Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hairong Bao | -- | 3116 | 2023-03-14 01:45:06 | | | |
| 2 | Conner Chen | Meta information modification | 3116 | 2023-03-15 03:23:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Li, M.; Guo, Q.; Lin, Y.; Bao, H.; Miao, S. Wall Materials of Peptide Microcapsules. Encyclopedia. Available online: https://encyclopedia.pub/entry/42143 (accessed on 07 February 2026).
Li M, Guo Q, Lin Y, Bao H, Miao S. Wall Materials of Peptide Microcapsules. Encyclopedia. Available at: https://encyclopedia.pub/entry/42143. Accessed February 07, 2026.
Li, Mengjie, Quanyou Guo, Yichen Lin, Hairong Bao, Song Miao. "Wall Materials of Peptide Microcapsules" Encyclopedia, https://encyclopedia.pub/entry/42143 (accessed February 07, 2026).
Li, M., Guo, Q., Lin, Y., Bao, H., & Miao, S. (2023, March 14). Wall Materials of Peptide Microcapsules. In Encyclopedia. https://encyclopedia.pub/entry/42143
Li, Mengjie, et al. "Wall Materials of Peptide Microcapsules." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Microencapsulation technology can improve the utilization rate of the active peptides. Microencapsulation technology is a kind of encapsulation technology based on nanocarriers. The microencapsulation of peptides refers to the selection of appropriate wall materials and the use of physical, chemical, or physicochemical methods to embed the active peptides, in order to give play to the advantages of isolating the interaction between the active peptide and the external environment.
active peptides
microcapsules
wall material
1. Natural Polymer Wall Materials
Common natural polymer wall materials can be divided into carbohydrates, proteins, and lipids [1]. Such materials are widely used in the preparation of peptides microcapsules, due to their broad variety of natural sources, non-toxicity, non-irritation, good biocompatibility, and film-forming properties.
1.1. Polysaccharides
Polysaccharides are green, nutritious, and healthy macromolecules, containing monomers with only three elements (carbon, hydrogen, and oxygen), which are polymerized through glycosidic bonds. Among them, sodium alginate, chitosan, pectin, and cellulose have promising applications in drug delivery.
Alginate is a natural polysaccharide extracted from brown algae or bacterial cell walls. Due to its good solubility, biocompatibility, gelation, ease of film formation, and natural non-toxicity, alginate has been widely used in the preparation of antibacterial films, dressings, microcapsule wall materials, hydrogels, and other aspects [2][3][4]. Calcium chloride solution is often added to sodium alginate, and alginate and metal particles are complexed to form calcium alginate gel systems to obtain stronger toughness and hardness. When Ca2+,-COO- and -OH work together to provide coordination bonds for other molecules in the system, the “egg box”-shaped three-dimensional network structure has a positive effect on the encapsulated active substance [5]. By increasing the temperature, the chain structure of sodium alginate can be expanded and complexed with Ca2+; when the temperature is too high, the structural unit of sodium alginate is destroyed, which is not conducive to the formation of the condensation system. The α-calcitonin gene-related peptide (α-CGRP) is a neuromodulatory peptide widely distributed in the central nervous system, which has a vasodilatory function and plays an important role in the pathogenesis of migraines. Its biological half-life is about 18 min, which means the time required to reduce the α-CGRP dosage in the body or the drug concentration in the blood by half is 18 min. Kumar et al. [6] studied alginate-based α- CGRP microcapsules and tested the efficacy of alginate -α-CGRP microcapsules in a TAC pressure overload heart failure mouse model. The results showed that alginate-α-CGRP microcapsules effectively prolonged the release of the peptide, while administering mice with significantly reduced cardiopulmonary weight, left ventricular cardiomyocyte size, apoptosis, and increased oxidative stress. Oki et al. [7] developed a sulfhydryl-maleimide-modified alginate microcapsule, and the modified alginate could be encapsulated by “in situ conjugation” of sulfhydryl-containing peptides. These in situ binding methods of functional peptides have promising applications in biomedicine, chemistry, and other aspects.
