Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mietek Jaroniec | -- | 2070 | 2023-03-14 04:14:44 | | | |
| 2 | Sirius Huang | Meta information modification | 2070 | 2023-03-15 01:06:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles. Encyclopedia. Available online: https://encyclopedia.pub/entry/42146 (accessed on 12 January 2026).
Dubadi R, Huang SD, Jaroniec M. Mechanochemical Synthesis of Nanoparticles. Encyclopedia. Available at: https://encyclopedia.pub/entry/42146. Accessed January 12, 2026.
Dubadi, Rabindra, Songping D. Huang, Mietek Jaroniec. "Mechanochemical Synthesis of Nanoparticles" Encyclopedia, https://encyclopedia.pub/entry/42146 (accessed January 12, 2026).
Dubadi, R., Huang, S.D., & Jaroniec, M. (2023, March 14). Mechanochemical Synthesis of Nanoparticles. In Encyclopedia. https://encyclopedia.pub/entry/42146
Dubadi, Rabindra, et al. "Mechanochemical Synthesis of Nanoparticles." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Various solvent-based approaches have been already used to synthesize porous materials. Mechanochemical synthesis is one of the effective eco-friendly alternatives to the conventional synthesis. It adopts the efficient mixing of reactants using ball milling without or with a very small volume of solvents, gives smaller size nanoparticles (NPs) and larger surface area, and facilitates their functionalization, which is highly beneficial for antimicrobial applications.
porous materials
mechanochemical synthesis
nanoparticles
1. Mechanochemistry: History and Advantages
The history of mechanochemistry is very long. The first use of the mortar and pestle as a grinding tool can be traced to the stone age. Later, these simple tools were replaced by more sophisticated devices that can be used for the preparation of materials for research and different practical applications. The mechanochemical process involves the chemical transformation of the reactant species by means of various forms of mechanical forces such as compression, shear strain, friction, etc. This process was found to be scripted from 315 BC by Theophrastus in his book, “On Stones” [1]. The working principles of mechanochemistry are still not fully explained, but systematic study was started around the middle of the 19th century and was significantly advanced after the 1960s. The important industrial applications of mechanochemistry include the processing of cement clinker, ores, and powder metallurgy, which adopt fine grinding as a mechanochemical tool and have been used since the 19th century until now [2]. Although the principles and methodologies of mechanochemistry are still being explored, the initial slow progress in this field was accelerated when mechanical alloying emerged. Nowadays, the popularity of mechanochemical synthesis is increasing in various fields, including organic, inorganic, and materials chemistry. Because of the growing popularity of mechanochemistry, IUPAC in 2003 defined mechanochemical reaction as “a chemical reaction that is induced by the direct absorption of mechanical energy” [3].
Mechanochemical synthesis is one of the safest ways to prepare nanomaterials. This synthesis is safer than wet chemical processing. The major advantages of this synthesis are:
- (i)
-
Reduction of particle size: ball milling is a physical method that affords the synthesis of particles with reduced sizes down to tens of nanometers.
- (ii)
-
Nanostructuring and activation of materials: mechanical grinding can be used for the synthesis of mesoporous materials via template-assisted methods. In addition, mechanochemistry can be applied for the nano-casting synthesis of nanoporous materials [4].
- (iii)
-
Doping of nanoparticles: the activity of nanomaterials mainly depends on their surface-to-volume ratio, size, and surface functionality, as well as the active sites present on the surface. The surface properties of NPs can be modified by doping, which is commonly used to enhance their catalytic activity, antimicrobial properties, etc. Moreover, doping permits the realization of desired properties for specific applications such as wastewater treatment, nuclear waste management, and adsorption-based removal of harmful dyes [5][6][7].
- (iv)
-
Reduction of reaction time: mechanochemical processing is quicker than conventional synthesis. The reduction of tungsten carbide particles from 2–3 mm sizes to 3 µm takes 70 h in conventional synthesis, whereas the same can be achieved in 3 min in a planetary ball mill [8].
- (v)
- (vi)
-
Low agglomeration: this approach helps to produce the NPs with narrow particle size distribution [11].
- (vii)
-
Medicinal value: the use of modern mechanochemistry in the medicinal field as medicinal mechanochemistry expands the scope of this approach [12].
