Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muthaiah Shellaiah | -- | 10776 | 2023-03-10 03:12:15 | | | |
| 2 | Muthaiah Shellaiah | -1924 word(s) | 8852 | 2023-03-10 07:12:13 | | | | |
| 3 | Muthaiah Shellaiah | + 42 word(s) | 8894 | 2023-03-10 07:16:44 | | | | |
| 4 | Rita Xu | -4891 word(s) | 4003 | 2023-03-10 11:14:01 | | | | |
| 5 | Rita Xu | Meta information modification | 4003 | 2023-03-10 11:23:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shellaiah, M.; Sun, K.W. Carbon Dot-Based Fluorescent Detection of Biothiols. Encyclopedia. Available online: https://encyclopedia.pub/entry/42060 (accessed on 03 March 2026).
Shellaiah M, Sun KW. Carbon Dot-Based Fluorescent Detection of Biothiols. Encyclopedia. Available at: https://encyclopedia.pub/entry/42060. Accessed March 03, 2026.
Shellaiah, Muthaiah, Kien Wen Sun. "Carbon Dot-Based Fluorescent Detection of Biothiols" Encyclopedia, https://encyclopedia.pub/entry/42060 (accessed March 03, 2026).
Shellaiah, M., & Sun, K.W. (2023, March 10). Carbon Dot-Based Fluorescent Detection of Biothiols. In Encyclopedia. https://encyclopedia.pub/entry/42060
Shellaiah, Muthaiah and Kien Wen Sun. "Carbon Dot-Based Fluorescent Detection of Biothiols." Encyclopedia. Web. 10 March, 2023.
Copy Citation
Biothiols, such as cysteine (Cys), homocysteine (Hcy), and glutathione (GSH), play a vital role in gene expression, maintaining redox homeostasis, reducing damages caused by free radicals/toxins, etc. Likewise, abnormal levels of biothiols can lead to severe diseases, such as Alzheimer’s disease (AD), neurotoxicity, hair depigmentation, liver/skin damage, etc. To quantify the biothiols in a biological system, numerous low-toxic probes, such as fluorescent quantum dots, emissive organic probes, composited nanomaterials, etc., have been reported with real-time applications.
carbon-dots
biothiols detection
fluorescence
complex-mediated sensors
1. Introduction
Detection and quantification of biologically important species are becoming important for treating infections and diseases existing in living systems [1][2][3]. Therefore, bioimaging of these affected tissues or cells was proposed by using fluorescent organic nanoparticles, inorganic nanostructures, hybrid nanosystems, and composites with authenticated evidence [4][5][6][7][8][9][10][11][12][13]. Among these biologically important species, non-protein biothiols, such as cysteine (Cys; normal blood plasma concentration is between 135 to 300 µM), homocysteine (Hcy; normal blood plasma concentration is between 5 to 15 µM), and glutathione (GSH normal blood plasma concentration is between 1 to 6 µM), play a vital role in many pathological process, clinical disorders, and diseases [14][15][16]. Cysteine plays an important role in protein/peptide synthesis, detoxification, cell metabolism, etc., and lack of cysteine may lead to hair depigmentation, liver damage, skin diseases, and cancer [17][18][19]. On the other hand, elevated cysteine levels can cause neurotoxic disorders [20][21]. Subsequently, homocysteine plays a role quite similar to cysteine. However, elevated concentrations of homocysteine in the blood plasma may lead to hyperhomocysteinemia, which is typically categorized into moderate (concentration = 15–30 µM of Hcy), intermediate (concentration = 30–100 µM of Hcy), and severe (concentration ≥ 100 µM of Hcy) disorders [22][23]. In fact, hyperhomocysteinemia can enhance other disorders, such as osteoporosis, dementia, Alzheimer’s disease, cardiac disorders, etc. [24]. Similarly, deficiency in glutathione decreases immunity and enhances the aging process [25]. Elevated levels of glutathione in the human body may enhance the resistance of cancerous cells to chemotherapy [26]. Individual biothiols play important roles in living systems. For example, they can coordinate with biomarkers to afford cancerous cell bioimaging and predict the therapeutic utilities of numerous drug delivery manuals [27][28]. Thereby, detection and quantification of biothiols is a highly important research topic in this field.
To detect and quantify the biothiols, numerous tactics have been proposed, including colorimetric assay, electrochemical methods, fluorescent imaging, surface enhanced Raman spectroscopy, etc. [29][30][31][32]. Among them, fluorescent imaging is rather impressive in terms of the real-time monitoring of biothiols in living tissues or cells [33][34]. Fluorescent sensing of biothiols can be achieved by using organic probes (undergo a reaction with biothiols), functionalized fluorescent quantum dots, hybrid composite nanomaterials, metal-organic frameworks (MOFs), etc. [35][36][37][38][39][40]. Recently, a smartphone-based surface plasmon-coupled emission (SPCE) platform and photonic crystal-coupled emission (PCCE) technology were also employed in biothiol quantification as well as in biosensing studies [41][42][43][44][45][46]. Among these materials, functionalized fluorescent quantum dots have attracted much attention due to their size, photostability, and unique optical properties (Stokes shifts, wide absorption and optimizable PL, etc.) with respect to surface stabilization [47][48]. The easily synthesizable carbon dots (CDs) with a size of <20 nm, which also belong to the quantum dots category, display exceptional opto-electronic properties and have been applied in energetic applications, sensing, bioimaging, therapy, etc. [49][50][51][52][53]. Numerous reports have discussed the detection ability of CDs towards biothiols in cellular imaging and real samples [54][55][56]. In fact, CDs-based detection of biothiols can be achieved by photoinduced electron transfer (PET), intramolecular charge transfer (ICT), Förster resonance energy transfer (FRET), internal filter effect (IFE), aggregation-caused quenching (ACQ), and aggregation-induced emission (AIE), as demonstrated in published works [48][50]. Similarly, fluorescent CDs-based sensing of biothiols can be performed by observing the “Turn-On” and “Turn-Off” florescent responses via the metal ion–CD pair or CDs-based nanocomposites when exposed to biothiols.
Recently, Khan et al. (2020) delivered a comprehensive review covering reports on both CDs and graphene dots (GQDs)-based biothiols sensing [55]. However, to date, the availability of a review focused on fluorescent CDs-based biothiols detection with information on recent trends, mechanistic aspects, linear ranges, LODs, and real applications is lacking, which allows researchers to deliver this comprehensive review. In this review, the use of emissive CDs in the assay of biothiols (Cys, Hcy, and GSH) is discussed with information on synthesis, photoluminescence quantum yield (PLQY), and demonstrative applications. Moreover, probe/CDs selections, sensory requirements, merits, limitations, and future opportunities for a fluorescent CDs-based biothiols assay are suggested for readers. Figure 1 illustrates schematics of applications and structures of fluorescent CDs-based assay of Cys, Hcy, and GSH.
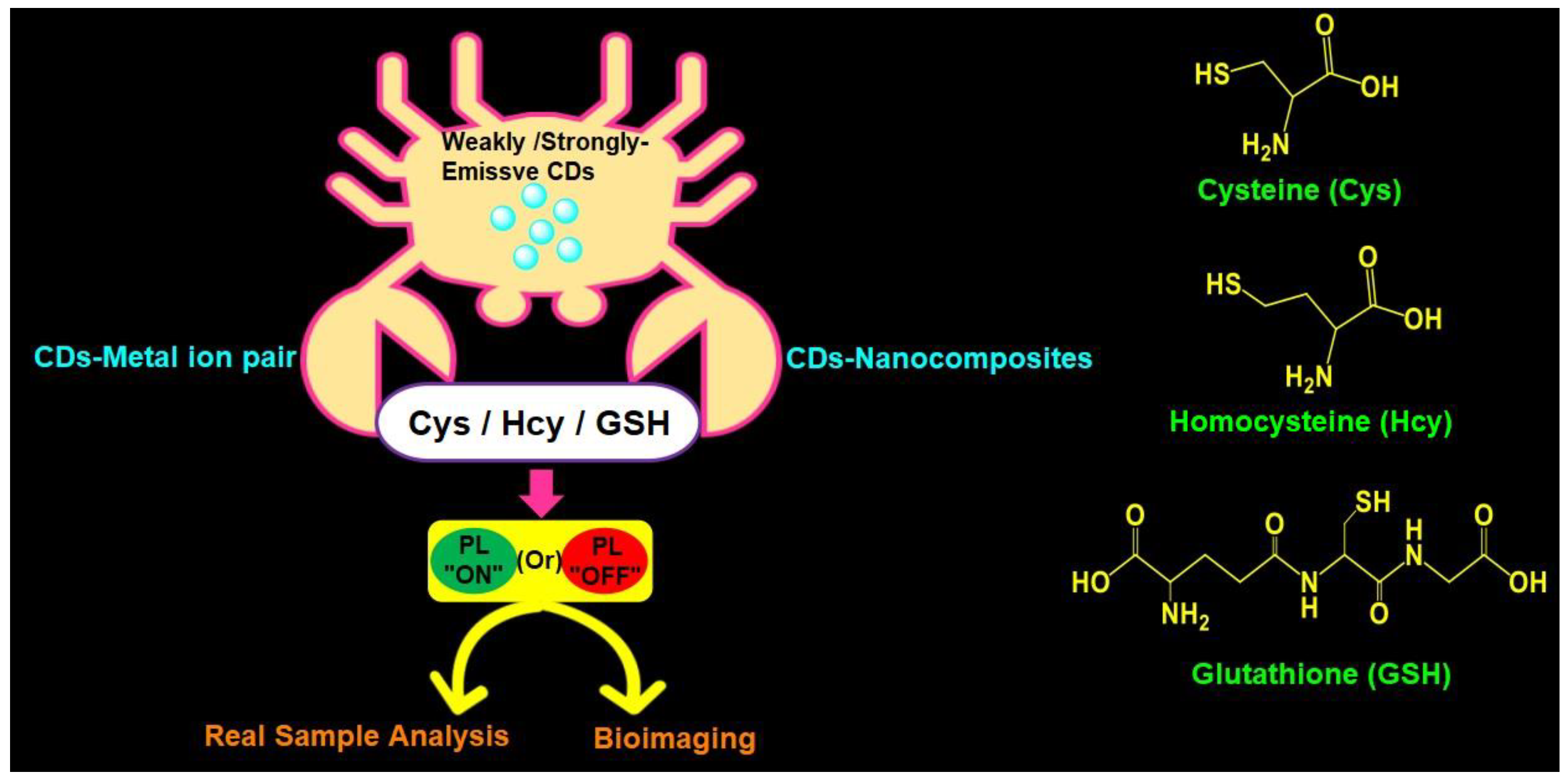
Figure 1. Schematic of fluorescent CDs-based assay of Cys, Hcy, and GSH with applications and structures of Cys, Hcy, and GSH.