Chitosan is also a kind of natural polymer material that is obtained by removing part of the acetyl group from chitin. It is biodegradable, biocompatible, non-toxic, hygroscopic, and film-forming and has various physiological functions, such as antibacterial, anticancer, lipid-lowering, etc., as the only alkaline polysaccharide among natural polysaccharides [8]. Zhao et al. [9] prepared microcapsules of desalinated duck egg white peptide calcium (DPS-Ca) with chitosan, which can prevent DPS-Ca from being digested by the stomach and make more calcium entering the intestine, promoting calcium absorption. Furthermore, since chitosan contains chemically modifiable -NH2 and -COOH, when dissolved in dilute acid, it will produce primary amino acids, which makes the molecular chain of chitosan carry a large amount of positive charge; the molecular chain of sodium alginate carries a large amount of negative charge in aqueous solution when a polyelectrolyte film is formed between the two through electrostatic interaction. Owing to the fact that this method does not require high temperature and chemical force for the preparation of films, it is an ideal encapsulation material for active substances, such as proteins and peptides, that are easily degradable and have a short half-life [10]. Salvatore et al. [11] constructed chitosan sodium alginate hollow capsule shells using the jet method and the layer-wise self-assembly method, in which fluorescently labeled peptide substrates were involved in the sortase-catalyzed trans-peptide reaction.
In addition, some polysaccharide gums from plants are also ideal materials for embedding active peptides. L-rhamnose, D-galactose, arabinose, etc., constitute these polysaccharide gums. The most widely used are Arabic gum, pectin, algae gum, and locust bean gum [12]. Different gums have different physical and chemical properties. Algae gum, for example, is a low-fat, high-dietary fiber polysaccharide material, including agar and alginate. Hambleton et al. [13] studied the interface characteristics of microcapsule products covered with carrageenan and found that the microcapsule of carrageenan wall material has the characteristics of a uniform surface, smoothness, and high strength. Joanna et al. [14] constructed the antioxidant peptides microcapsules embedded by algae gum and evaluated the lipid distribution, antioxidant blood status, and mRNA expression of some genes related to antioxidant status in rats in vitro experiments and confirmed that the serum level of total antioxidant status was significantly increased in animals that consumed microcapsules without causing oxidative stress. Raúl E et al. [15] found that acacia bean gum and algae gum as composite wall materials had better encapsulation rates and resistance to digestive enzymes for ACE-I inhibitory peptides, compared to both of them used independently.
Dextrins are oligosaccharides formed by the physical, chemical, or enzymatic decomposition of starch, of which, cyclodextrins are small glucose molecules with cavities in rings formed by straight-chain starch under the action of enzymes produced by Bacillus, with the advantage of non-toxic and easily absorbed by humans. The structural characteristics of cyclodextrins allow them to form a complex structure by combining specific compounds of compatible size in an aqueous solution to achieve the functions of stabilization, sustained release, oxidation resistance, and odor masking of the object [16]. β-cyclodextrin is an ideal wall material for hydrophobic substances, due to the hydrophilic properties of the outer wall and hydrophobic properties of the inner cavity [17]. The nano-sponge is a colloidal structure composed of solid nanoparticles with holes and a reticular structure, which is used to encapsulate various substances. The β-cyclodextrin-based nano-sponge has potential application value in the targeted drug delivery of the liver, spleen, and lung and is an ideal carrier for protein and polypeptide drug delivery [18]. Neuropeptide Y (NPY) is a hormone with a stabilizing effect on the human body. Desai et al. [19] aimed to compound NPY with hydroxypropyl β-cyclodextrin (HPβ-CD) to finally achieve NPY microcapsules with an encapsulation rate of 84.68 ± 5.47% and a 24 h release of 85.16 ± 6.13% in vitro.
1.2. Protein
Protein is an appropriate wall material for embedding active substances, due to its good biocompatibility and biodegradability, and is regarded as “Generally recognized as safe” by the FDA [20]. Soybean protein, gelatin, whey protein, and zein can be used as the embedding materials of active peptides. Different proteins have different abilities for drug loading and controlled release, due to their different properties, structures, and functions. However, the spontaneous aggregation behavior of proteins and the protease in the body can both damage the structure of microcapsules. The combination of protein and other materials can optimize the embedding effect [21].
Gelatin is a macromolecular colloid produced from the decomposition of animal collagen. Its amphiphilic makes it effectively act as a binding agent between composite walls and between walls and core materials. Compared with plant proteins, animal proteins have better film-forming properties [22], considered a high-quality natural encapsulation carrier. Nevertheless, the application of gelatin is often limited by its low mechanical properties and poor bio-adhesiveness, and it is usually used in the form of hard gelatin capsule shells. These kinds of defects can be optimized by compounding with other materials to achieve their wall properties [23][24]. Favaro et al. [25] prepared a gelatin compounded with soybean isolate as the wall material, and the peptides microcapsules were proved to be more stable and to have lower bitterness than the raw peptides. According to Niu et al. [26], the composite wall material of gelatin and sodium alginate was used to embed methionine, which realized the controlled release of methionine and simultaneously stimulated the synchronous absorption of other amino acids to synthesize proteins.