Along with these advantages, some disadvantages of this process are known too. Namely, this method requires high-energy mechanochemical equipment, is prone to particle contamination originating from the container and grinding balls, and it is often difficult to achieve ordered porosity, precise shape, and size due to high energy milling [13][14].
2. Mechanochemical Synthesis of Nanoparticles
The basic principle of mechanical synthesis is the grinding of solid materials, which involves the reduction of particle sizes. The essence of mechanochemical processing involves the induction of chemical reactions between raw materials by the input of mechanical energy. This is the most important difference between grinding (top-down approach) and mechanochemical processing [15][16]. The close contact between the milled particles highly enhances the diffusion and chemical reactivity of the reactants [8]. During the ball milling process the plastic deformation, shear stress or shock impact, fracture, and friction due to the collisions induce structural defects and can break chemical bonds. After multiple processes, a new and active state of the material is produced [17].
Mechanochemistry can be used to facilitate reactions at different interfacial systems such as solid-solid, solid-gas, and solid-liquid systems. Specifically, mechanochemical ball milling is extensively used for the synthesis of different types of metallic NPs, metal oxide nanocomposites, and different types of doping processes. There are various types of mills in use for synthesis. Some of them are [17]:
- SPEX shaker mills
- Planetary ball mills
- Attritor mills
- Modern mills (rod mills, vibrating frame mills)
2.1. Synthesis of Metal Nanoparticles
AgNPs were successfully synthesized via mechanochemistry (ball milling) by using lignin as a biodegradable reducing agent without solvents. The synthesized AgNPs showed a very efficient antimicrobial property for both gram-positive and gram-negative bacteria [18]. Some of the studies showed that mechanochemistry can be successfully used for the synthesis of ultrafine Fe, Co, Ni, and Cu NPs [19]. The mechanochemical reduction of binary sulfides of copper, chalcocite (Cu2S), and covellite (CuS) by elemental iron resulted in the formation of copper nanoparticles [20]. This semi-industrial approach can also be used in laboratories as well as large-scale production [9][20]. Mechanochemistry helps solve the problems associated with coalescence and oxidation of metallic particles and facilitates particle size reduction by extending the milling time. It also helps to generate the products within a short time, even within a few seconds [21]. The synthesis of AgNPs in the presence of graphite as a reducing agent is the next successful example of ball milling [22]. The latest emerging area of mechanochemistry for the synthesis of nanomaterials is the use of green-type precursors. In this case, mechanochemical processing can be considered bio-mechanochemical synthesis. An example of such processing is the synthesis of AgNPs in the presence of natural products as reducing agents, i.e., Origanum vulgare leaf extract [18].
2.2. Synthesis of Metal Oxide Nanoparticles
Synthesis of ZnO NPs in an eco-friendly mechanochemical way is based on chemical Reactions (1) and (2). During this process, Zn (OH)2 formed after milling and subsequent heat treatment, gives the ZnO NPs [23].
Zn (CH3COO)2 + NaOH → 2CH3COONa + Zn(OH)2
Zn (OH)2 → ZnO +H2O
There are various routes for the synthesis of metal oxide NPs such as hydrothermal synthesis, chemical bath deposition (CBD), sol-gel method, etc. Most of these syntheses are carried out in the liquid phase and require a large volume of solvents. In contrast, high-energy ball milling converts the bulk materials into fine powder without solvents or with an extremely small volume of solvents. The mechanical energy activates the chemical reagents, which results in producing nanoparticles as the final products [15]. An easy, fast, and green synthetic route for the preparation of different metal oxide NPs makes the mechanochemical process very useful. For instance, the synthesis of Gd2O3 by mechanochemical processing and subsequent heat treatment was reported [24]. Similarly, other metal oxide nanoparticles including Cr2O3 [25], ZnO [26][27], ZrO2 [28], CeO2 [29], SnO2 [30], CdO [31], CoO [32], and TiO2 [33] were effectively synthesized by this method.
The biochar (carbonaceous and porous material) exhibits limited adsorption ability to anionic species. For instance, modification of biochar with metal oxide species to form nanocomposites significantly enhances its adsorption capacity. The formation of these nanocomposites by different processes may discharge some contaminants either as a byproduct, or impose contamination risk on the final product. High-energy ball milling can greatly reduce the contamination risk of the final product. It also decreases the particle size and increases the specific surface area and thus introduces plenty of active sites for adsorption. Specifically, combining CuO with biochar can increase the porosity of the resulting composite, enlarge specific surface area, and introduce hydrophilicity, which greatly enhances the adsorption capacity of the composite [34]. The comparison study for the synthesis of some metal and metal oxide NPs through mechanochemical and solvent-based methods is shown in Table 1. It includes the chemicals used, and particle size.