2. Tactics Involved in CDs Synthesis
Before discussing the CDs-based sensory reports for biothiols, this section briefly describes tactics involved in CDs synthesis. Highly emissive CDs can be synthesized by both (A) top-down approaches and (B) bottom-up approaches. Top-down approaches are categorized into (i) arc discharge, (ii) laser ablation, (iii) chemical oxidation, (iv) electrochemical method, and (v) ultrasonic synthesis. Likewise, the bottom-up approaches can be categorized into (i) microwave synthesis, (ii) hydrothermal method, (iii) solvothermal method, (iv) thermal decomposition, and (v) carbonization/pyrolysis method [57][58].
2.1. Top-Down Approaches
2.1.1. Arc Discharge
With this method, CDs were synthesized by applying a direct-current arc voltage across two graphite electrodes immersed in an inert gas atmosphere. Chao-Mujica et al. reported synthesis of the fluorescent CQDs using this tactic in water [59]. After purification, these CQDs were consumed in cellular imaging studies; therefore, it was noted as a unique top-down approach.
2.1.2. Laser Ablation
With this method, fluorescent CDs were produced by ablating nanosecond pulse laser over a solid carbon target. The as-synthesized CDs were engaged in cellular imaging studies [60]. Doñate-Buendia et al. synthesized the CQDs with a size of 3 nm via laser irradiation in a continuous flow jet and applied them to cellular imaging studies for prolonged periods of time [61]. In fact, this tactic can produce low toxic CDs for numerous bioimaging/biosensing applications [60][61].
2.1.3. Chemical Oxidation
Chemical oxidation, or exfoliation of a disintegrating bulk carbon source, can be achieved by using a strong oxidizing agent, such as H2SO4, HNO3, NaClO3, etc., to produce fluorescent CDs [62]. Desai et al. synthesized fluorescent CDs from muskmelon fruit using sulfuric acid and phosphoric acid as the oxidizing agents [63]. The prepared CDs in the above report were engaged in Hg2+ detection and cellular imaging studies, which have motivated researchers to engage in this synthetic tactic.
2.1.4. Electrochemical Method
In the electrochemical method, the oxidation/carbonization takes place by applying an electric field in a chemical environment to produce the fluorescent CDs [64]. This is a rather straight forward method and has been adopted widely in the production of CDs. Lee et al. synthesized fluorescent CDs by the electrochemical method and employed them in the turn-on recognition of chlortetracycline [65], thereby confirming the effectiveness of this tactic.
2.1.5. Ultrasonic Synthesize
In an ultrasonic process, formation and collapsing of small bubbles in liquid produces a strong hydrodynamic shear force to cut the macroscopic carbon materials into nanoscale CDs [66]. Moreover, CDs with diverse properties can be attained by adjusting the ultrasonic power, reaction time, ratio of carbon sources, solvents, etc. Xu et al. developed the multicolour N-doped CDs from kiwi-fruit juice by the ultrasonic synthesis approach and applied these CDs in investigations of fluorescent inks, sensors, and logic gate operations [67].
2.2. Bottom-Up Approaches
2.2.1. Microwave Synthesis
By irradiating the electromagnetic wave over the sample at a high temperature, CDs can be produced with exceptional PL quantum yield. In fact, electric dipoles in materials are aligned via microwave-assisted excitation. By optimizing precursor and solvent interactions in the microwave synthesis, CDs with hydrophilic, hydrophobic, or amphiphilic properties can be produced for multiple applications [68]. For instance, Liu et al. demonstrated microwave-assisted synthesis of emissive CDs from citric acid, L-cysteine, and dextrin, and employed them in the real-time detection of Cu2+ [69].
2.2.2. Hydrothermal Method
In this method, the reaction mixture in water is placed in a Teflon container and kept in an oven to react hydrothermally at a high pressure and high temperature to produce fluorescent CDs for distinguished applications [57]. For example, Lee et al. synthesized the fluorescent CDs via the hydrothermal method from citric acid, ethylenediamine, and methyl blue and applied them in the “Turn-Off” detection of Hg2+ and ClO− [70].
2.2.3. Solvothermal Method
In contrast to the hydrothermal method, the solvothermal tactic replaces the water with one or more organic solvents. The mixtures are sealed with Teflon and subjected to a steel autoclave under a high temperature and high pressure [71]. This method produces highly fluorescent CDs cost-effectively for various applications. Omer et al. discussed the use of solvothermally prepared phosphorous and nitrogen-doped CDs towards Fe3+ detection [72], and attested the affordability of the tactic.
2.2.4. Thermal Decomposition
Thermal decomposition (via chemical decomposition) by heating the material or compound was engaged in the production of CDs [73]. This tactic is classified as an endothermic process; however, it was rarely used for CDs synthesis due to its complexity. CDs produced from thermal decomposition were also employed in optoelectronic studies. Wan et al. employed the thermal decomposition tactic to synthesize CDs and graphene-like carbon nanosheets and applied them in optoelectronic device fabrication [74].
2.2.5. Carbonization/Pyrolysis
This is a the most cost-effective, facile, and ultrafast method to synthesize CDs. When organic materials are subjected to prolonged pyrolysis in an inert atmosphere, solid residues with a high carbon content or CDs can be produced with a high yield [75]. Esfandiari et al. synthesized fluorescent CDs by pyrolyzing citric acid in different time periods and temperature ranges. The as-synthesized fluorescent CDs employed in cellular imaging studies showed low toxicity, thereby validating the pyrolysis mediated fluorescent CDs synthesis and suggesting feasible drug delivery applications in the future [76].
3. Fluorescence Mechanism, Importance of PLQY, and Desired Size of CDs
3.1. Fluorescence Mechanism of CDs
Synthesized CDs may possess strong, moderate, and weak emission due to the quantum confinement effect, conjugate effect, surface passivation/functionalization effect, surface state, and molecular/carbon-core state properties [77]. Further, the fluorescence of CDs can be tuned via surface passivation, functionalization, doping, and compositing with nanomaterials [78]. In fact, emission of CDs produced by top-down approaches are mostly dependent on surface passivation. On the other hand, bottom-up approaches can produce emissive CDs even without surface passivation [77].
3.2. Importance of PLQY of CDs
The PLQY of CDs defines its capability to convert every absorbed photon into fluorescence emission. The PLQY of CDs serves as a correction factor for the determination of multiparameter fluorescence spectroscopy (MFS) parameters, such as FRET, PL quenching efficacy, incorporation of diverse doping/compositing fluorophores and nanomaterials, complex stoichiometry, and decay profiles, etc. [79]. Further, the use of CDs with a higher PLQY affords the high feasibility of long-term bioimaging and tracking of CDs-based drug delivery systems [80]. To achieve CDs with a high PLQY, surface passivation, functionalization, and doping/compositing with nanomaterials can be used, as stated earlier [77].
3.3. Desired Size of CDs for Biothiols Quantification
Both Top-down and Bottom-up approaches can produce CDs with a size ranging 1–30 nm [81]. The biothiols assay in real water samples can be performed with emissive CDs with a size ranging 1–30 nm [55]. On the other hand, for the detection and quantification of biothiols in intracellular studies, the size of emissive biocompatible CDs must range 1–10 nm [82]. However, in both cases, the lower the size, the greater the emissive properties of CDs to deliver effective analytical results.
4. Representative Mechanism of CDs-Based Fluorescent Biothiols Assay
The CDs-based fluorescent biothiols assay was illustrated by (1) the CD–metal ion pair system [83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120] and (2) CD-nanocomposites [121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143]. Both proposed systems/models deliver the fluorescent response by means of fluorescence recovery or the quenching principle.
4.1. Representative PL Mechanism of CD–Metal Ion Pair in the Biothiols Assay
In general, the interaction of emissive CDs with metal ions (Hg2+, Ag+, Cu2+, Fe3+, and Au3+) led to fluorescent quenching, which recovers due to the effective interaction of biothiols with metal ions, as shown in Figure 2A [87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][116][117][118][119][120]. In fact, the metal ions present in the CD–metal ion pair strongly bind with biothiols via an Mn+–S interaction to release emissive CDs and exhibit fluorescence recovery. In contrast, CD–metal ion pair with a certain emission may led to fluorescence quenching upon interaction with biothiols, as seen in Figure 2B; however, it has been reported very rarely [115].