As a common protein of plant origin, soy protein has an amino acid composition similar to that of milk protein and has a nutritional value equivalent to that of animal protein, which also has isoflavones linked to lower cholesterol levels [27]. Through industrial processing, soy protein flour (SPF), soy protein concentrate (SPC), soy protein isolate (SPI), and tissue soybean protein (TSP) exhibit remarkable emulsification and film-forming properties, and their unfolding structure in the emulsion wrap around the oil phase to stabilize the water–oil interface. It is worth mentioning that SPI has high protein concentration, emulsification, and cohesion, and the macromolecular complex formed by electrostatic self-assembly with acid-resistant SPSS is a good wall material for embedding hydrophobic active substances. It has been shown that the pre-heating treatment of SPI results in stronger emulsification and the interfacial elasticity of it, as well as tighter complexation with polysaccharides; moreover, heating exposes more internal hydrophobic groups by unfolding the structure of SPI, which is beneficial to the embedding of hydrophobic peptides [28]. Wei et al. [29] utilized the self-assembly ability of protein hydrolysate and polyphenol curcumin to mask the bitter hydrophobic residue of protein hydrolysate through hydrophobic combinations. By embedding with wall material SPI and SPC, the soybean peptide–curcumin nanoparticles microcapsules were prepared with an embedding rate of 50.92%, bitterness reduction of 2.68 times, and moisture absorption reduction of 1.61 times.
Whey protein is a high-quality protein that is easily absorbed, low in sugar, and has the highest nutritional value of all proteins. Industrial whey proteins are mainly derived from by-products of cheese production and are dominated by whey protein concentrate (WPC) and whey protein isolate (WPI). Whey protein has high nutritional value, and as the wall material of microcapsules, it also has pH-sensitive characteristics specific to the controlled release of the cores [30]. Considering whey as a natural bacterial medium, Farizano et al. [31] investigated the use of WPC and chitosan derivatives as composite wall materials to encapsulate Gram-positive bacteria with bacteriocin-erythromycin CRL35, which not only maintained bacteriocin activity, but also provided a scheme for the green treatment of whey.
1.3. Lipids
The lipids used as microcapsule wall materials are generally fatty acids and lecithin. The advantages of this type of wall material are that it can encapsulate both hydrophilic and lipophilic core materials and it has good biocompatibility, and targeting, among which, stearic acid also shows good oxidation resistance. Despite so many benefits, lipids still have some disadvantages: the preparation efficiency is not high, and the preparation method is limited by the properties of lipids [32]. N. Blanco-Pascual et al. [33] used a mixture of stearic acid and Brazilian carnauba wax (3:1) as a wall material and peptide solution as a core material to produce microcapsules with a shell material/peptide dry base ratio of 13.3:1. This product was realized by the microencapsulation printing technique. The microcapsules were found to have good stability (<30% peptides release within 3 h) under experimental conditions of pH5 and pH7. Further research revealed that the modifying properties of lipids and the chemical interaction with other biopolymers both allow lipids to be worked as wall fillers to promote the formation of dense walls; at the same time, the lipid–peptide complex makes the core material more stable, similar to the model in Figure 1 [32].
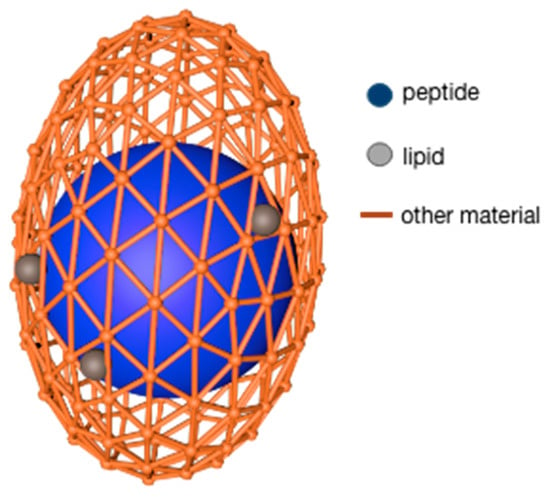
Figure 1. Microcapsule wall materials of alginate filled by lipid.
