Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | mikhail artamonov | -- | 2557 | 2023-03-09 21:00:56 | | | |
| 2 | Rita Xu | -3 word(s) | 2554 | 2023-03-10 02:31:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Artamonov, M.Y.; Martusevich, A.K.; Pyatakovich, F.A.; Minenko, I.A.; Dlin, S.V.; Lebaron, T.W. Molecular Hydrogen into the Body. Encyclopedia. Available online: https://encyclopedia.pub/entry/42045 (accessed on 08 February 2026).
Artamonov MY, Martusevich AK, Pyatakovich FA, Minenko IA, Dlin SV, Lebaron TW. Molecular Hydrogen into the Body. Encyclopedia. Available at: https://encyclopedia.pub/entry/42045. Accessed February 08, 2026.
Artamonov, Mikhail Yu., Andrew K. Martusevich, Felix A. Pyatakovich, Inessa A. Minenko, Sergei V. Dlin, Tyler W. Lebaron. "Molecular Hydrogen into the Body" Encyclopedia, https://encyclopedia.pub/entry/42045 (accessed February 08, 2026).
Artamonov, M.Y., Martusevich, A.K., Pyatakovich, F.A., Minenko, I.A., Dlin, S.V., & Lebaron, T.W. (2023, March 09). Molecular Hydrogen into the Body. In Encyclopedia. https://encyclopedia.pub/entry/42045
Artamonov, Mikhail Yu., et al. "Molecular Hydrogen into the Body." Encyclopedia. Web. 09 March, 2023.
Copy Citation
It is known that molecular hydrogen is a relatively stable, ubiquitous gas that is a minor component of the atmosphere. At the same time, molecular hydrogen has been shown to have diverse biological effects. By the end of 2022, more than 2000 articles have been published in the field of hydrogen medicine, many of which are original studies.
molecular hydrogen
oxidative stress
mechanisms
mesenchymal stem cells
1. Introduction
In recent decades, medical gases have deservedly attracted much attention from specialists in the field of biomedicine. At the same time, the spectrum of gases for which biological effects have been discovered and a beneficial effects have been observed is quite wide and continues to increase [1][2][3]. Initially, the concept of “medical gases” extended only to oxygen and hyperbaric O2 procedures, followed by the introduction of the inhalation of anesthetics and ozone into medical practice. At the same time, among these gases, only ozone had specific bioregulatory effects, while other factors either contributed to the elimination of hypoxia, or had a systemic anesthetic effect, being considered as a pharmacological (synthetic) agent.
The prospect of using endogenously formed low-molecular-weight gaseous compounds as regulators began to arise only after the establishment of the biological activity of nitrogen monoxide (i.e., nitric oxide; NO•), for which the Nobel Prize in Medicine and Physiology was awarded [4]. Later, the ability of other substances—previously considered exclusively as toxic—to act as endogenous gaso-transmitters was discovered. These substances formed a triumvirate, which, in addition to nitric oxide, included hydrogen sulfide and carbon monoxide [5].
In parallel, ideas were developed about the biological properties of inert gases (helium, argon, xenon, etc.) which are chemically inactive under standard conditions, but show a modulating effect on cells and tissues [6][7][8][9]. These ideas served as the basis for the creation of innovative therapeutic technologies, the prospects of which continue to be studied and expanded upon.
Recently, molecular hydrogen, which has several unique characteristics, has occupied a special place in medical gas therapy. This gas, which has no specific color or smell, is created from the lightest chemical element, is ubiquitous, and, due to its size and minimal molecular weight, can penetrate through any biological barrier [10][11][12]. Many supplements and antioxidants require specific transporters to enter the cells and realize their effect, whereas H2 does not need them due it is smaller molecular weight/size (Figure 1). The high bioavailability of H2 satisfies the first requirement of any pharmacological agent to have a biological effect. However, there are additional properties that are needed to explain the breadth of the biomodulating and, consequently, therapeutic effect of this gas.