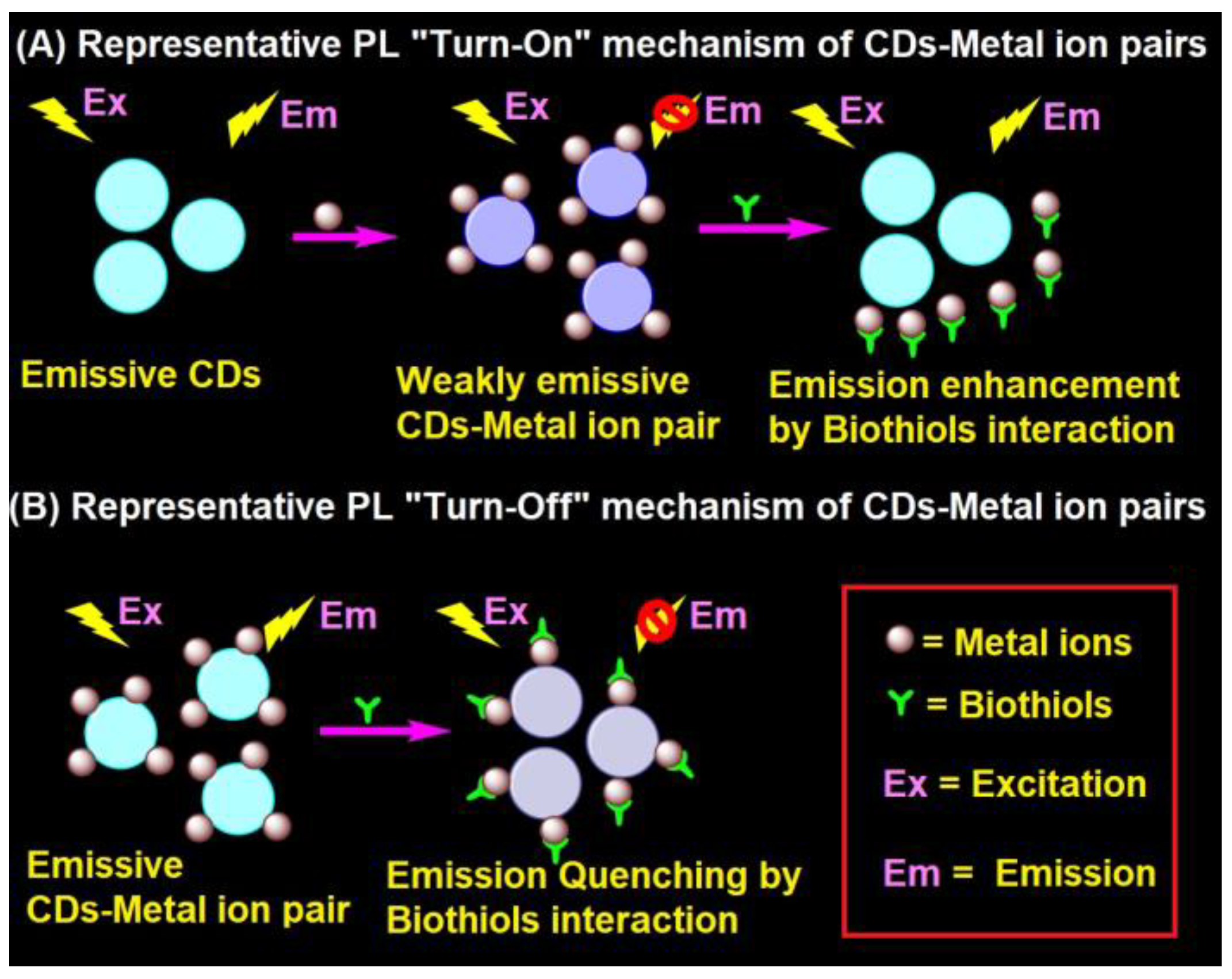
Figure 2. Representative (A) PL “Turn-On” and (B) PL “Turn-Off” mechanism of the CD–metal ion pair in the biothiols assay.
4.2. Representative PL Mechanism of CD–Nanocomposites in the Biothiols Assay
CD–nanocomposites can be formed by compositing diverse inorganic nanomaterials, conjugation of organic moiety, and doping of metal ions etc., with emissive CDs to afford weakly emissive composites via FRET. These weakly emissive CD–nanocomposites interacts with biothiols to afford two kinds of reaction-based mechanisms, as shown in Figure 3A,B. Biothiols may react with compositing moiety to release emissive CDs (Figure 3A) or interact over the surface of CD–nanocomposites to recovers the fluorescence (Figure 3B). In general, CD–nanocomposites produced by compositing inorganic nanomaterials (such as Au NPs, Ag NPs, and MnO2, etc.) and few organic molecule functionalized composite models [121][122][123][124][125][129][130][131][132][133][134][135][136][137] follows the reaction-based mechanism-2 (Figure 3B). On the other hand, few organic moiety functionalized CD–nanocomposite models [138][139][140] follows the reaction-based mechanism-1 (Figure 3A). Subsequently, CD–DTNB (DTNB = 5,5′-dithiobis-(2-nitrobenzoic acid)) dispersed composite model [141][142] has an initial emission due to IFE, which gets disturbed when it interacts with biothiols, resulting in fluorescence quenching, as visualized in Figure 3C. In fact, biothiols react with DTNB and break it into 2-nitro-5-thiobenzoic acid (TNB), which functionalizes over CDs to afford fluorescent quenching (Figure 3C). A rare report on CD–nanocomposites to afford both PL recovery and quenching for discrimination between biothiols via IFE is available [131]. Therefore, the metal ion doped CD–nanocomposite model for biothiols interactive reaction-based fluorescence quenching was also proposed in Figure 3D. However, to date, only cobalt-doped CDs [143] follows the proposed mechanism.
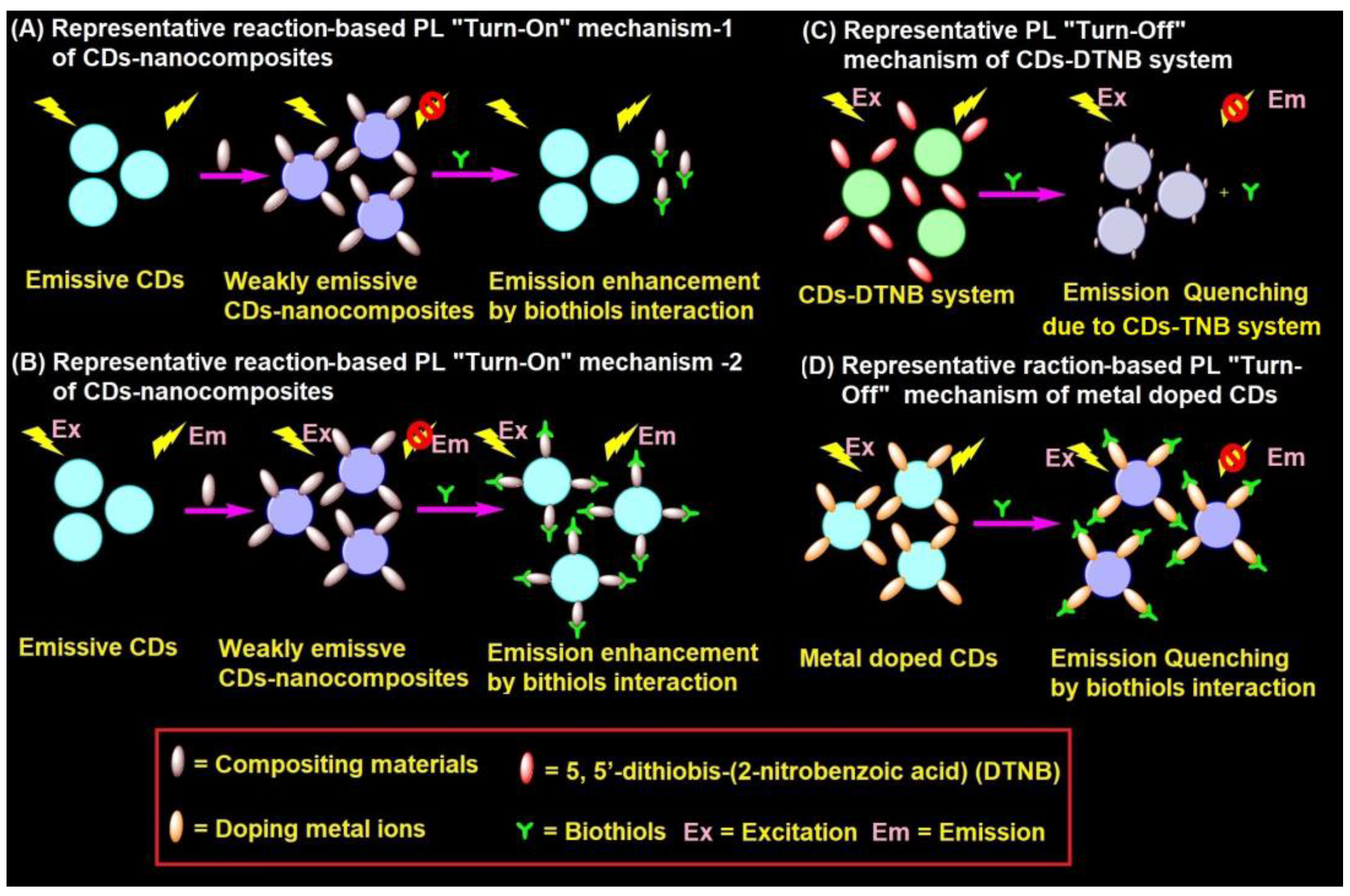
Figure 3. Representative (A,B) reaction-based PL “Turn-On” mechanism-1 and mechanism-2 of CD–nanocomposites, (C) PL “Turn-Off” mechanism of CD–DTNB system, and (D) reaction-based PL “Turn-Off” mechanism of metal doped CDs in the biothiols assay.
5. Probe/CDs Selection and Sensory Requirements
The development of fluorescent CDs-based probes towards selective detection and quantification of Cys, Hcy, and GSH must fulfil certain requirements, as illustrated below.
-
The uniqueness of a CDs-based fluorescent assay of biothiols depends on the size and PLQY. Therefore, to obtain CDs with a proper size and PLQY, it is essential to identify the precursor reactants and suitable synthetic tactics.