2. Modified Polymer Wall Materials
Through the modification of natural materials, the mechanical strength and stability of natural materials are enhanced, and the limitation of the application of natural materials is made up, which makes natural materials a good tool to protect the inner substance. Researchers usually use modified protein or modified cellulose as the shell of the microencapsulated peptides.
2.1. Modified Cellulose
Cellulose is the most popular food packaging material now—it is non-toxic, biodegradable, and has good barrier properties, making it a substitute for traditional petroleum-based plastics. The hydrophilicity of cellulose is the biggest limitation as a packaging material, which can be solved by modifying it. The researchers reported, in detail, the cellulose modification technology and the application prospect of chemically modified cellulose in food packaging [34]. The modified cellulose used for embedding peptides is introduced below.
Hydroxyethyl cellulose (HEC), hydroxypropyl methyl cellulose (HPMC), carboxymethyl cellulose (CMC), and nanocellulose are all common materials for embedding peptides. As early as 1999, Ohyama et al. [35] tried to embed islets with agarose, polystyrene sulfonic acid, and polybrene carboxymethyl cellulose to realize xenotransplantation of islets. Microencapsulation proved to be successful in prolonging islet survival. Up until now, great progress has been made in embedding delivery materials for islets of Langerhans [36]. Cesar et al. [37] prepared Ctx(Ile21)-Ha antimicrobial peptide microcapsules encapsulated by hydroxypropylmethyl cellulose phthalate (HPMCP) as an alternative to traditional antibiotics and as a production enhancer for chick rearing.
2.2. Modified Protein
The modification of amino acid components, charged properties, and spatial structure of proteins through physical, chemical, enzymatic, or genetic engineering is called protein modification, leading to a broad foundation for the application of proteins in many fields [38][39][40]. The modified proteins are less sensitive to the environment and obtain better stability, emulsification, and solubility.
Wang et al. [41] studied the chemical, enzymatic, and physical modifications of rapeseed isolate protein (RPI) to improve its mechanical properties as a wall material for spray-dried rapeseed peptide (RP) microcapsules. The experimental results showed that both acylation of the wall material RPI (acylation degree (DA) of 47%) and high pressure (HP) of 400 MPa resulted in greater encapsulation of microcapsules, with 99% and 94%, respectively.
3. Synthetic Polymer Wall Materials
The synthetic polymer materials used for peptide microcapsules wall materials mainly include degradable polymers, polyesters, polydextrose, and non-degradable polymers (polyacrylamides). Such materials have stronger manipulability and specificity and have become potential materials in the medical, biological, and food fields in recent years. Polymer-embedded microcapsules tend to have a more homogeneous particle size and higher mechanical strength for a wide range of applications.
3.1. Polylactic Acid
Polylactic acid (PLA), a polymer formed by dehydration of lactic acid (LA), is the most widely used polyester carrier for protein and peptide drugs, due to its adjustable molecular weight and good biocompatibility. It is often used in copolymers with other materials (e.g., PLGA and PLA-PEG-PLA) (Figure 2). PLGA carriers act as “delayed” excipients: when PLGA degrades, it produces acidic degradation products that cause a drop in the internal pH of the polyacrylate matrix. The decrease in pH value weakens the peptides–polymer interaction and enables the release of the peptides [42][43]. Justin et al. [44] investigated the thermodynamics of cationic octapeptide octreotide binding to PLGA using nanoisothermal titration calorimetry (NanoITC), revealing that the actual driving force for octreotide-PLGA interaction is ion pairing. Cationic peptides readily bind PLA-PLGAs to carboxylic acid (-COOH) end groups, and such binding is key to leading to PLGA-based peptide acylation, which poses a significant challenge for both drug microencapsulation and delivery systems. Lim et al. [45] found that the encapsulation of exenatide acetate in PLGA microspheres using the w/o/w double-emulsion solvent evaporation method and the determination of peptide release profiles and acylation products of PLGA polymer degradation in PLGA microspheres showed that this method significantly improved the stability of exenatide acetate. Meanwhile, the acylation of the PLGA polymer was eliminated during the degradation process in vitro, and the immunogenicity was reduced in vivo. PEG is a polyether polymer with a flexible hydrophilic surface, which has the function of extending the circulation time and retaining the half-life of nanoparticles as a microcapsular wall material. Zhang et al. [46] used thymopentin as a model drug and the PLA-PEG (polyethylene glycol)-PLA triblock copolymer as a carrier to prepare microcapsules for cancer therapy, and this dense shell structure significantly delayed the abrupt release of the core material, which has good prospects for application as an anticancer drug.