Table 1. Comparative study of solvent-based synthesis and mechanochemical synthesis of various NPs.
| Solvent-Based Synthesis | Mechanochemical Synthesis | ||||
|---|---|---|---|---|---|
| Samples (NPs) | Size (nm) |
Hazardous Chemicals Used | Refs. | Size (nm) |
Ref. |
| Ag | 8–50 | Hydrazine hydrate, Sodium hypophosphite | [35][36] | 39–100 | [37] |
| Au | 22 ± 4.6 | NaBH4 | [38] | 14.8 ± 6.8 | [23] |
| Cu2O | 7.5 ± 1.8 | NaBH4, NaOH | [39] | 11 | [40] |
| Fe2O3 | 50 | H2O2, N2H4 | [41] | 4.21 | [42] |
| ZnO | 45–76 | Ammonia | [43] | <20 ± 5 | [44] |
2.3. Synthesis of Nanoalloys and Nanocomposites
Mechanical alloying is the next advantageous strategy to synthesize mixed metal nanoparticles (alloy nanoparticles). These types of nanoparticles are widely used in catalytic applications as they show some synergetic effects. There are various methods for the preparation of bi- or multi-metallic nanoalloys. Many of the synthetic procedures are analogous to those used for the formation of monometallic NPs. Due to the various technical difficulties and laborious conventional synthetic procedures, mechanochemistry is one of the alternative and easy ways to prepare the metal oxide nanocomposites, supported metal nanoparticles, mesoporous materials, and different coordination polymers because of its simplicity and low cost [45]. For instance, Fe/CaO and Co/CaO nanocomposites were synthesized by inexpensive mechanochemical processing using non-toxic metal oxide precursors [46]. Mechanochemical synthesis can also be used for the synthesis of mixed metal oxide NPs such as ceria-zirconia [47].
2.4. Use of Mechanochemistry for Doping and Incorporating Various Species
TiO2 has been extensively studied as a photocatalyst and it can be synthesized by different approaches such as sol-gel, hydrothermal, and mechanochemical (ball milling) methods. Doping and co-doping with suitable metallic or nonmetallic elements or coupling with another semiconductor have been used to enhance its properties. Silver-doped TiO2 has Schottky defects and behaves as an electron trap. Various methods have already been proposed to dope Ag on the titania surface. However, ball milling is cost-effective, less time-consuming, and an eco-friendly approach. Doped titania nanoparticles are photo-catalytically and biologically active [48]. Another example is a conventional synthesis of porous carbon, which involves a multi-step and expensive process, and produces many wastes as a byproduct. Mechanochemical synthesis of carbons eliminates these shortcomings. Thus, a mechanically-induced self-sustaining reaction can be performed at room temperature to get the N-doped porous carbon or nitrogen-rich carbon materials – specifically, one-pot mechanochemical process involving calcium carbide and cyanuric chloride [49]. Reaction (3) was used to obtain nitrogen-rich carbon material, i.e., C6N3 carbon nitride:
3CaC2 + 2C3Cl3N3 ⟶ 3CaCl2 + 2C6N3
Similarly, an easy, efficient, and safe method of doping of Mg on hydroxyapatite was achieved by the dry mechanochemical method [50]. Additionally, the mechanochemical synthesis of transition metal doped ZnO for photocatalytic applications was performed. Co-doping of ZnO highly reduced its photocatalytic activity as the Co ions substituted the Zn ions in the ZnO wurtzite phase. On the other hand, the Mn dopant showed an increased photocatalytic activity at low levels of doping, which was reversed at a higher level of doping [51].
