Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Karolina A. Wojtunik-Kulesza | -- | 1851 | 2023-03-14 01:31:42 | | | |
| 2 | Jessie Wu | -4 word(s) | 1847 | 2023-03-14 04:22:10 | | | | |
| 3 | Jessie Wu | Meta information modification | 1847 | 2023-03-14 04:23:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wojtunik-Kulesza, K.; Rudkowska, M.; Orzeł-Sajdłowska, A. Aducanumab for Positive and Negative Sides of Therapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/42141 (accessed on 07 February 2026).
Wojtunik-Kulesza K, Rudkowska M, Orzeł-Sajdłowska A. Aducanumab for Positive and Negative Sides of Therapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/42141. Accessed February 07, 2026.
Wojtunik-Kulesza, Karolina, Monika Rudkowska, Anna Orzeł-Sajdłowska. "Aducanumab for Positive and Negative Sides of Therapy" Encyclopedia, https://encyclopedia.pub/entry/42141 (accessed February 07, 2026).
Wojtunik-Kulesza, K., Rudkowska, M., & Orzeł-Sajdłowska, A. (2023, March 14). Aducanumab for Positive and Negative Sides of Therapy. In Encyclopedia. https://encyclopedia.pub/entry/42141
Wojtunik-Kulesza, Karolina, et al. "Aducanumab for Positive and Negative Sides of Therapy." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Aducanumab (BIIB037, ADU), being a monoclonal antibody IgG1, is the newest AD treatment. The activity of the drug is targeted towards amyloid β, which is considered one of the main causes of Alzheimer’s disease. Clinical trials have revealed time- and dose-dependent activity towards Aβ reduction, as well as cognition improvement.
Alzheimer’s disease
aducanumab
plaques
1. Introduction
Alzheimer’s disease is an irreversible CNS disorder. Up to June 2021, medicine had in its arsenal only four drugs based on AChE inhibitors and an NMDA agonist. The situation changed when the FDA approved a new drug based on the amyloid hypothesis.
Aducanumab is a recombinant, human immunoglobulin G1 monoclonal antibody targeting soluble amyloid beta and insoluble fibrils [1]. The antibody was derived from a blood lymphocyte library from elderly people without cognitive impairment or with unusually slow cognitive decline. In 2018, Arndt et al. presented the structure of the aducanumab amyloid beta complex [2]. Further research provided a structural rationale for the low affinity of the molecule for non-pathogenic monomers (Figure 1). In later work, in silico studies allowed the analysis of the structure of the molecule, along with its interaction with the amyloid (residues 1–11). It is now known that aducanumab is able to bind Aβ residues 3–7 in an external conformation. Further work has led to the crystallization of Fab from aducanumab (AduFab). The most important amyloid residues interacting with AduFab are Phe4 and His6, along with Glu3, while the main-chain carbonyl of Arg5 makes additional contributions to the binding interaction.