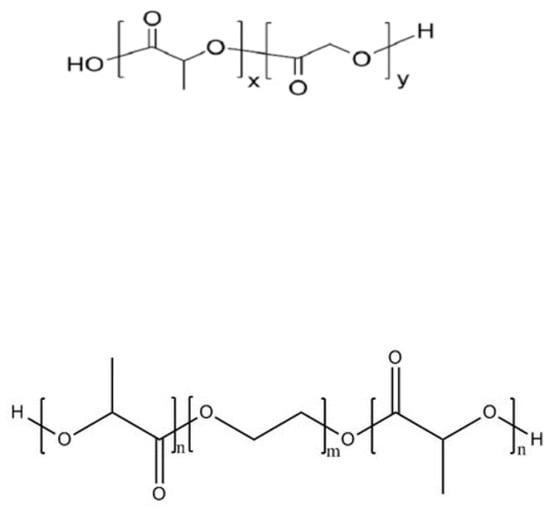
Figure 2. Molecular structure formulas of PLGA (up) and PLA-PEG-PLA (down).
3.2. Polydextrose
Polydextrose (PD) is a water-soluble dietary fiber produced by the vacuum condensation of glucose and small amounts of sorbitol, citric acid, or phosphoric acid in the molten state, and it is not toxic to humans [47]. Oligoglucose with a molecular weight of 162–5000 has a regulatory effect on human intestinal flora and is most widely used in practice. Marilia et al. [48] used PD and maltodextrin (MD) polymers as wall materials and ferrous sulfate with organic peptides as core materials and improved the absorption of iron in humans by the spray drying method of encapsulated microcapsules.
3.3. Polypropylene
Polypropylene is a common package material, and polymeric materials prepared by polymerization with its derivatives or reaction with other substances are widely used in microcapsules, hydrogels, flocculants, etc.
The hepatitis B surface antigen (HBsAg) is a mixture of peptides, and oral delivery is one of the most effective ways to deliver it. However, factors such as intestinal pH and bacterial flora pose challenges to drug delivery. In this study, HBsAg was manually filled into the commercial hard gelatin microcapsule shell, and then Eudragit S100 and Eudragit L100 (4:1 W/W) were used as the secondary coating for embedding the microcapsules [23]. The experimental results confirmed that HBsAg microcapsules constructed by this method achieved targeted delivery in the colon and produced considerable antibody volume. Gunay et al. [49] studied a peptides microcapsule with the poly(N-(2-hydroxypropyl) methacrylamide) (PHPMA) copolymer as the aromatophile and polyurethane as the microcapsule shell, and the experimental results showed that the incorporation of peptides increased the deposition of PHPMA copolymer by 3.5–5.0 times and enhanced the deposition of the fragrance system in hair.
4. Potential Material
In addition to the materials mentioned above, several substances with potential as active peptide wall materials are provided below.
Polyhydroxyalkanoates (PHA) and their derivatives (PHB, PHO, etc.) are a kind of natural polyester materials synthesized by microbial fermentation from carbon sources, which can be degraded both in and out of cells quickly [50]. Compared with other natural materials, polyhydroxyalkanoates exhibit better high-temperature stability, lower surface porosity, and enhanced toughness and elasticity, making them popular in the medical field as raw material of nanoparticles (Figure 3) [51]. In the study, Cao et al. [52] encapsulated trifluralin with PHB polymer as the carrier, and the microcapsules formed showed better photostability and herbicidal activity. The release rule could be adjusted by changing the preparation parameters, such as the shear rate and emulsifier concentration.
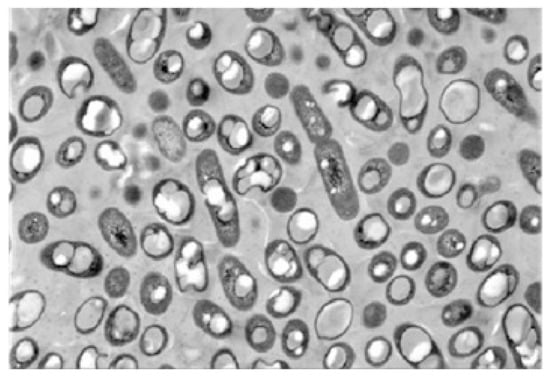
Figure 3. Microscopic image of PHA granules [51].