-
To attain high biothiols selectivity in CD–metal complex-mediated detection, the CDs must possess specific selectivity to metal ions with thiophilicity nature (such as Hg2+, Ag+, Cu2+, Fe3+, Au3+, etc.). Therefore, to be able to interact with those metal ions, CDs must possess functional units, such as -NH2 and -COOH, or be doped with N, S, P, etc. However, in the case of doping, the concentration must be carefully tuned to achieve the expected results.
-
Dye-incorporated CDs towards consecutive ratiometric discrimination of metal ions and biothiols depends on the precise concentration of dye molecules. Thus, it is essential to optimize the dye concentration before designing such innovative probes.
-
To attain greater sensory responses to biothiols using the CDs incorporated in composites, it is necessary to choose compositing material involved in the detection process/mechanism with thiophilicity.
-
For dual readout fluorescence and colorimetric detection of biothiols, the CDs must be composited with the colorimetric probe, such as Au NPs. The composition ratio must be fixed to achieve significant results.
-
Reaction-based sensory responses of CDs to biothiols depend on the reacting units functionalized over the carbon dot surface. Thus, it is necessary to identify molecules to be functionalized over the CD surface at required concentrations that are highly reactive to specific biothiols.
-
It is essential to categorize the exact mechanisms of the selective sensing of biothiols with CDs-based probes. The coordinative bindings and mechanistic approaches, such as PET, FRET, IFE, and NSET, must be clarified for the emerging new designs.
-
To commercialize the CDs-based biothiols assay, the exact pH conditions with given details on buffer solutions and concentrations, incubation time, operative temperature, and interference effect must be clarified for researchers.
6. Advantages
CDs-based fluorescent probes and their utilization in biothiols assays have the following advantages, as stated below.
-
CDs hold the promise as potentially safe vehicles for biological sample-based biothiols assays due to low in toxicity and high biocompatibility. Moreover, toxicity of CDs can be further reduced by compositing with low toxic nanomaterials, such as Ag NPs, Au NPs, and nanoclusters, to engage in bioimaging and therapeutic applications.
-
CDs-nanocomposites are comprised of highly selective reactive species (in the presence of biothiols), which can avoid the interference effect. Likewise, CDs are also able to discriminate Cys, Hcy, and GSH via tuning of the pH environment.
-
Construction of the red-green-blue (RGB) emitting CDs-nanocomposites is possible by mixing CDs with red to blue emissive nanomaterials, which can be utilized for biothiols assay over a broad PL range.
-
CDs-based fluorometric discrimination of biothiols can be effectively applied in real samples, such as human serum, FBS, plasma, urine, etc. This can be noted as a great advantage towards the development of a unique analytical method.
7. Limitations
CDs-based fluorometric biothiols detection and quantification also have the following limitations, as mentioned below.
-
In CD–metal ion pair-based biothiols assays, the formation of metal complexes, such as CD–Hg2+, CD–Ag2+, and CD–Cu2+, may increase toxicity. Hence, the use of such complex-mediated biothiols assays may harm the biological environment or cell lines, which should be carefully examined.
-
In general, CDs-based specific sensory responses to biothiols are limited by the functional units or doped elements. Thus, careful optimization is mandatory to ensure the existing functional units or doped elements are at required concentrations.
-
Dye molecules combined with CDs for ratiometric sequential detection of metal ions and biothiols is limited by the concentration and overlapping efficacy of dye molecule, which requires great attention.
-
CDs-nanocomposites formation for FRET/IFE-based biothiols assays is limited by the composition ratio of CDs and composting material. Otherwise, the primary quenching by FRET or IFE can affected significantly. In case of IFE, it is also essential to clarify the absorbance overlapping of the compositing materials.
-
The reaction-based biothiols assay is limited by the solid evidence of the mechanistic pathway. In such cases, a model reaction must be conducted to support the proposed mechanism.
-
Characterization of CDs and detailed mechanistic studies on CDs-based biothiols assay require instruments such as high-resolution transmission electron microscopy (HRTEM), dynamic light scattering (DLS) analyzer, X-ray photoelectron spectroscopy (XPS), fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), etc. Thus, CDs-based biothiols discrimination is limited by the available instruments and cost-effectiveness.
-
In many reports, CDs-based biothiols quantification was not demonstrated by an interference effect. and discrimination between Cys, Hcy, and GSH was unavailable. Therefore, real sample-based recoveries and bioimaging remain a concern.
8. Conclusions and Perspectives
Fluorescent CDs-based biothiols detection and quantification are discussed in detail. The synthetic methods involved in the fabrication of fluorescent CDs were clearly delivered. Thereafter, the use of (1) a CD–metal ion system and (2) CD–nanocomposites towards biothiols quantification were comprehensively illustrated with their real-time applications in real samples (such as human serum, plasma, and urine) and bioimaging studies. The uniqueness and deficiencies of each report was clearly stated and commented. Finally, the selection of CDs/sensory probe, sensory requirements, merits, and limitations were discussed for the readers. However, some perspective points must be given more attention, as noted below.
-
Many reports of CDs-based fluorescent detection of biothiols followed difficult procedures and did not provide reliable information on the interference effect, which should be rectified to be considered “state-of-the-art”.
-
Up until now, reports on green and red emissive CDs-based assays of biothiols are insufficient, which should be the focus for future research towards a wide range of applications.
-
Although research on using CD–metal ion pairs or composites for detecting Cys, Hcy, and GSH has become the mainstream, not much detail was given on how to distinguish among them. Because Cys, Hcy, and GSH are involved in many different biological processes, this issue should be addressed in the future.
-
In some reports, information regarding the PLQY, exact cause of CDs emission, and PL quenching type (static/dynamic) of CDs with metal ions and during composites formation was not clarified for the readers. These issues should be stated more clearly in future studies.
-
The majority of reports delivered recoveries of CD–Hg2+ complex-mediated biothiols assays in biological samples (such as human serum, plasma, urine, etc.) without giving information on the toxicity of the CD–Hg2+ metal complex, which should be clearly addressed in the future.
-
So far, only Hg2+, Ag+, Cu2+, Fe3+, and Au3+ were reported in CD–metal ion pair enabled biothiols assays based on the thiophilicity of metal ions. This approach should be expanded with other thiophilic metal ions, such as Pb2+, Cd2+, Mo4+, etc.
-
Reports on dye molecules incorporated in the CD–metal ion pair system for ratiometric detection of biothiols are still insufficient. Future research should focus on using other dye molecules and justifying the role of dye molecules.
-
The CDs–nanocomposites system for FRET-tuned PL “Turn-On” detection of biothiols can be improved by encouraging more research.
-
To date, only one report is available on the reaction-based PL “Turn-On” dual channel discrimination between Cys, Hcy, and GSH [124], which should be expanded with other biothiols reactive species.
-
Reports on the fabrication of microfluidic paper-based analytical devices from vinyl sulfone clicked CDs for fluorescent assays of biothiols were impressive and could be commercialized. Thus, a similar approach should be strongly encouraged.
-
Only one report is available so far on CD–nanocomposite (Ag NPs/N, S-CDs)-based pH dependence discrimination between Cys, Hcy, and GSH [129], which should be a future research focus.
-
Au NPs and CDs composites displayed dual readout (fluorescent and colorimetric) responses to a specific analyte GSH against Cys and Hcy, which requires more attention in future research.
-
The CD–MnO2 composite system and CD–Br system selectively detects the GSH against Cys and Hcy via redox or specific reactions, thereby such approach can be anticipated for biological applications and towards commercialization.
-
CD–DTNB and Co–CDs (metal doped CDs) composite models showed IFE and reaction-tuned direct recognition of biothiols and Cys, respectively, via the PL “Turn-Off” response against Hcy and GSH. This approach must be improved by more similar research.
-
Reports on CDs-based discrimination of Cys against Hcy and GSH, and GSH against Cys and Hcy are available. However, there is no report on the discrimination of Hcy against Cys and Hcy, which should be the focus towards groundbreaking achievements.
-
The emission of CDs can be enhanced by combining a surface plasmon-coupled emission (SPCE) platform and photonic crystal-coupled emission (PCCE) technology for distinct detection of biothiols at a lower concentration (<nM).
Currently, many research groups are working on developing new CDs-based sensory probes to rectify the aforementioned issues. In terms of PL “On” or “Off” responses to biothiols with real-time applicability, research on CDs-based biothiols assay tactics can be noted as exceptional with great anticipation and excitement.
References
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and In Vivo. Nat. Metab. 2022, 4, 651–662.
- Shieh, M.; Xu, S.; Lederberg, O.L.; Xian, M. Detection of Sulfane Sulfur Species in Biological Systems. Redox Biol. 2022, 57, 102502.
- Paramasivam, K.; Shen, Y.; Yuan, J.; Waheed, I.; Mao, C.; Zhou, X. Advances in the Development of Phage-Based Probes for Detection of Bio-Species. Biosensors 2022, 12, 30.
- Zhang, K.; Gao, Y.-J.; Yang, P.-P.; Qi, G.-B.; Zhang, J.-P.; Wang, L.; Wang, H. Self-Assembled Fluorescent Organic Nanomaterials for Biomedical Imaging. Adv. Healthc. Mater. 2018, 7, 1800344.
- Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550.
- Shen, C.-L.; Liu, H.-R.; Lou, Q.; Wang, F.; Liu, K.-K.; Dong, L.; Shan, C.-X. Recent progress of Carbon Dots in Targeted Bioimaging and Cancer Therapy. Theranostics 2022, 12, 2860–2893.
- Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Inorganic Nanomaterials for Bioimaging, Targeted Drug Delivery and Therapeutics. Chem. Commun. 2014, 50, 14071–14081.
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent Development of Inorganic Nanoparticles for Biomedical Imaging. ACS Cent. Sci. 2018, 4, 324–336.