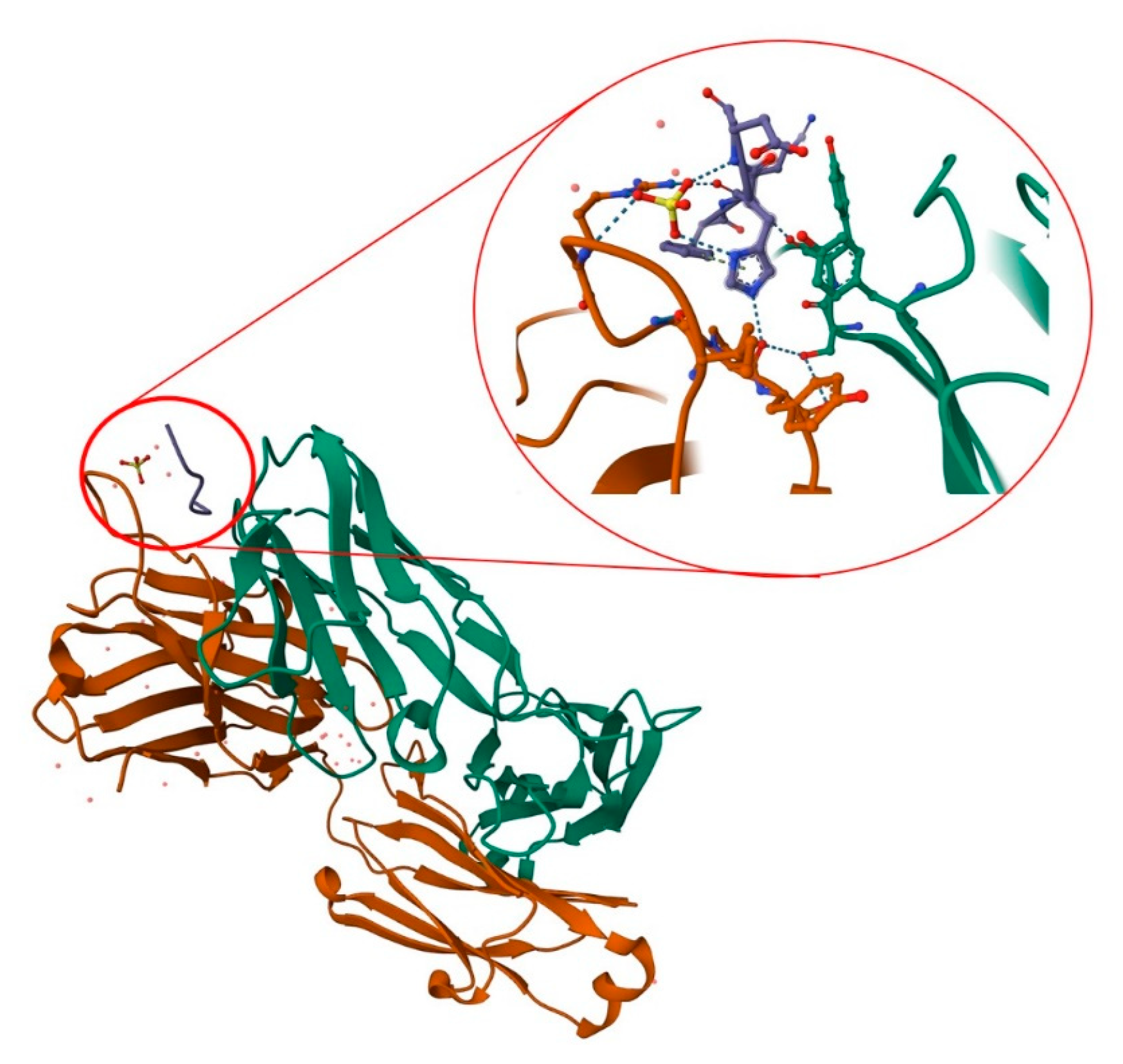
Figure 1. Structure of AduFab with bound Aβ (1–11) peptide. The figure shows Fab light chain in green, Fab heavy chain in brown, and amyloid β in blue, along with hydrogen interactions between AduFab and amyloid. Prepared with the use of Protein Data Bank.
A characteristic feature of aducanumab is its ability to bind oligomeric and fibrillar states of amyloid rather than monomers. The monoclonal antibody provides specific amino acid interactions, which allow for more shallow and compact binding in comparison to other monoclonal antibodies [3].
In vivo research is an obligatory stage of research on a new drug. Similar to other studied substances, the activity of aducanumab was analyzed with the use of mice models (22-month-old mice genetically modified to overexpress APP). The studies were performed in acute and chronic (6 months) models. Changes in the brain were observed with the use of fluorescent microscopy to tag Aβ plaques. Similarly, before and after treatment, the inositol triphosphate receptor, NMDA receptor, ryanodine receptor, and visinin-like-protein activity were observed. Detailed analysis of the obtained results revealed that acute treatment caused a greater decrease in amyloid plaques than the placebo. A reduction was observed to 48% of the total number of Aβ plaques, whereas the control group revealed only a 14% reduction. However, chronic treatment did not bring a significant reduction of amyloid plaques [3][4].