2.5. Mechanochemical Synthesis of Highly Porous Nanoparticles
Adsorption is an important physical phenomenon that results in attracting atoms or molecules of gas, liquid, or solid phase on the surface. The porosity of material means the presence of various interconnected voids and/or channels in its matrix. IUPAC defines porosity in terms of the size (diameter) of pores and distinguishes three classes of materials: (i) microporous, having pore sizes below 2 nm; (ii) mesoporous, having pores in the range of 2–50 nm; and (iii) macroporous, possessing pores with sizes larger than 50 nm [52]. Microporous materials are further sub-classified as ultra-microporous materials having pore sizes of 0.7 nm or smaller [53]. Mechanochemical synthesis is an emerging method for the preparation of various porous materials [1]. This method overshadows the phenol-formaldehyde polycondensation approach for the formation of porous carbon. The uniform and scalable ordered mesoporous carbons (OMCs) were synthesized using bio polyphenols (tannin), and block-copolymer. This method was modified to incorporate Ni and Zn species into carbons [54]. Similarly, mesoporous crystalline γ-alumina and modified alumina with a high specific surface area and pore volume were synthesized from boehmite as an alumina precursor via high-energy mechanochemical ball milling [55][56].
Additionally, ball milling was used to synthesize FeO(OH) nanoflake/graphene and nano Fe3O4/graphene composites from commercially-available graphite oxide and iron powders [57]. Mechanochemical approach facilitates the synthesis of two- and three-dimensional metal-organic compounds. Figure 1 represents the chemical reaction for the formation of Cu3(BTC)2 and Cu3(BTB)2 [58]. A comparison of the mechanochemical activation of metal-organic framework (MOF) (HKUST-1) (SBET = 1713 m2/g) with the sample without activation (SBET = 758 m2/g), and commercial sample (SBET = 1836 m2/g) has been reported elsewhere [58].
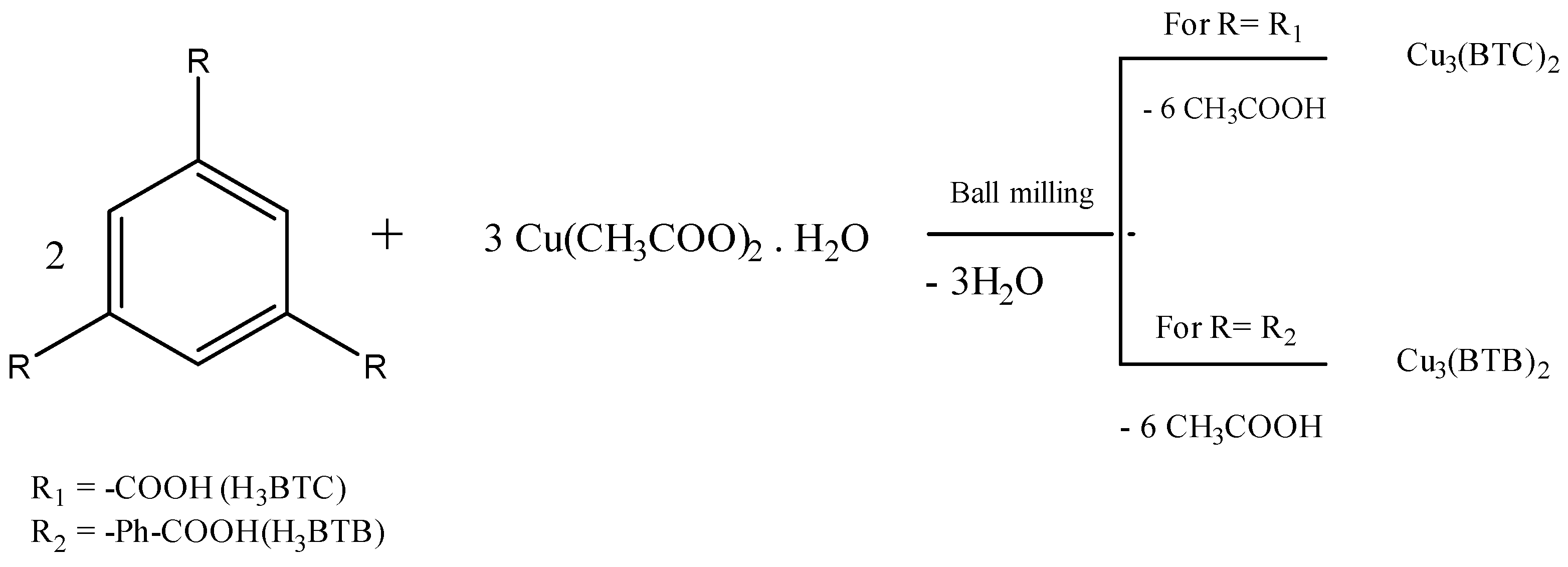
Figure 1. Mechanochemical synthesis of HKUST-1 (copper benzene-1,3,5-tricarboxylate) and MOF-14 {[Cu3(BTB)2(H2O)3] (DMF)9(H2O)2}.