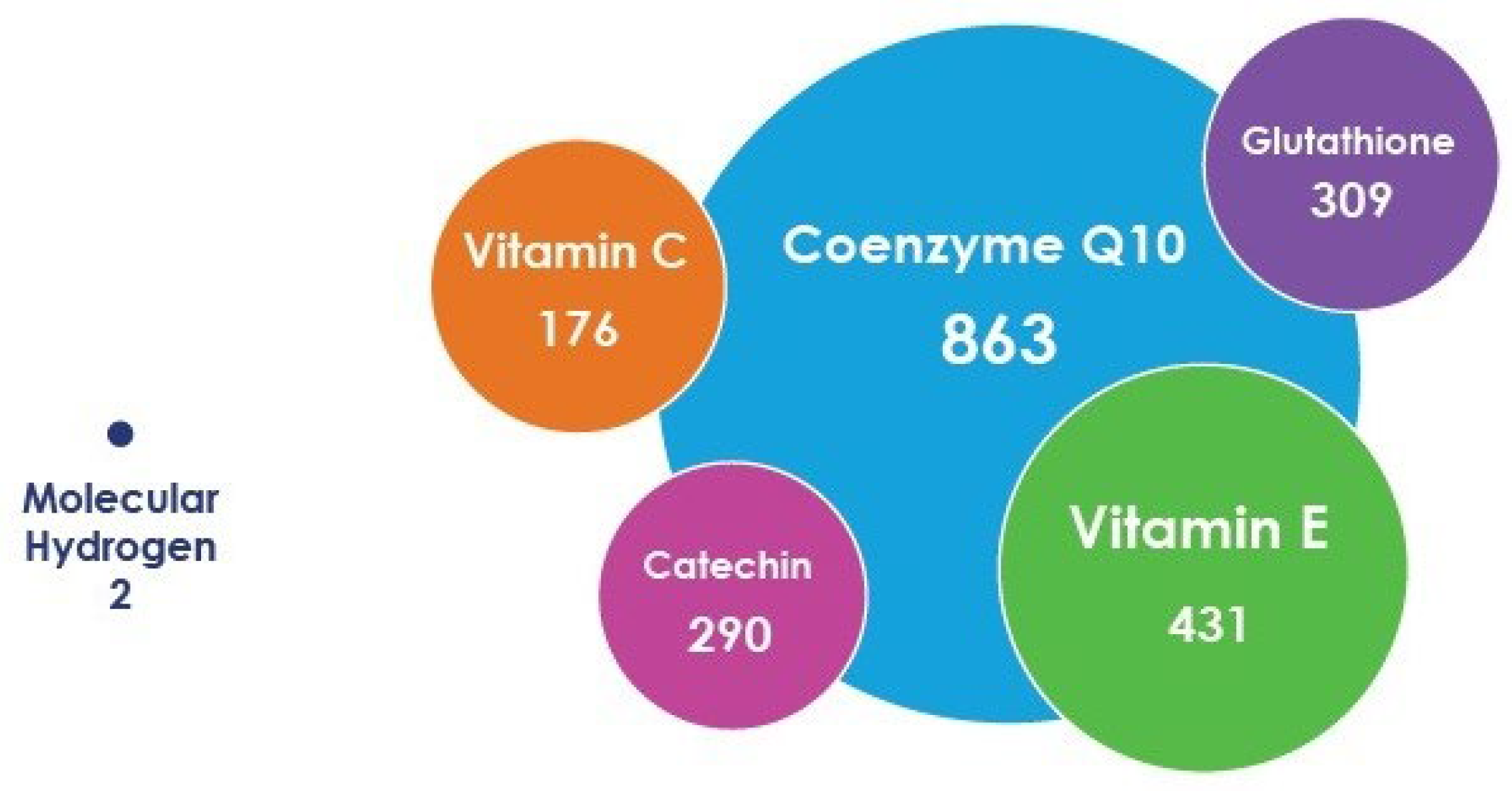
Figure 1. Relative size of molecular hydrogen and some antioxidants based on differences in their molecular weights, not their actual effective diameter size (e.g., kinetic diameter or Stokes–Einstein radius).
The body of knowledge in the field of H2 biomedicine is being actively updated, including about 100 randomized controlled trials and more than 2000 articles that have been published by the end of 2022 (Figure 2). Various aspects of the issue are considered in detail, but the focus of research is largely shifted towards the cardiac [11][13], neurological [14][15], and radioprotective effects [16][17] of hydrogen. Several studies assessed the possibility and expediency of using hydrogen in oncological diseases [18][19][20]. On the other hand, cellular effects, which are fundamentally significant for regenerative medicine, are revealed only indirectly. In this regard, the purpose of this research is to systematize ideas about the nature, features, and mechanisms of the influence of H2 on cells, specifically stem cells.