The specificity of aducanumab is the fact that it is the only drug based on Aβ. The amyloid hypothesis has many contradictions and therefore the drug is controversial. The drug’s history had its start in 2016 when Biogen reported data from the phase 1b PRIME trial, which revealed a reduction of the amyloid burden in the brain (10 mg/kg). Additionally, a positive influence on cognition was observed [5]. Aducanumab was subjected to subsequent trials (EMERGE and ENGAGE), and while ENGAGE did not reveal a positive effect in comparison with the placebo, EMERGE revealed amyloid reduction and was supported by an ad hoc analysis [1]. The study results revealed dose-dependent and time-dependent amyloid reduction. It is important to mention that EMERGE was based on a small group of patients who were in treatment for at least 14 months. Despite the controversy surrounding the conducted clinical trials, the FDA approved aducanumab (ADUHELM®, 100 mg/mL solution) for the treatment of AD [6].
2. Phase 1b: PRIME
It is commonly known that the approval of a new medicinal substance and drugs is preceded by many years of research based on in vitro, in silico, and in vivo tests, which end with clinical tests. Significant in vivo studies of aducanumab were presented by Sevigny et al. in 2016 [7]. To date (January 2023), the paper has been cited 1591 times, which underlines the importance of the presented study results. The researchers presented the interim results from a double-blind, placebo-controlled phase 1b trial. The aim of the studies was to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of aducanumab. The PRIME phase was based on 165 patients with diagnosed prodromal or mild Alzheimer’s disease and confirmed by positive emission tomography (PET) scans of amyloid beta in the brain. The outcome of the study explicitly indicated a positive impact of aducanumab on Aβ reduction in a dose- and time-dependent fashion. The trial, which lasted 54 weeks, brought about a significant decrease in the PET standard uptake value ratio (SUVR) in the 3, 6, and 10 mg/kg dose groups, in comparison to the baseline, whereas the placebo group was not significant. Equally interesting and important is that the trial established that aducanumab can penetrate the brain to a sufficient extent to allow the accumulation of Aβ plaques. What is more, aducanumab was found to be able to clear plaques of all sizes, which suggests that the substance is able to prevent the formation of new plaques [7].
Nevertheless, it is worth mentioning the limitation of the studies. The PRIME phase 1b was based on a small sample size, was conducted in the USA only, had a staggered parallel-group design, and indicated possible partial unblinding due to ARIA-E (vasogenic edema). Moreover, ARIA-E was observed in 1 (3), 2 (6%), 11 (37%), and 13 (41%) participants who were treated with 1, 3, 6, and 10 mg/kg, respectively. The trial was also continued with more than half (56%) of all participants displaying the aforementioned side effect. However, referring to these limitations, the researchers underlined the results of their post hoc analysis, which indicated no apparent differences in treatment effect when comparing patients with and without ARIA-E. Other side effects of the therapy were headaches, urinary tract infections, and upper respiratory tract infections [7].
3. Phase 3: ENGAGE and EMERGE
Phase 3 studies were conducted with 1600 amyloid-positive participants with early AD in each trial. The trials involved adults and older adults (50 years to 85 years) who met a number of criteria. Among these were the following: Objective evidence of cognitive impairment at screening, a Mini-Mental State Examination (MMSE) score between 24 and 30, a positive amyloid PET scan, and a Clinical Dementia Rating (CDR)–Global score of 0.5, as well as having a reliable informant or caregiver. In the case of patients who were treated with AD drugs, doses had to be stable for at least 8 weeks prior to their first screening visit [8]. The most important exclusion criteria were the following: Clinically significant unstable psychiatric illness in the past 6 months, impaired renal or liver function, taking blood thinners, except aspirin, at a prophylactic dose or less, brain hemorrhage, bleeding disorder, and cerebrovascular abnormalities, any condition other than AD conditions that can influence cognitive impairment, and having a stroke or Transient Ischemic Attack or unexplained loss of consciousness in the past 1 year. Dosage differed from the recommendation for the trials, namely, EMERGE participants were treated with higher doses for longer periods.