Inorganic materials usually have good chemical and thermal stability, so they are often used in the field of biodegradation and environmental protection [53]. Among them, bimetallic hydroxide, calcium carbonate, and phosphate can be used as microcapsule wall materials. Hollow microcapsules show good performance in drug delivery and personalized medicine. However, microcapsules made of polyelectrolytes are soft and unstable in harsh environments. Jin et al. [54] reported an improved Ca2+ cross-linked hard-shell microcapsule to improve the instability of microcapsules made of polyelectrolytes in harsh environments. The experimental results confirm that the Ca2+ embedded hard-shell microcapsules have good mechanical strength and a two-stage sustained release effect.
References
- Jéssica, S.R.; Cristiane, M.V. Microencapsulation of natural dyes with biopolymers for application in food: A review. Food Hydrocoll. 2021, 112, 106374.
- Ramprakash, B.; Incharoensakdi, A. Alginate encapsulated nanobio-hybrid system enables improvement of photocatalytic biohydrogen production in the presence of oxygen. Int. J. Hydrog. Energy 2022, 47, 11492–11499.
- Abdel, A.M.S.; Salama, H.E. Developing multifunctional edible coatings based on alginate for active food packaging. Int. J. Biol. Macromol. 2021, 190, 837–844.
- Pratiksha, S.; Pankaj, B.; Omprakash, S.Y. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, e03026.
- Sikorski, P.; Mo, F.; Skjak, B.G.; Stokke, B.T. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber X-ray diffraction. Biomacromolecules 2007, 8, 2098–2103.
- Kumar, A.; Belhaj, M.; DiPette, D.J.; Potts, J.D. A Novel Alginate-Based Delivery System for the Prevention and Treatment of Pressure-Overload Induced Heart Failure. Front. Pharmacol. 2021, 11, 602952.
- Oki, Y.; Kirita, K.; Ohta, S.; Ohba, S.; Horiguchi, I.; Sakai, Y.; Ito, T. Switching of Cell Proliferation/ Differentiation in Thiol-Maleimide Clickable Microcapsules Triggered by in Situ Conjugation of Biomimetic Peptides. Biomacromolecules 2019, 20, 2350–2359.
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850.
- Zhao, M.G.; He, H.; Guo, D.J.; Zhang, X.; Jia, L.; Hou, T.; Ma, A.M. Chitosan oligosaccharides-tripolyphosphate microcapsules as efficient vehicles for desalted duck egg white peptides-calcium: Fabrication, entrapment mechanism and in vivo calcium absorption studies. LWT-Food Sci. Technol. 2022, 154, 112869.
- Li, Z.L.; Chen, P.; Xu, X.Z.; Ye, X.; Wang, J. Preparation of chitosan-sodium alginate microcapsules containing ZnS nanoparticles and its effect on the drug release. Mater. Sci. Eng. C 2009, 29, 2250–2253.
- Salvatore, D.G.; Chasper, P.; Lipps, G. Stable and selective permeable hydrogel microcapsules for high-throughput cell cultivation and enzymatic analysis. Microb. Cell Factories 2020, 19, 170.
- Ansari, Z.; Goomer, S. Natural Gums and Carbohydrate-Based Polymers: Potential Encapsulants. Indo Glob. J. Pharm. Sci. 2022, 12, 1–20.
- Alicia, H.; Fabra, M.J.; Frédéric, D.; Cécile, D.B.; Andrée, V. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88.
- Joanna, T.; Ewelina, J.; Ewa, P.; Barbara, B.; Joanna, K.D. Furcellaran-Coated Microcapsules as Carriers of Cyprinus carpio Skin-Derived Antioxidant Hydrolysate: An In Vitro and In Vivo Study. Nutrients 2019, 11, 2502.
- Raú, l.E.C.; Pablo, R.S.; Adriana, N.M.; Silvina, R.D. Pyropia columbina phycocolloids as microencapsulating material improve bioaccessibility of brewers’ spent grain peptides with ACE-I inhibitory activity. Int. J. Food Sci. Technol. 2020, 55, 1311–1317.
- Nazia, T.; Suhani, D.K. Synthesis, characterization and applications of copolymer of β-cyclodextrin: A review. J. Polym. Res. 2020, 27, 1–30.