References
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical synthesis of highly porous materials. Mater. Horiz. 2020, 7, 1457–1473.
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Advances in microwave synthesis of nanoporous materials. Adv. Mater. 2021, 33, 2103477–2103505.
- McNaught, A.D. Compendium of chemical terminology. Oxf. Blackwell Sci. 1997, 1669, 1–464.
- Xiao, W.; Yang, S.; Zhang, P.; Li, P.; Wu, P.; Li, M.; Chen, N.; Jie, K.; Huang, C.; Zhang, N.; et al. Facile synthesis of highly porous metal oxides by mechanochemical nanocasting. Chem. Mater. 2018, 30, 2924–2929.
- Bulina, N.V.; Vinokurova, O.B.; Eremina, N.V.; Prosanov, I.Y.; Khusnutdinov, V.R.; Chaikina, M.V. Features of solid phase mechanochemical synthesis of hydroxyapatite doped by copper and zinc ions. J. Solid State Chem. 2021, 296, 121973–121980.
- Subramonian, W.; Wu, T.Y.; Chai, S.P. Using one-step facile and solvent-free mechanochemical process to synthesize photoactive Fe2O3-TiO2 for treating industrial wastewater. J. Alloys Compd. 2017, 695, 496–507.
- Ulbrich, K.F.; Nishida, E.N.; Souza, B.S.; Campos, C.E.M. NiS2-NiS nanocrystalline composite synthesized by mechanochemistry and its performance for methylene blue dye adsorption. Mater. Chem. Phys. 2020, 252, 123226–123235.
- Wieczorek-Ciurowa, K.; Gamrat, K. Some aspects of mechanochemical reactions. Mater. Sci.-Pol. 2007, 25, 219–232.
- He, X.; Deng, Y.; Zhang, Y.; He, Q.; Xiao, D.; Peng, M.; Zhao, Y.; Zhang, H.; Luo, R.; Gan, T.; et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts. Cell Rep. Phys. Sci. 2020, 1, 100004–100017.
- Zhang, L.; Shi, H.; Tan, X.; Jiang, Z.; Wang, P.; Qin, J. Ten-gram-scale mechanochemical synthesis of ternary lanthanum coordination polymers for antibacterial and antitumor activities. Front. Chem. 2022, 10, 898324.
- Stanković, A.; Veselinović, L.J.; Škapin, S.D.; Marković, S.; Uskoković, D. Controlled mechanochemically assisted synthesis of ZnO nanopowders in the presence of oxalic acid. J. Mater. Sci. 2011, 46, 3716–3724.
- Tan, D.; Loots, L.; Friščić, T. Towards medicinal mechanochemistry: Evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Comm. 2016, 52, 7760–7781.
- Chin, P.P.; Ding, J.; Yi, J.B.; Liu, B.H. Synthesis of FeS2 and FeS nanoparticles by high-energy mechanical milling and mechanochemical processing. J. Alloys Compd. 2005, 390, 255–260.
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174–101185.
- McCormick, P.G.; Froes, F.H. The fundamentals of mechanochemical processing. J. Osteopath. Med. 1998, 50, 61–65.
- de Oliveira, P.F.; Torresi, R.M.; Emmerling, F.; Camargo, P.H. Challenges, and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141.
- Suryanarayana, C. Mechanical alloying, and milling. Prog. Mater. Sci. 2001, 46, 1–184.
- Baláž, M.; Daeu, N.; Balážová, Ľ.; Dutková, E.; Tkáčiková, Ľ.; Briančin, J.; Vargová, M.; Balážová, M.; Zorkovská, A.; Baláž, P. Bio-mechanochemical synthesis of silver nanoparticles with antibacterial activity. Adv. Powder Technol. 2017, 28, 3307–3312.
- Tsuzuki, T.; McCormick, P.G. Mechanochemical synthesis of nanoparticles. J. Mater. Sci. 2004, 39, 5143–5146.
- Baláž, M.; Tešinský, M.; Marquardt, J.; Škrobian, M.; Daneu, N.; Rajňák, M.; Baláž, P. Synthesis of copper nanoparticles from refractory sulfides using a semi-industrial mechanochemical approach. Adv. Powder Technol. 2000, 31, 782–791.