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Modified Diamond Nanoparticles Applied in Cellular Imaging and Hg2+ Ions Detection. Appl. Surf. Sci. 2019, 465, 340–350.
- de la Encarnación, C.; Jimenez de Aberasturi, D.; Liz-Marzán, L.M. Multifunctional Plasmonic-Magnetic Nanoparticles for Bioimaging and Hyperthermia. Adv. Drug Delivery Rev. 2022, 189, 114484.
- Li, Z.; Gao, Z.; Wang, C.; Zou, D.; Zhou, H.; Yi, Y.; Wang, J.; Wang, L. Recent Progress on Bioimaging Strategies Based on Janus Nanoparticles. Nanoscale 2022, 14, 12560–12568.
- Sohrabi, H.; Javanbakht, S.; Oroojalian, F.; Rouhani, F.; Shaabani, A.; Majidi, M.R.; Hashemzaei, M.; Hanifehpour, Y.; Mokhtarzadeh, A.; Morsali, A. Nanoscale Metal-Organic Frameworks: Recent Developments in Synthesis, Modifications and Bioimaging Applications. Chemosphere 2021, 281, 130717.
- Guo, Z.; Zeng, J.; Liu, W.; Chen, Y.; Jiang, H.; Weizmann, Y.; Wang, X. Formation of Bio-Responsive Nanocomposites for Targeted Bacterial Bioimaging and Disinfection. Chem. Eng. J. 2021, 426, 130726.
- Zhang, H.-F.; Klein Geltink, R.I.; Parker, S.J.; Sorensen, P.H. Transsulfuration, Minor Player or Crucial for Cysteine Homeostasis in Cancer. Trends Cell Biol. 2022, 32, 800–814.
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78.
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624.
- Carter, R.N.; Morton, N.M. Cysteine and Hydrogen Sulphide in the Regulation of Metabolism: Insights from Genetics and Pharmacology. J. Pathol. 2016, 238, 321–332.
- Chen, M.; Zhu, J.-Y.; Mu, W.-J.; Guo, L. Cysteine Dioxygenase Type 1 (CDO1): Its Functional Role in Physiological and Pathophysiological Processes. Genes Dis. 2022, in press.
- Cho, I.-J.; Kim, D.; Kim, E.-O.; Jegal, K.-H.; Kim, J.-K.; Park, S.-M.; Zhao, R.; Ki, S.-H.; Kim, S.-C.; Ku, S.-K. Cystine and Methionine Deficiency Promotes Ferroptosis by Inducing B-Cell Translocation Gene 1. Antioxidants 2021, 10, 1543.
- Paul, B.D.; Sbodio, J.I.; Snyder, S.H. Cysteine Metabolism in Neuronal Redox Homeostasis. Trends Pharmacol. Sci. 2018, 39, 513–524.
- Pham, T.K.; Buczek, W.A.; Mead, R.J.; Shaw, P.J.; Collins, M.O. Proteomic Approaches to Study Cysteine Oxidation: Applications in Neurodegenerative Diseases. Front. Mol. Neurosci. 2021, 14, 678837.
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine and Age-Associated Disorders. Ageing Res. Rev. 2019, 49, 144–164.
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421.
- Tsogas, G.Z.; Kappi, F.A.; Vlessidis, A.G.; Giokas, D.L. Recent Advances in Nanomaterial Probes for Optical Biothiol Sensing: A Review. Anal. Lett. 2018, 51, 443–468.
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324.
- Vairetti, M.; Di Pasqua, L.G.; Cagna, M.; Richelmi, P.; Ferrigno, A.; Berardo, C. Changes in Glutathione Content in Liver Diseases: An Update. Antioxidants 2021, 10, 364.
- Liu, Z.; Zhou, W.; Li, J.; Zhang, H.; Dai, X.; Liu, Y.; Liu, Y. High-Efficiency Dynamic Sensing of Biothiols in Cancer Cells with A Fluorescent β-Cyclodextrin Supramolecular Assembly. Chem. Sci. 2020, 11, 4791–4800.
- Zhu, H.; Zhang, H.; Liang, C.; Liu, C.; Jia, P.; Li, Z.; Yu, Y.; Zhang, X.; Zhu, B.; Sheng, W. A Novel Highly Sensitive Fluorescent Probe for Bioimaging Biothiols and Its Applications in Distinguishing Cancer Cells from Normal Cells. Analyst 2019, 144, 7010–7016.
- Li, X.; Li, S.; Liu, Q.; Cui, Z.; Chen, Z. A Triple-Channel Colorimetric Sensor Array for Identification of Biothiols Based on Color RGB (Red/Green/Blue) as Signal Readout. ACS Sustain. Chem. Eng. 2019, 7, 17482–17490.
- Mostafa, I.M.; Liu, H.; Hanif, S.; Gilani, M.R.H.S.; Guan, Y.; Xu, G. Synthesis of a Novel Electrochemical Probe for the Sensitive and Selective Detection of Biothiols and Its Clinical Applications. Anal. Chem. 2022, 94, 6853–6859.
- Dong, J.; Lu, G.; Tu, Y.; Fan, C. Recent Research Progress of Red-Emitting/Near-Infrared Fluorescent Probes for Biothiols. New J. Chem. 2022, 46, 10995–11020.
- Kuligowski, J.; El-Zahry, M.R.; Sánchez-Illana, Á.; Quintás, G.; Vento, M.; Lendl, B. Surface Enhanced Raman Spectroscopic Direct Determination of Low Molecular Weight Biothiols in Umbilical Cord Whole Blood. Analyst 2016, 141, 2165–2174.
- Hou, H.; Liu, Q.; Liu, X.; Fu, S.; Zhang, H.; Li, S.; Chen, S.; Hou, P. Dual Response Site Fluorescent Probe for Highly Sensitive Detection of Cys/Hcy and GSH In Vivo through Two Different Emission Channels. Biosensors 2022, 12, 1056.
- Wang, J.; Zhang, F.; Yang, L.; Wang, B.; Song, X. A Red-Emitting Fluorescent Probe for Sensing and Imaging Biothiols in Living cells. J. Lumin. 2021, 234, 117994.
- Kang, Y.-F.; Niu, L.-Y.; Yang, Q.-Z. Fluorescent Probes for Detection of Biothiols Based on “Aromatic Nucleophilic Substitution-Rearrangement” Mechanism. Chin. Chem. Lett. 2019, 30, 1791–1798.
- dos Santos, A.P.A.; da Silva, J.K.; Neri, J.M.; Neves, A.C.O.; de Lima, D.F.; Menezes, F.G. Nucleophilicity of Cysteine and Related Biothiols and the Development of Fluorogenic Probes and Other Applications. Org. Biomol. Chem. 2020, 18, 9398–9427.
- Wu, Z.; Li, W.; Chen, J.; Yu, C. A Graphene Quantum Dot-Based Method for the Highly Sensitive and Selective Fluorescence Turn-On Detection of Biothiols. Talanta 2014, 119, 538–543.
- Assi, N.; Nejdl, L.; Zemankova, K.; Pavelicova, K.; Bezdekova, J.; Macka, M.; Adam, V.; Vaculovicova, M. UV-Induced Zn:Cd/S Quantum Dots In-Situ Formed in the Presence of Thiols for Sensitive and Selective Fluorescence Detection of Thiols. Sci. Rep. 2021, 11, 13806.
- Zhang, H.; Nie, C.; Wang, J.; Guan, R.; Cao, D. Synthesis of Novel Organic-Inorganic Hybrid Fluorescent Microspheres and Their Applications as Fe(III), Hg(II) and Biothiols Probes. Talanta 2019, 195, 713–719.
- Sharma, S.; Ghosh, S.K. Metal–Organic Framework-Based Selective Sensing of Biothiols via Chemidosimetric Approach in Water. ACS Omega 2018, 3, 254–258.
- Rai, A.; Bhaskar, S.; Ganesh, K.M.; Ramamurthy, S.S. Gelucire®-Mediated Heterometallic AgAu Nanohybrid Engineering for Femtomolar Cysteine Detection Using Smartphone-Based Plasmonics Technology. Mater. Chem. Phys. 2022, 279, 125747.
- Bhaskar, S.; Moronshing, M.; Srinivasan, V.; Badiya, P.K.; Subramaniam, C.; Ramamurthy, S.S. Silver Soret Nanoparticles for Femtomolar Sensing of Glutathione in a Surface Plasmon-Coupled Emission Platform. ACS Appl. Nano Mater. 2020, 3, 4329–4341.
- Shen, P.; Shi, Y.; Li, R.; Han, B.; Ma, H.; Hou, X.; Zhang, Y.; Jiang, L. Multi-Factors Cooperatively Actuated Photonic Hydrogel Aptasensors for Facile, Label-Free and Colorimetric Detection of Lysozyme. Biosensors 2022, 12, 662.
- Liao, Z.; Zhou, Q.; Gao, B. AIEgens-Doped Photonic Crystals for High Sensitivity Fluorescence Detection of Tumor Markers. Biosensors 2023, 13, 276.
- Jia, X.; Xiao, T.; Hou, Z.; Xiao, L.; Qi, Y.; Hou, Z.; Zhu, J. Chemically Responsive Photonic Crystal Hydrogels for Selective and Visual Sensing of Thiol-Containing Biomolecules. ACS Omega 2019, 4, 12043–12048.
- Qin, J.; Li, X.; Cao, L.; Du, S.; Wang, W.; Yao, S.Q. Competition-Based Universal Photonic Crystal Biosensors by Using Antibody–Antigen Interaction. J. Am. Chem. Soc. 2020, 142, 417–423.
- Lin, F.; Jia, C.; Wu, F.-G. Carbon Dots for Intracellular Sensing. Small Struct. 2022, 3, 2200033.
- Pawar, S.; Duadi, H.; Fleger, Y.; Fixler, D. Carbon Dots-Based Logic Gates. Nanomaterials 2021, 11, 232.
