonella Typhimurium Requires Villin to Remodel the Brush Border Actin Cytoskeleton. Cell Host Microbe 2015, 17, 164–177.
- Fàbrega, A.; Vila, J. Salmonella enterica Serovar Typhimurium Skills To Succeed in the Host: Virulence and Regulation. Clin. Microbiol. Rev. 2013, 26, 308–341.
- Mil-Homens, D.; Barahona, S.; Moreira, R.N.; Silva, I.J.; Pinto, S.N.; Fialho, A.M.; Arraiano, C.M. Stress Response Protein BolA Influences Fitness and Promotes Salmonella enterica Serovar Typhimurium Virulence. Appl. Environ. Microbiol. 2018, 84, e02850-17.
- Graça-Lopes, G.; Graça, G.; Barahona, S.; Moreira, R.N.; Arraiano, C.M.; Gonçalves, L.G. NMR-Metabolomics Shows That BolA Is an Important Modulator of Salmonella Typhimurium Metabolic Processes under Virulence Conditions. Metabolites 2019, 9, 243.
- Zhang, F.; Yan, X.; Bai, J.; Xiang, L.; Ding, M.; Li, Q.; Zhang, B.; Liang, Q.; Zhou, Y. Identification of the BolA Protein Reveals a Novel Virulence Factor in K. pneumoniae that Contributes to Survival in Host. Microbiol. Spectr. 2022, 10, e00378-22.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
705
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