- Baláž, M.; Zorkovská, A.; Urakaev, F.; Baláž, P.; Briančin, J.; Bujňáková, Z.; Achimovičová, M.; Gock, E. Ultrafast mechanochemical synthesis of copper sulfides. R. Soc. Chem. Adv. 2016, 6, 87836–87842.
- Khayati, G.R.; Janghorban, K. The nanostructure evolution of Ag powder synthesized by high energy ball milling. Adv. Powder Technol. 2012, 23, 393–397.
- Nguyen, T.A.; Mai, T.Y.; Nguyen, T.X.M.; Huynh, K.P.H.; Le, M.V.; Nguyen, T. Mechanochemical synthesis of zinc oxide nanoparticles and their antibacterial activity against escherichia coli. Mater. Sci. Forum 2020, 1007, 59–64.
- Tsuzuki, T.; Pirault, E.; McCormick, P.G. Mechanochemical synthesis of gadolinium oxide nanoparticles. Nanostructured Mater. 1999, 11, 125–131.
- Tsuzuki, T.; McCormick, P.G. Synthesis of Cr2O3 nanoparticles by mechanochemical processing. Acta Mater. 2000, 48, 2795–2801.
- Ao, W.; Li, J.; Yang, H.; Zeng, X.; Ma, X. Mechanochemical synthesis of zinc oxide nanocrystalline. Powder Technol. 2006, 168, 148–151.
- Aghababazadeh, R.; Mazinani, B.; Mirhabibi, A.; Tamizifar, M. ZnO nanoparticles synthesized by mechanochemical processing. J. Phys. Conf. Ser. 2006, 26, 312–314.
- Dodd, A.C.; McCormick, P.G. Synthesis of nanocrystalline ZrO2 powders by mechanochemical reaction of ZrCl4 with LiOH. J. Eur. Ceram. Soc. 2002, 22, 1823–1829.
- Li, Y.X.; Zhou, X.Z.; Wang, Y.; You, X.Z. Preparation of nano sized CeO2 by mechanochemical reaction of cerium carbonate with sodium hydroxide. Mater. Lett. 2004, 58, 245–249.
- Yang, H.; Hu, Y.; Tang, A.; Jin, S.; Qiu, G. Synthesis of tin oxide nanoparticles by mechanochemical reaction. J. Alloys Compd. 2004, 363, 276–279.
- Yang, H.; Qiu, G.; Zhang, X.; Tang, A.; Yang, W. Preparation of CdO nanoparticles by mechanochemical reaction. J. Nanopart. Res. 2004, 6, 539–542.
- Yang, H.; Hu, Y.; Zhang, X.; Qiu, G. Mechanochemical synthesis of cobalt oxide nanoparticles. Mater. Lett. 2004, 58, 387–389.
- Dodd, A.; McKinley, A.; Tsuzuki, T.; Saunders, M. Optical and photocatalytic propertie of nanocrystalline TiO2 synthesized by solid-state chemical reaction. J. Phys. Chem. Solids 2007, 68, 2341–2348.
- Wei, X.; Wang, X.; Gao, B.; Zou, W.; Dong, L. Facile ball-milling synthesis of CuO/biochar nanocomposites for efficient removal of reactive red 120. ACS Omega 2020, 5, 5748–5755.
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnology. 2016, 14, 73.
- Li, Z.; Wang, Y.; Yu, Q. Significant parameters in the optimization of synthesis of silver nanoparticles by chemical reduction method. J. Mater. Eng. Perform. 2010, 19, 252–256.
- Baláž, M.; Goga, M.; Hegdüs, M.; Daneu, N.; Kováčová, M.; Tkáčiková, L.U.; Balážová, L.U.; Bačkor, M. Biomechanochemical solid-state synthesis of silver nanoparticles with antibacterial activity using lichens. ACS Sustain. Chem. Eng. 2020, 8, 13945–13955.
- Itoh, H.; Naka, K.; Chujo, Y. Synthesis of gold nanoparticles modified with ionic liquid based on the imidazolium cation. J. Am. Chem. Soc. 2004, 126, 3026–3027.
- Mallik, M.; Monia, S.; Gupta, M.; Ghosh, A.; Toppo, M.P.; Roy, H. Synthesis, and characterization of Cu2O nanoparticles. J. Alloys Compd. 2020, 829, 154623–154629.