The study results revealed no drug–placebo difference for primary and secondary clinical outcomes in the final dataset of the ENGAGE trial. Moreover, differences were observed between the ENGAGE and EMERGE trials; notably, ENGAGE did not have positive results. The trial did not reveal a benefit in comparison to the placebo. In contrast, the EMERGE trial revealed a 22% decreased rate of cognition impairment in the group of patients treated with high-dose of aducanumab (10 mg/kg) [3]. The FDA then performed post hoc analysis, which revealed a decrease in the amyloid burden: Low-dose aducanumab = 0.179 reduction in mean SUVR; high-dose aducanumab: 0.278 reduction in mean SUVR [n = 109], placebo = no change [n = 93] [9]. In both trials, adverse events were observed. Herein, amyloid-related imaging abnormalities occurred in 34% of the test population in the EMERGE group and 35.5% in the ENGAGE group. Both ENGAGE and EMERGE trials also revealed ARIAs, which occurred within eight doses (7 months of initiation). It should be underlined that almost all ARIA-E cases were resolved within 3 months (69%) and 4 months (83%). Patients who suffered from ARIAs also revealed other symptoms such as headache (47%), confusion (15%), dizziness (11%), and nausea (8%). All patients were treated with the highest dose of 10 mg/kg [10].
Clinical trials of aducanumab in patients with Alzheimer’s diseases listed on Clinicaltrials.gov (accessed on 16 January 2023) are presented in Table 1.
Table 1. Data obtained during aducanumab clinical trials.
| Study Name/Identification | Number Enrolled | Key Inclusion Criteria | Level of Evidence Statement |
|---|---|---|---|
| Single ascending dose study of BIIB037 in participants with AD | 53 | Clinically confirmed AD, age: 55–85 years old, others: Good health, reliable informant or caregiver | Single dose of aducanumab (up to 30 mg/kg) was safe and tolerable |
| PRIME (Multiple dose study of aducanumab) | 197 | Prodromal or mild AD, Age: 50–90 years old; others: Good health, reliable informant or caregiver |
Decreasing amyloid value studied with the use of PET SUVR at 1 year vs. placebo (dosage: 3–10 mg/kg) |
| ENGAGE (Phase 3 Study) | 1647 | MCI due to AD or mild AD; Age: 50–85 years old; MMSE 24–30; others: Positive amyloid PET scan, stable doses of drugs treating AD symptoms, reliable informant or caregiver |
Aducanumab (3–10 mg/kg) did not significantly affect mean change in CDR-SB scores vs. placebo over 78 weeks whereas the same doses caused decrease in amyloid PET SUVR at 78 weeks vs. placebo |
| EMERGE (Phase 3 study) | 1638 | MCI due to AD or mild AD; Age: 50–85 years old; MMSE 24–30; others: Positive amyloid PET scan, stable doses of drugs treating AD symptoms, reliable informant or caregiver |
Aducanumab at a dose of 10 mg/kg results in less worsening of the CDR-SB vs. placebo at 78 weeks; degree less than a clinically relevant change; doses of 3–10 mg/kg caused decrease in amyloid PET SUVR at 78 weeks vs. placebo |
| EVOLVE | 52 | MCI due to AD or mild AD; Age: 50–85 years old; MMSE 24–30 others: Positive amyloid PET scan |
NA |
| PROPEL (Single and multiple ascending dose study in Japanese participants with AD) | 21 | Clinical diagnosis of mild-moderate AD; age: 55–85 years old; others: Good health, reliable informant or caregiver | NA |
An interesting fact is that aducanumab can impact calcium homeostasis, of which dysregulation is one of the possible pro-AD factors. Based on in vivo studies performed on 2756 transgenic mice, aducanumab caused restoration of calcium homeostasis. Treatment of cognitive impairment resulting from the mitigation of overload of calcium was observed [4][11][12]. Aducanumab administration in 22-month-old mice did not clear existing plaques whereas calcium overload was ameliorated over time. Analysis of the obtained results suggests that expression of the intracellular store channel was reduced in Tg2576 mice treated with the control antibody and restored with aducanumab immunotherapy, which suggests that intracellular calcium stores may contribute to calcium dyshomeostasis [4].