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin, C.N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375.
- Pawar, S.; Shende, P. A Comprehensive Patent Review on β-cyclodextrin Cross-linked Nanosponges for Multiple Applications. Recent Pat. Nanotechnol. 2020, 14, 75–89.
- Desai, D.; Shende, P. Monodispersed cyclodextrin-based nanocomplex of neuropeptide Y for targeting MCF-7 cells using a central composite design. J. Drug Deliv. Sci. Technol. 2021, 65, 102692.
- Chen, L.Y.; Gabriel, E.R.; Muriel, S. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283.
- Dave, J.; Ye, X.; Jethro, M.; Xiao, H. Protein-Based Drug-Delivery Materials. Materials 2017, 5, 517.
- Fan, Q.Q.; Ma, J.Z.; Xu, Q.; Zhang, J.; Demetra, S.; Gaidău, C.; Guo, C. Animal-derived natural products review: Focus on novel modifications and applications. Colloids Surf. B Biointerfaces 2015, 128, 181–190.
- Kantrol, K.S.; Monika, K.; Ravi, S.P. Chylomicron mimicking solid lipid nanoemulsions encapsulated enteric minicapsules targeted to colon for immunization against hepatitis B. Int. Immunopharmacol. 2019, 66, 317–329.
- Ahmady, A.; Hayati, A.S.N. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037.
- Favaro, C.S.; Santana, A.S.; Monterrey, E.S.; Trindade, M.A.; Netto, F.M. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2009, 24, 336–340.
- Niu, H.X.; Chang, J.; Jia, Y.D. Microencapsulation of crystalline-methionine enclosed with gelatine and sodium alginate by spray-drying. Mater. Res. Innov. 2015, 19, 257–262.
- Ashaolu, T.J. Applications of soy protein hydrolysates in the emerging functional foods: A review. Int. J. Food Sci. Technol. 2020, 55, 421–428.
- Gao, X.Q.; Xiong, G.Y.; Fu, L.; Liu, S.L. Water distribution of raw and heat-induced gelation of minced pork paste prepared by soy protein isolates and carrageenan: Ingredients modify the gelation of minced pork. J. Food Process. Preserv. 2019, 43, e14221.
- Wei, C.L. Construction of soybean peptide-curcumin nanoparticles and their microencapsulation. South China Univ. Technol. 2019, 24, 1–86.
- Zhao, C.H.; Chen, N.; Ashaolu, T.J. Whey proteins and peptides in health-promoting functions—A review. Int. Dairy J. 2022, 126, 105269.
- Farizano, J.V.; Díaz, V.L.I.; Masias, E.; Baillo, A.A.; Torino, M.I.; Fadda, S.; Vanden, B.N.L.; Montenegro, M.A.; Saavedra, L.; Minahk, C. Biotechnological use of dairy by-products for the production and microencapsulation of the food preservative enterocin CRL35. FEMS Microbiol. Lett. 2022, 369, fnac033.
- Zubair, M.; Pradhan, R.A.; Arshad, M.; Ullah, A. Recent Advances in Lipid Derived Bio-Based Materials for Food Packaging Applications. Macromol. Mater. Eng. 2021, 306, 1–35.
- Blanco-Pascual, N.; Koldeweij, R.B.J.; Stevens, R.S.A.; Montero, M.P.; Gómez-Guillén, M.C.; Cate, A.T. Peptide Microencapsulation by Core-Shell Printing Technology for Edible Film Application. Food Bioprocess Technol. 2014, 7, 2472–2483.
- Jiang, Z.L.; To, N. Recent advances in chemically modified cellulose and its derivatives for food packaging applications: A review. Polymers 2022, 14, 1533.
- Aomatsu, Y.; Nakajima, Y.; Ohyama, T.; Kin, T.; Kanehiro, H.; Hisanaga, M.; Ko, S.; Nagao, M.; Tatekawa, Y.; Sho, M.; et al. Efficacy of agarose/polystyrene sulfonic acid microencapsulation for islet xenotransplantation. Transplant. Proc. 2000, 32, 1071–1072.
- Nishimura, M.; Iizuka, N.; Fujita, Y.; Sawamoto, O.; Matsumoto, S. Effects of encapsulated porcine islets on glucose and C-peptide concentrations in diabetic nude mice 6 months after intraperitoneal transplantation. Xenotransplantation 2017, 24, e12313.