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 6515–6541.
- Cao, L.; Fernando, K.A.S.; Liang, W.; Seilkop, A.; Veca, L.M.; Sun, Y.-P.; Bunker, C.E. Carbon dots for Energy Conversion Applications. J. Appl. Phys. 2019, 125, 220903.
- Nazri, N.A.A.; Azeman, N.H.; Luo, Y.; A Bakar, A.A. Carbon Quantum dots for Optical Sensor Applications: A Review. Opt. Laser Technol. 2021, 139, 106928.
- Li, J.; Hu, Z.-E.; We, Y.-J.; Liu, Y.-H.; Wang, N.; Yu, X.-Q. Multifunctional Carbon Quantum Dots as A Theranostic Nanomedicine for Fluorescence Imaging-Guided Glutathione Depletion to Improve Chemodynamic Therapy. J. Colloid Inter. Sci. 2022, 606, 1219–1228.
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Facile Synthesis of Surface-Modified Carbon Quantum Dots (CQDs) for Biosensing and Bioimaging. Materials 2020, 13, 3313.
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and Perspectives in Carbon Dot-Based Fluorescent Probes: Mechanism, and Application. Coord. Chem. Rev. 2021, 431, 213686.
- Khan, Z.G.; Patil, P.O. A Comprehensive Review on Carbon Dots and Graphene Quantum Dots Based Fluorescent Sensor for Biothiols. Microchem. J. 2020, 157, 105011.
- Molaei, M.J. Principles, Mechanisms, and Application of Carbon Quantum Dots in Sensors: A Review. Anal. Methods 2020, 12, 1266–1287.
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419.
- Sharma, A.; Das, J. Small Molecules Derived Carbon Dots: Synthesis and Applications in Sensing, Catalysis, Imaging, and Biomedicine. J. Nanobiotechnol. 2019, 17, 92.
- Chao-Mujica, F.J.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.A.; Contreras, D.R.; Pérez-Rodríguez, A.; Peña-Caravaca, J.P.; Páez-Rodríguez, A.; Darias-Gonzalez, J.G.; et al. Carbon Quantum Dots by Submerged Arc Discharge in Water: Synthesis, Characterization, and Mechanism of Formation. J. Appl. Phys. 2021, 129, 163301.
- Doñate-Buendía, C.; Fernández-Alonso, M.; Lancis, J.; Mínguez-Vega, G. Pulsed Laser Ablation in Liquids for the Production of Gold Nanoparticles and Carbon Quantum Dots: From Plasmonic to Fluorescence and Cell Labelling. J. Phys. Confer. Ser. 2020, 1537, 012013.
- Doñate-Buendia, C.; Torres-Mendieta, R.; Pyatenko, A.; Falomir, E.; Fernández-Alonso, M.; Mínguez-Vega, G. Fabrication by Laser Irradiation in a Continuous Flow Jet of Carbon Quantum Dots for Fluorescence Imaging. ACS Omega 2018, 3, 2735–2742.
- Magesh, V.; Sundramoorthy, A.K.; Ganapathy, D. Recent Advances on Synthesis and Potential Applications of Carbon Quantum Dots. Front. Mater. 2022, 9, 906838.
- Desai, M.L.; Jha, S.; Basu, H.; Singhal, R.K.; Park, T.-J.; Kailasa, S.K. Acid Oxidation of Muskmelon Fruit for the Fabrication of Carbon Dots with Specific Emission Colors for Recognition of Hg2+ Ions and Cell Imaging. ACS Omega 2019, 4, 19332–19340.
- Deng, J.; Lu, Q.; Mi, N.; Li, H.; Liu, M.; Xu, M.; Tan, L.; Xie, Q.; Zhang, Y.; Yao, S. Electrochemical Synthesis of Carbon Nanodots Directly from Alcohols. Chem. Eur. J. 2014, 20, 4993–4999.
- Lee, Y.-S.; Hu, C.-C.; Chiu, T.-C. Electrochemical Synthesis of Fluorescent Carbon Dots for the Selective Detection of Chlortetracycline. J. Environ. Chem. Eng. 2022, 10, 107413.
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical Synthesis of Carbon Dots, Mechanism, Effect of Parameters, and Catalytic, Energy, Biomedical and Tissue Engineering Applications. Ultrason. Sonochem. 2020, 64, 105009.
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.u.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312.
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-Assisted Synthesis of Carbon Dots and Their Applications. J. Mater. Chem. C 2019, 7, 7175–7195.
- Liu, Q.; Zhang, N.; Shi, H.; Ji, W.; Guo, X.; Yuan, W.; Hu, Q. One-Step Microwave Synthesis of Carbon Dots for Highly Sensitive and Selective Detection of Copper Ions in Aqueous Solution. New J. Chem. 2018, 42, 3097–3101.
- Lee, H.; Su, Y.-C.; Tang, H.-H.; Lee, Y.-S.; Lee, J.-Y.; Hu, C.-C.; Chiu, T.-C. One-Pot Hydrothermal Synthesis of Carbon Dots as Fluorescent Probes for the Determination of Mercuric and Hypochlorite Ions. Nanomaterials 2021, 11, 1831.
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094.
- Omer, K.M.; Tofiq, D.I.; Hassan, A.Q. Solvothermal Synthesis of Phosphorus and Nitrogen Doped Carbon Quantum Dots as A Fluorescent Probe for Iron(III). Microchim. Acta 2018, 185, 466.
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190.
- Wan, J.-Y.; Yang, Z.; Liu, Z.-G.; Wang, H.-X. Ionic Liquid-Assisted Thermal Decomposition Synthesis of Carbon Dots and Graphene-Like Carbon Sheets for Optoelectronic Application. RSC Adv. 2016, 6, 61292–61300.
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316.
- Esfandiari, N.; Bagheri, Z.; Ehtesabi, H.; Fatahi, Z.; Tavana, H.; Latifi, H. Effect of Carbonization Degree of Carbon Dots on Cytotoxicity and Photo-Induced Toxicity to Cells. Heliyon 2019, 5, e02940.
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreenivasan Sreeprasad, T.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-Doped Carbon Dots: Synthesis, Characterization, Properties, Photoluminescence Mechanism and Biological Applications. J. Mater. Chem. B 2016, 4, 7204–7219.
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939.
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence Mechanism of Carbon Dots: Triggering High-Color-Purity Red Fluorescence Emission Through Edge Amino Protonation. Nat. Commun. 2021, 12, 6856.
- Debnath, S.K.; Srivastava, R. Drug Delivery with Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564.
- Zhang, J.; Yu, S.-H. Carbon dots: Large-scale synthesis, sensing and bioimaging. Mater. Today 2016, 19, 382–393.
- Wang, B.; Cai, H.; Waterhouse, G.I.N.; Qu, X.; Yang, B.; Lu, S. Carbon Dots in Bioimaging, Biosensing and Therapeutics: A Comprehensive Review. Small Sci. 2022, 2, 2200012.
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626.
- Shellaiah, M.; Sun, K.-W. Review on Anti-Aggregation-Enabled Colorimetric Sensing Applications of Gold and Silver Nanoparticles. Chemosensors 2022, 10, 536.
- Shellaiah, M.; Sun, K.-W. Progress in Metal-Organic Frameworks Facilitated Mercury Detection and Removal. Chemosensors 2021, 9, 101.
- Fang, H.-P.; Shellaiah, M.; Singh, A.; Raju, M.V.R.; Wu, Y.-H.; Lin, H.-C. Naked Eye and Fluorescent Detections of Hg2+ Ions and Cysteine Via J-Aggregation and Deaggregation of a Perylene Bisimide Derivative. Sens. Actuators B 2014, 194, 229–237.
- Zhou, L.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Carbon Nanodots as Fluorescence Probes for Rapid, Sensitive, and Label-Free Detection of Hg2+ and Biothiols in Complex Matrices. Chem. Commun. 2012, 48, 1147–1149.
- Liang, J.Y.; Han, L.; Liu, S.G.; Ju, Y.J.; Li, N.B.; Luo, H.Q. Carbon Dots-Based Fluorescent Turn Off/On Sensor for Highly Selective and Sensitive Detection of Hg2+ and Biothiols. Spectrochim. Acta A 2019, 222, 117260.
- Zhang, H.; You, J.; Wang, J.; Dong, X.; Guan, R.; Cao, D. Highly Luminescent Carbon Dots as Temperature Sensors and “Off-On” Sensing of Hg2+ and Biothiols. Dyes Pig. 2020, 173, 107950.
- Gao, X.; Zhang, Y.; Fu, Z.; Cui, F. One Step Synthesis of Ultra-High Quantum Yield Fluorescent Carbon Dots for “On-Off-On” Detection of Hg2+ and Biothiols. J. Fluoresc. 2022, 32, 1921–1930.
- Xu, S.; Liu, Y.; Yang, H.; Zhao, K.; Li, J.; Deng, A. Fluorescent Nitrogen and Sulfur Co-Doped Carbon Dots from Casein and Their Applications for Sensitive Detection of Hg2+ and Biothiols and Cellular Imaging. Anal. Chim. Acta 2017, 964, 150–160.
- Du, H.; Xu, H.; Zhao, Y.; Li, D.; Wang, Y. Mercury Ions Mediated Phosphorus Containing Carbon Dots as Fluorescent Probe for Biothiols Screening. NANO Brief Rep. Rev. 2018, 13, 1850116.
- Pang, L.-F.; Wu, H.; Fu, M.-J.; Guo, X.-F.; Wang, H. Red Emissive Boron and Nitrogen Co-Doped “On-Off-On” Carbon Dots for Detecting and Imaging of Mercury(II) and Biothiols. Microchim. Acta 2019, 186, 708.