- Khayati, G.R.; Nourafkan, E.; Karimi, G.; Moradgholi, J. Synthesis of cuprous oxide nanoparticles by mechanochemical oxidation of copper in high planetary energy ball mill. Adv. Powder Technol. 2013, 24, 301–305.
- Wang, W.W.; Zhu, Y.J.; Ruan, M.L. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J. Nanopart. Res. 2007, 9, 419–426.
- Seyedi, M.; Haratian, S.; Khaki, J.V. Mechanochemical synthesis of Fe2O3 nanoparticles. Procedia Mater. Sci. 2015, 11, 309–313.
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73.
- Manzoor, U.; Siddique, S.; Ahmed, R.; Noreen, Z.; Bokhari, H.; Ahmad, I. Antibacterial, structural, and optical characterization of mechano-chemically prepared ZnO nanoparticles. PLoS ONE 2016, 11, 0154704–0154716.
- De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical synthesis of supported bimetallic catalysts. Chem. Mater. 2021, 33, 2037–2045.
- Chaudhary, V.; Zhong, Y.; Parmar, H.; Sharma, V.; Tan, X.; Ramanujan, R.V. Mechanochemical synthesis of iron and cobalt magnetic metal nanoparticles and iron/calcium oxide and cobalt/calcium oxide nanocomposites. Chem. Open 2018, 7, 590–598.
- Shah, P.M.; Day, A.N.; Davies, T.E.; Morgan, D.J.; Taylor, S.H. Mechanochemical preparation of ceria-zirconia catalysts for the total oxidation of propane and naphthalene volatile organic compounds. Appl. Catal. B Environ. 2019, 253, 331–340.
- Aysin, B.; Ozturk, A.; Park, J. Silver-loaded TiO2 powders prepared through mechanical ball milling. Ceram. Int. 2013, 39, 7119–7126.
- Casco, M.E.; Kirchhoff, S.; Leistenschneider, D.; Rauche, M.; Brunner, E.; Borchardt, L. Mechanochemical synthesis of N-doped porous carbon at room temperature. Nanoscale 2019, 11, 4712–4718.
- Abu Bakar, S.A.S.; Ramesh, S.; Sopyan, I.; Tan, C.Y.; Hamdi, M.; Teng, W.D. Mechanochemical synthesis of magnesium doped hydroxyapatite: Powder characterization. Appl. Mech. Mater. 2013, 372, 62–65.
- He, R.; Hocking, R.K.; Tsuzuki, T. Local structure, and photocatalytic property of mechanochemical synthesized ZnO doped with transition metal oxides. J. Aust. Ceram. Soc. 2013, 49, 70–75. Available online: http://hdl.handle.net/10536/DRO/DU:30057625 (accessed on 31 December 2012).
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069.
- Du, S.; Wu, Y.; Wang, X.; Xia, Q.; Xiao, J.; Zhou, X.; Li, Z. Facile synthesis of ultramicroporous carbon adsorbents with ultra-high CH4 uptake by in situ ionic activation. AIChE J. 2020, 66, 16231–16240.
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nat.Commun. 2017, 8, 15020.
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Facile mechanochemical synthesis of highly mesoporous γ-Al2O3 using boehmite. Microporous Mesoporous Mater. 2021, 312, 110792–110799.
- Weidner, E.; Dubadi, R.; Samojeden, B.; Piasecki, A.; Jesionowski, T.; Jaroniec, M.; Ciesielczyk, F. Mechanochemical synthesis of alumina-based catalysts enriched with vanadia and lanthana for selective catalytic reduction of nitrogen oxides. Sci. Rep. 2022, 12, 21294.
- Zhao, B.; Zheng, Y.; Ye, F.; Deng, X.; Xu, X.; Liu, M.; Shao, Z. Multifunctional iron oxide nanoflake/graphene composites derived from mechanochemical synthesis for enhanced lithium storage and electrocatalysis. ACS Appl. Mater. Interfaces. 2015, 7, 14446–14455.
- Klimakow, M.; Klobes, P.; Thünemann, A.F.; Rademann, K.; Emmerling, F. Mechanochemical synthesis of metal− organic frameworks: A fast and facile approach toward quantitative yields and high specific surface areas. Chem. Mater. 2010, 22, 5216–5221.
More
Information
Subjects:
Chemistry, Inorganic & Nuclear
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.8K
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