- Cesar, A.R.B.; Larissa, P.P.; Elisabete, A.L.G.; Nilce, M.S.; Priscilla, A.B.M.L.; Douglas, D.A.S.; Andreia, B.M.; Marlus, C.; Eduardo, F.V. HPMCP-coated microcapsules containing the ctx (Ile21)-ha antimicrobial peptide reduce the mortality rate caused by resistant salmonella enteritidis in laying hens. Antibiotics 2021, 10, 616.
- Jenny, K.R.; Luz, S.; Mary, A.A. Stabilization of oils by microencapsulation with heated protein-glucose syrup mixtures. J. Am. Oil Chem. Soc. 2006, 83, 965–972.
- Pavel, S.; Vladimir, M. Protein interaction with charged macromolecules: From model polymers to unfolded proteins and post- translational modifications. Int. J. Mol. Sci. 2019, 20, 1252.
- Swati, K.; Aasima, R.; Savita, S. Protein engineering and its applications in food industry. Taylor Fr. 2017, 57, 2321–2329.
- Wang, Z.G.; Ju, X.R.; He, R.; Yuan, J.; Wang, L.F. Effect of rapeseed protein structural modification on microstructural properties of peptide microcapsules. Food Bioprocess Technol. 2015, 8, 1305–1318.
- Deborah, M.S.; Joachim, K. A synthetic polymer matrix for the delayed or pulsatile release of water-soluble peptides. J. Control. Release 2002, 78, 143–153.
- Li, X.M.; Xu, Y.L.; Chen, G.G.; Wei, P.; Ping, Q.N. PLGA nanoparticles for the oral delivery of 5-Fluorouracil using high pressure homogenization-emulsification as the preparation method and in vitro/in vivo studies. Drug Dev. Ind. Pharm. 2008, 34, 107–115.
- Justin, K.Y.H.; Steven, P.S. Characterization of octreotide-PLGA binding by isothermal titration calorimetry. Biomacromolecules 2020, 21, 4087–4093.
- Lim, S.M.; Eom, H.N.; Jiang, H.H.; Sohn, M.J.; Lee, K.C. Evaluation of PEGylated exendin-4 released from poly (lactic-co-glycolic acid) microspheres for antidiabetic therapy. J. Pharm. Sci. 2015, 104, 72–80.
- Zhang, Y.; Wu, X.H.; Han, Y.R.; Mo, F.; Duan, Y.R.; Li, S.M. Novel thymopentin release systems prepared from bioresorbable PLA-PEG-PLA hydrogels. Int. J. Pharm. 2010, 386, 15–22.
- Burdock, G.A.; Flamm, W.G. A review of the studies of the safety of polydextrose in food. Food Chem. Toxicol. 1999, 37, 233–264.
- Marília, P.F.; Bruna, G.; Maria, E.C.S.; Izabela, D.A.; Maria, T.B.P. Microencapsulation performance of Fe-peptide complexes and stability monitoring. Food Res. Int. 2019, 125, 108505.
- Günay, K.A.; Berthier, D.L.; Jerri, H.A.; Benczédi, D.; Klok, H.-A.; Herrmann, A. Selective Peptide-Mediated Enhanced Deposition of Polymer Fragrance Delivery Systems on Human Hair. ACS Appl. Mater. Interfaces 2017, 9, 24238–24249.
- Shashi, K.B.; Ranjit, G.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Song, H.S.; Jeon, J.M.; Kim, J.S.; Lee, Y.K.; Yang, Y.H. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) production from engineered Ralstonia eutropha using synthetic and anaerobically digested food waste derived volatile fatty acids. Int. J. Biol. Macromol. 2019, 133, 1–10.
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2020, 212, 123161.
- Cao, L.D.; Liu, Y.J.; Xu, C.L.; Zhou, Z.L.; Zhao, P.Y.; Niu, S.J.; Huang, Q.L. Biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) microcapsules for controlled release of trifluralin with improved photostability and herbicidal activity. Mater. Sci. Eng. C 2019, 102, 134–141.
- Svetlana, U.; Cruz, M.J.; Cabeza, L.F.; Grágeda, M. Preparation and Characterization of Inorganic PCM Microcapsules by Fluidized Bed Method. Materials 2016, 9, 24.
- Jin, Y.; Zhou, Q.; Li, Z.H.; Yang, Z.H.; Fan, H.J. Calcium-cross linked polysaccharide microcapsules for controlled release and antimicrobial applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 125025.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
979
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