It is known that the effective action of drugs is possible after reaching the appropriate concentration in the treated organ, which, in the case of the brain, is very difficult due to the blood–brain barrier. Study results revealed that the maximal effectiveness of aducanumab was observed around the fifth month of the therapy, which results from the establishment of the appropriate concentration of the substance that will be able to induce the destruction of amyloid aggregates. Pharmacodynamic analysis revealed that aducanumab binds fibrils and targets them for microglial-mediated removal, interrupting the bridge between neuroprotective amyloid monomers and neurotoxic oligomers [3][13].
References
- Coerver, K.; Yu, M.M.; D’Abreu, A.; Wasserman, M.; Nair, K.V. Practical Considerations in the Administration of Aducanumab for the Neurologist. Neurol. Clin. Pract. 2022, 12, 169–175.
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S.; et al. Structural and Kinetic Basis for the Selectivity of Aducanumab for Aggregated Forms of Amyloid-β. Sci. Rep. 2018, 8, 6412.
- Haddad, H.W.; Malone, G.W.; Comardelle, N.J.; Degueure, A.E.; Kaye, A.M.; Kaye, A.D. Aducanumab, a Novel Anti-Amyloid Monoclonal Antibody, for the Treatment of Alzheimer’s Disease: A Comprehensive Review. Health Psychol. Res. 2022, 10.
- Kastanenka, K.V.; Bussiere, T.; Shakerdge, N.; Qian, F.; Weinreb, P.H.; Rhodes, K.; Bacskai, B.J. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J. Neurosci. 2016, 36, 12549–12558.
- Musiek, E.S.; Bennett, D.A. Aducanumab and the “Post-Amyloid” Era of Alzheimer Research? Neuron 2021, 109, 3045–3047.
- Terao, I.; Honyashiki, M.; Inoue, T. Comparative Efficacy of Lithium and Aducanumab for Cognitive Decline in Patients with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Ageing Res. Rev. 2022, 81, 101709.
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56, reprinted in Nature 2017, 546, 564.
- Biogen, A. Phase 3 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy and Safety of Aducanumab (BIIB037) in Subjects with Early Alzheimer’s Disease; 2021. Available online: clinicaltrials.gov (accessed on 15 January 2023).
- Day, G.S.; Scarmeas, N.; Dubinsky, R.; Coerver, K.; Mostacero, A.; West, B.; Wessels, S.R.; Armstrong, M.J. Aducanumab Use in Symptomatic Alzheimer Disease Evidence in Focus: A Report of the AAN Guidelines Subcommittee. Neurology 2022, 98, 619–631.
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21.
- DeMattos, R.B.; Lu, J.; Tang, Y.; Racke, M.M.; DeLong, C.A.; Tzaferis, J.A.; Hole, J.T.; Forster, B.M.; McDonnell, P.C.; Liu, F.; et al. A Plaque-Specific Antibody Clears Existing β-Amyloid Plaques in Alzheimer’s Disease Mice. Neuron 2012, 76, 908–920.
- Behl, T.; Kaur, I.; Sehgal, A.; Singh, S.; Sharma, N.; Makeen, H.A.; Albratty, M.; Alhazmi, H.A.; Felemban, S.G.; Alsubayiel, A.M.; et al. “Aducanumab” Making a Comeback in Alzheimer’s Disease: An Old Wine in a New Bottle. Biomed. Pharm. 2022, 148, 112746.
- Linse, S.; Scheidt, T.; Bernfur, K.; Vendruscolo, M.; Dobson, C.M.; Cohen, S.I.A.; Sileikis, E.; Lundqvist, M.; Qian, F.; O’Malley, T.; et al. Kinetic Fingerprints Differentiate the Mechanisms of Action of Anti-Aβ Antibodies. Nat. Struct. Mol. Biol. 2020, 27, 1125–1133.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
932
Revisions:
3 times
(View History)
Update Date:
14 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