- Hou, J.; Zhang, F.; Yan, X.; Wang, L.; Yan, J.; Ding, H.; Ding, L. Sensitive Detection of Biothiols and Histidine Based on the Recovered Fluorescence of the Carbon Quantum Dots–Hg(II) System. Anal. Chim. Acta 2015, 859, 72–78.
- Lan, M.; Zhang, J.; Chui, Y.-S.; Wang, H.; Yang, Q.; Zhu, X.; Wei, H.; Liu, W.; Ge, J.; Wang, P.; et al. A Recyclable Carbon Nanoparticle-Based Fluorescent Probe for HighlyS and Sensitive Detection of Mercapto Biomolecules. J. Mater. Chem. B 2015, 3, 127–134.
- Zhang, Y.; Cui, P.; Zhang, F.; Feng, X.; Wang, Y.; Yang, Y.; Liu, X. Fluorescent Probes for “Off–On” Highly Sensitive Detection of Hg2+ and L-Cysteine Based on Nitrogen-Doped Carbon Dots. Talanta 2016, 152, 288–300.
- Xavier, S.S.J.; Siva, G.; Annaraj, J.; Kim, A.R.; Yoo, D.J.; Kumar, G.G. Sensitive and Selective Turn-Off-On Fluorescence Detection of Hg2+ and Cysteine Using Nitrogen Doped Carbon Nanodots Derived from Citron and Urine. Sens. Actuators B 2018, 259, 1133–1143.
- Tabaraki, R.; Abdi, O. Green and Simple Turn Off/On Fluorescence Sensor for Mercury (II), Cysteine and Histidine. J. Mol. Liq. 2018, 251, 77–82.
- Zhu, S.; He, L.; Zhang, F.; Li, M.; Jiao, S.; Li, Y.; Chen, M.; Zhao, X.-E.; Wang, H. Fluorimetric Evaluation of Glutathione Reductase Activity and Its Inhibitors Using Carbon Quantum Dots. Talanta 2016, 161, 769–774.
- Iqbal, A.; Iqbal, K.; Xu, L.; Li, B.; Gong, D.; Liu, X.; Guo, Y.; Liu, W.; Qin, W.; Guo, H. Heterogeneous Synthesis of Nitrogen-Doped Carbon Dots Prepared Via Anhydrous Citric Acid and Melamine for Selective and Sensitive Turn On-Off-On Detection of Hg (II), Glutathione and Its Cellular Imaging. Sens. Actuators B 2018, 255, 1130–1138.
- Li, X.; Chai, C.; Zhang, Y.; Wang, Y.; Lv, J.; Bian, W.; Choi, M.M.F. Microwave Synthesis of Nitrogen and Sulfur Co-Doped Carbon Dots for the Selective Detection of Hg2+ and Glutathione. Opt. Mater. 2020, 99, 109559.
- Chang, D.; Zhao, Z.; Li, W.; Shi, H.; Yang, Y.; Shi, L.; Shuang, S. Hg2+-Mediated Ratiometric Fluorescent Carbon Dots for Imaging Glutathione in Living Cells and Zebrafish. ACS Sustain. Chem. Eng. 2022, 10, 10068–10076.
- Shen, L.-M.; Chen, Q.; Sun, Z.-Y.; Chen, X.-W.; Wang, J.-H. Assay of Biothiols by Regulating the Growth of Silver Nanoparticles with C-Dots as Reducing Agent. Anal. Chem. 2014, 86, 5002–5008.
- Thirumalaivasan, N.; Wu, S.-P. Bright Luminescent Carbon Dots for Multifunctional Selective Sensing and Imaging Applications in Living Cells. ACS Appl. Bio Mater. 2020, 3, 6439–6446.
- Borse, V.; Thakur, M.; Sengupta, S.; Srivastava, R. N-Doped Multi-Fluorescent Carbon Dots for ‘Turn Off-On’ Silver-Biothiol Dual Sensing and Mammalian Cell Imaging Application. Sens. Actuators B 2017, 248, 481–492.
- Liu, X.; Zhang, S.; Xu, H.; Wang, R.; Dong, L.; Gao, S.; Tang, B.; Fang, W.; Hou, F.; Zhong, L.; et al. Nitrogen-Doped Carbon Quantum Dots from Poly(ethyleneimine) for Optical Dual-Mode Determination of Cu2+ and L-Cysteine and Their Logic Gate Operation. ACS Appl. Mater. Interfaces 2020, 12, 47245–47255.
- Zhou, W.; Mo, F.; Sun, Z.; Luo, J.; Fan, J.; Zhu, H.; Zhu, Z.; Huang, J.; Zhang, X. Bright Red-Emitting P, Br Co-Doped Carbon Dots as “OFF-ON” Fluorescent Probe for Cu2+ and L-Cysteine Detection. J. Alloys Comp. 2022, 897, 162731.
- Guo, Y.; Yang, L.; Li, W.; Wang, X.; Shang, Y.; Li, B. Carbon Dots Doped with Nitrogen and Sulfur and Loaded with Copper(II) as a “Turn-On” Fluorescent Probe for Cystein, Glutathione and Homocysteine. Microchim. Acta 2016, 183, 1409–1416.
- Wang, Y.; Feng, M.; He, B.; Chen, X.; Zeng, J.; Sun, J. Ionothermal Synthesis of Carbon Dots from Cellulose in Deep Eutectic Solvent: A Sensitive Probe for Detecting Cu2+ and Glutathione with “Off-On” Pattern. Appl. Surf. Sci. 2022, 599, 153705.
- Zhang, B.; Duan, Q.; Li, Y.; Zhang, Y.; Che, M.; Zhang, W.; Sang, S. A “Turn-On” Fluorescent Probe for Glutathione Detection Based on the Polyethylenimine-Carbon Dots-Cu2+ System. J. Photochem. Photobiol. B 2019, 197, 111532.
- Zhang, D.; Zhang, F.; Liao, Y.; Wang, F.; Liu, H. Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off–On” High-Sensitivity Detection of Fe3+ and L-Cysteine. Molecules 2022, 27, 4099.
- Lu, X.; Liu, C.; Wang, Z.; Yang, J.; Xu, M.; Dong, J.; Wang, P.; Gu, J.; Cao, F. Nitrogen-Doped Carbon Nanoparticles Derived from Silkworm Excrement as On–Off–On Fluorescent Sensors to Detect Fe(III) and Biothiols. Nanomaterials 2018, 8, 443.
- Xu, H.; Liu, X.; Wang, R.; Gao, S.; Hou, F.; Liang, K.; Luo, S. A Yellow-Emitting Carbon Quantum Dot-Based Fluorescent Logic Gate for the Continuous Detection of Au3+ and Biothiols. Chem. Commun. 2021, 57, 11549–11552.
- Gu, J.; Hu, D.; Wang, W.; Zhang, Q.; Meng, Z.; Jia, X.; Xi, K. Carbon Dot Cluster as An Efficient “Off–On” Fluorescent Probe to Detect Au(III) and Glutathione. Biosens. Bioelectron. 2015, 68, 27–33.
- Gupta, A.; Verma, N.C.; Khan, S.; Nandi, C.K. Carbon Dots for Naked Eye Colorimetric Ultrasensitive Arsenic and Glutathione Detection. Biosens. Bioelectron. 2016, 81, 465–472.
- Wang, Y.; Xu, J.; Lei, L.; Wang, F.; Xu, Z.; Zhang, W. Multi-Functional Carbon Dots-Based Nanoprobe for Ratiometric Enzyme Reaction Monitoring and Biothiol Analysis. Sens. Actuators B 2018, 264, 296–303.
- Lu, S.; Wu, D.; Li, G.; Lv, Z.; Chen, Z.; Chen, L.; Chen, G.; Xia, L.; You, J.; Wu, Y. Carbon Dots-Based Ratiometric Nanosensor for Highly Sensitive and Selective Detection of Mercury(ii) Ions and Glutathione. RSC Adv. 2016, 6, 103169–103177.
- Fu, H.; Ji, Z.; Chen, X.; Cheng, A.; Liu, S.; Gong, P.; Li, G.; Chen, G.; Sun, Z.; Zhao, X.; et al. A Versatile Ratiometric Nanosensing Approach for Sensitive and Accurate Detection of Hg2+ and Biological Thiols Based on New Fluorescent Carbon Quantum Dots. Anal. Bioanal. Chem. 2017, 409, 2373–2382.
- Wu, Y.; Liu, X.; Wu, Q.; Yi, J.; Zhang, G. Carbon Nanodots-Based Fluorescent Turn-On Sensor Array for Biothiols. Anal. Chem. 2017, 89, 7084–7089.
- Chen, S.; Xu, C.-H.; Yu, Y.-L.; Wang, J.-H. Multichannel Fluorescent Sensor Array for Discrimination of Thiols Using Carbon Dot–Metal Ion Pairs. Sens. Actuators B 2018, 266, 553–560.
- Mandani, S.; Sharma, B.; Dey, D.; Sarma, T.K. Carbon Nanodots as Ligand Exchange Probes in Nanobeacons for Fluorescent Turn-On Detection of Biothiols. Nanoscale 2015, 7, 1802–1808.
- Garg, D.; Mehta, A.; Mishra, A.; Basu, S. A Sensitive Turn-On Fluorescent Probe for Detection of Biothiols Using MnO2@Carbon Dots Nanocomposites. Spectrochim. Acta A 2018, 192, 411–419.
- Sun, J.; Wang, Q.; Yang, J.; Zhang, J.; Li, Z.; Li, H.; Yang, X.-F. 2,4-Dinitrobenzenesulfonate-Functionalized Carbon Dots as a Turn-On Fluorescent Probe for Imaging of Biothiols in Living Cells. Microchim. Acta 2019, 186, 402.
- Zhang, J.; Jia, H.; Liu, W.; Wang, J.; Fang, D. A Novel Dual-Excitation and Dual-Emission Fluorescent Probe (CQDs-O-NBD) Based on Carbon Quantum Dots for Detection and Discrimination of Cys/Hcy and GSH/H2S in Living Cells. Dyes Pig. 2021, 193, 109554.
- Ortiz-Gomez, I.; Ortega-Muñoz, M.; Marín-Sánchez, A.; de Orbe-Payá, I.; Hernandez-Mateo, F.; Capitan-Vallvey, L.F.; Santoyo-Gonzalez, F.; Salinas-Castillo, A. A Vinyl Sulfone Clicked Carbon Dot-Engineered Microfluidic Paper-Based Analytical Device for Fluorometric Determination of Biothiols. Microchim. Acta 2020, 187, 421.
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Nanodiamonds Conjugated to Gold Nanoparticles for Colorimetric Detection of Clenbuterol and Chromium(III) in Urine. Microchim. Acta 2017, 185, 74.
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.-P. A pH Cooperative Strategy for Enhanced Colorimetric Sensing of Cr(III) Ions Using Biocompatible L-Glutamic Acid Stabilized Gold Nanoparticles. Microchem. J. 2021, 160, 105754.
- Shellaiah, M.; Sun, K.W. Conjugation of Cysteamine Functionalized Nanodiamond to Gold Nanoparticles for pH Enhanced Colorimetric Detection of Cr3+ Ions Demonstrated by Real Water Sample Analysis. Spectrochim. Acta A 2023, 286, 121962.
- Xiang, F.; Li, J.; Liu, Z. pH-Dependent Photoluminescence “Switch-On” Nanosensors Composed of Silver Nanoparticles and Nitrogen and Sulphur Co-Doped Carbon Dots for Discriminative Detection of Biothiols. Analyst 2019, 144, 7057–7063.
- Amjadi, M.; Abolghasemi-Fakhri, Z.; Hallaj, T. Carbon Dots-Silver Nanoparticles Fluorescence Resonance Energy Transfer System as a Novel Turn-On Fluorescent Probe for Selective Determination of Cysteine. J. Photochem. Photobiol. A 2015, 309, 8–14.
- Zhou, N.; Shi, Y.; Sun, C.; Zhang, X.; Zhao, W. Carbon Quantum Dot-AgOH Colloid Fluorescent Probe for Selective Detection of Biothiols Based on the Inner Filter Effect. Spectrochim. Acta A 2020, 228, 117847.
- Fu, X.; Gu, D.; Zhao, S.; Zhou, N.; Zhang, H. A Dual-Readout Method for Biothiols Detection Based on the NSET of Nitrogen-Doped Carbon Quantum Dots–Au Nanoparticles System. J. Fluoresc. 2017, 27, 1597–1605.
- Shi, Y.; Pan, Y.; Zhang, H.; Zhang, Z.; Li, M.-J.; Yi, C.; Yang, M. A Dual-Mode Nanosensor Based on Carbon Quantum Dots and Gold Nanoparticles for Discriminative Detection of Glutathione in Human Plasma. Biosens. Bioelectron. 2014, 56, 39–45.
- Li, J.; Rao, X.; Xiang, F.; Wei, J.; Yuan, M.; Liu, Z. A Photoluminescence “Switch-On” Nanosensor Composed of Nitrogen and Sulphur Co-Doped Carbon Dots and Gold Nanoparticles for Discriminative Detection of Glutathione. Analyst 2018, 143, 2083–2089.
- Cai, Q.-Y.; Li, J.; Ge, J.; Zhang, L.; Hu, Y.-L.; Li, Z.-H.; Qu, L.-B. A Rapid Fluorescence “Switch-On” Assay for Glutathione Detection by Using Carbon Dots–MnO2 Nanocomposites. Biosens. Bioelectron. 2015, 72, 31–36.
- Wang, D.; Meng, Y.-t.; Zhang, Y.; Wang, Q.; Lu, W.-j.; Shuang, S.-m.; Dong, C. A Specific Discriminating GSH from Cys/Hcy Fluorescence Nanosensor: The Carbon Dots-MnO2 Nanocomposites. Sens. Actuators B 2022, 367, 132135.
- Xu, Y.; Chen, X.; Chai, R.; Xing, C.; Li, H.; Yin, X.-B. A Magnetic/Fluorometric Bimodal Sensor Based on a Carbon Dots–MnO2 Platform for Glutathione Detection. Nanoscale 2016, 8, 13414–13421.
- Kong, D.; Yan, F.; Luo, Y.; Wang, Y.; Chen, L.; Cui, F. Carbon Nanodots Prepared for Cellular Imaging and Turn-On Detection of Glutathione. Anal. Methods 2016, 8, 4736–4743.
- Yan, F.; Ye, Q.; Xu, J.; He, J.; Chen, L.; Zhou, X. Carbon Dots-Bromoacetyl Bromide Conjugates as Fluorescence Probe for the Detection of Glutathione Over Cysteine and Homocysteine. Sens. Actuators B 2017, 251, 753–762.
- Zhu, H.; Wang, E.; Li, J.; Wang, J. L-Tyrosine Methyl Ester-Stabilized Carbon Dots as Fluorescent Probes for the Assays of Biothiols. Anal. Chim. Acta 2018, 1006, 83–89.
- Wu, D.; Li, G.; Chen, X.; Qiu, N.; Shi, X.; Chen, G.; Sun, Z.; You, J.; Wu, Y. Fluorometric Determination and Imaging of Glutathione Based on a Thiol-Triggered Inner Filter Effect on the Fluorescence of Carbon Dots. Microchim. Acta 2017, 184, 1923–1931.
- Yang, J.; Wu, H.; Yang, P.; Hou, C.; Huo, D. A High-Performance N-Doped Carbon Quantum Dots/5,5′-Dithiobis-(2-nitrobenzoic acid) Fluorescent Sensor for Biothiols Detection. Sens. Actuators B 2018, 255, 3179–3186.
- Liu, H.; Sun, Y.; Yang, J.; Hu, Y.; Yang, R.; Li, Z.; Qu, L.; Lin, Y. High Performance Fluorescence Biosensing of Cysteine in Human Serum with Superior Specificity Based on Carbon Dots and Cobalt-Derived Recognition. Sens. Actuators B 2019, 280, 62–68.
- Wang, N.; Yu, X.; Zhang, K.; Mirkin, C.A.; Li, J. Upconversion Nanoprobes for the Ratiometric Luminescent Sensing of Nitric Oxide. J. Am. Chem. Soc. 2017, 139, 12354–12357.
- Shellaiah, M.; Sun, K.W. Luminescent Metal Nanoclusters for Potential Chemosensor Applications. Chemosensors 2017, 5, 36.
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55.
- Madhu, M.; Santhoshkumar, S.; Tseng, W.-B.; Tseng, W.-L. Optical Nanoprobes for Aminothiols Sensing in Real-World Samples. Sens. Actuators Rep. 2022, 4, 100123.
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788.
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874.
- Huang, Z.-Q.; Zhao, S.-M.; Chen, J.-Q.; Zhao, Y.; Sun, W.-Y. Pillar-Layered Metal-Organic Frameworks for Sensing Specific Amino Acid and Photocatalyzing Rhodamine B Degradation. Molecules 2022, 27, 7551.
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors 2022, 10, 381.
- Wang, H.-B.; Mao, A.-L.; Li, Y.-H.; Gan, T.; Liu, Y.-M. A Turn-On Fluorescence Strategy for Biothiols Determination by Blocking Hg(II)-Mediated Fluorescence Quenching of Adenine-Rich DNA-Templated Gold Nanoclusters. Luminescence 2020, 35, 1296–1303.
- Arumugaperumal, R.; Srinivasadesikan, V.; Lin, M.-C.; Shellaiah, M.; Shukla, T.; Lin, H.-C. Facile Rhodamine-Based Colorimetric Sensors for Sequential Detections of Cu(ii) Ions and Pyrophosphate (P2O74−) Anions. RSC Adv. 2016, 6, 106631–106640.
- Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Novel Rhodamine Probe for Colorimetric and Fluorescent Detection of Fe3+ Ions in Aqueous Media with Cellular Imaging. Spectrochim. Acta A 2020, 242, 118757.
- Wang, G.; Xu, W.; Guo, Y.; Fu, N. Near-Infrared Squaraine Dye as a Selective Protein Sensor Based on Self-Assembly. Sens. Actuators B 2017, 245, 932–937.
- Putro, P.A.; Roza, L.; Isnaeni, I. Precursor concentration effect on optical properties of carbon dots from Cassava’s peels. J. Phys. Theor. Appl. 2018, 2, 43–52.
- Chu, K.-W.; Lee, S.L.; Chang, C.-J.; Liu, L. Recent Progress of Carbon Dot Precursors and Photocatalysis Applications. Polymers 2019, 11, 689.
- Liang, W.; Ge, L.; Hou, X.; Ren, X.; Yang, L.; Bunker, C.E.; Overton, C.M.; Wang, P.; Sun, Y.-P. Evaluation of Commercial “Carbon Quantum Dots” Sample on Origins of Red Absorption and Emission Features. J. Carbon Res. 2019, 5, 70.
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195.
- Ambrusi, R.E.; Arroyave, J.M.; Centurión, M.E.; Di Nezio, M.S.; Pistonesi, M.F.; Juan, A.; Pronsato, M.E. Density Functional Theory Model for Carbon Dot Surfaces and Their Interaction with Silver Nanoparticles. Phys. E Low-Dimen. Sys. Nanostruct. 2019, 114, 113640.
- Wang, C.; Ding, Y.; Bi, X.; Luo, J.; Wang, G.; Lin, Y. Carbon quantum dots-Ag nanoparticle complex as a highly sensitive “turn-on” fluorescent probe for hydrogen sulfide: A DFT/TD-DFT study of electronic transitions and mechanism of sensing. Sens. Actuators B 2018, 264, 404–409.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
5 times
(View History)
Update Date:
13 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