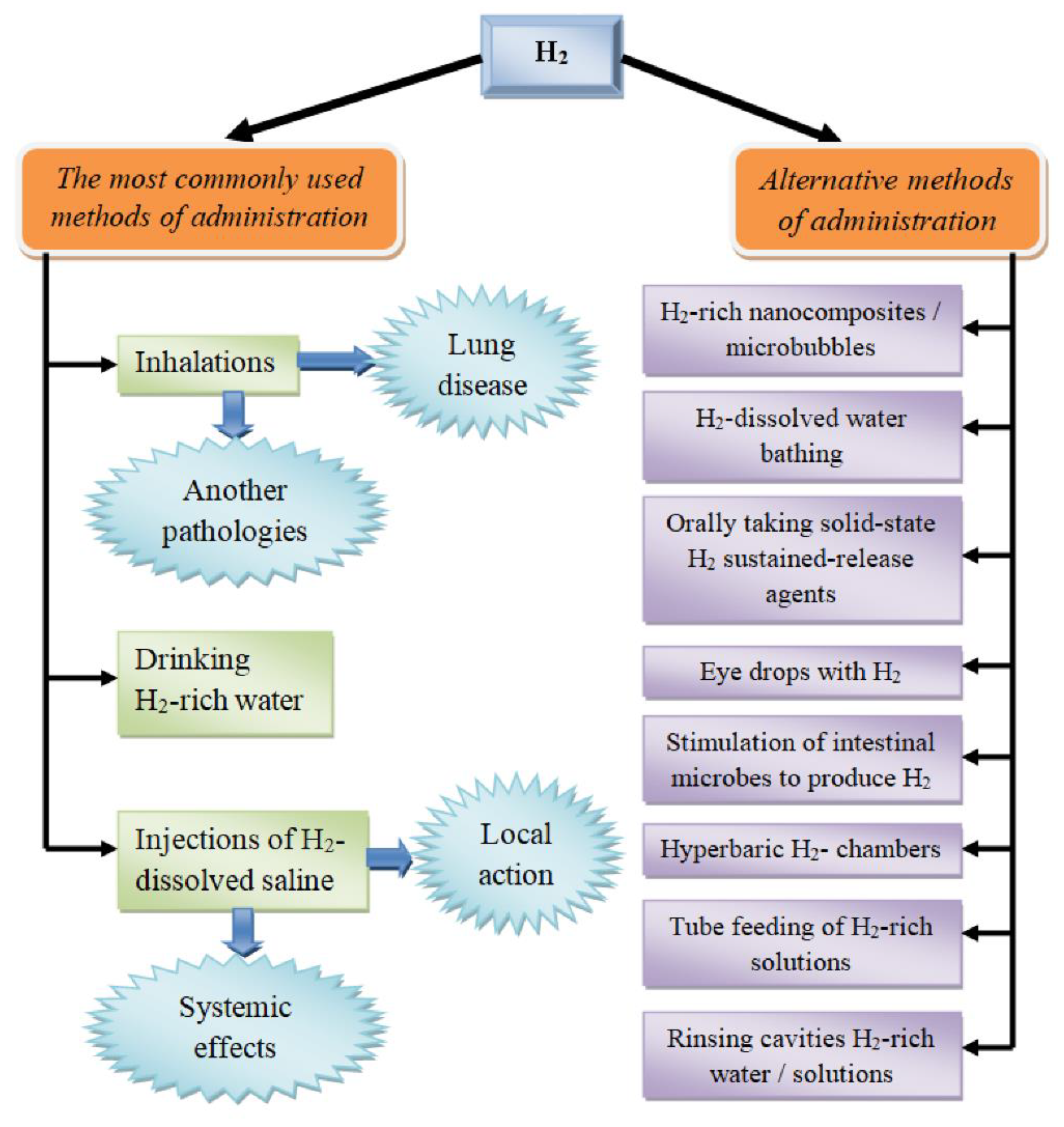
Figure 2. Routes of molecular hydrogen administration.
2. A Brief Outline of the History of the Discovery of Molecular Hydrogen as a Biological Agent and the Formation of Hydrogen Biomedicine
Hydrogen in free form was first experimentally obtained in 1671 by Robert Boyle [21] and identified as an independent chemical element in 1766 by Henry Cavendish [22]. Interestingly, by the end of the eighteenth century, Thomas Beddoes had completed the first documented attempt at the medical use of hydrogen for the treatment of patients with tuberculosis [23][24]. Until around 1969, the possibility of endogenous production of H2 in the human body from intestinal bacteria was not known [25][26].
One of the earliest direct uses of hydrogen was in deep sea diving. For example, Lanphier (1972) used Hydroliox (a mixture of hydrogen, helium, and oxygen) to prevent the development of decompression syndrome and nitrogen anesthesia in divers working at great depths [27]. One of the most famous early publications in this field belongs to Doyle et al. (1975), who showed a marked regression of squamous skin carcinoma in mice under the influence of H2 supplied under increased pressure (8 ATM) [26]. They used a gas mixture containing 97.5% hydrogen and 2.5% oxygen. Since that time, isolated reports have appeared on the effectiveness of the use of H2 for other applications [28][29]. However, an article in 2007 by Ohsawa et al. was the trigger for an rapid increase in the interest of specialists into the biological and medical effects of hydrogen [30]. The research presents the results of a successful treatment using inhalation of H2 to prevent the damage induced from ischemia–reperfusion after ischemic stroke in a rat model [30]. The researchers suggested that the main mechanism for achieving this clinical effect is the relief of oxidative stress induced by these pathological conditions.
Most recently, hydrogen therapy was included in the protocol for the management of patients with COVID-19 in China (Chinese Clinical Guidelines (7th edition) for COVID-19 Pneumonia Diagnosis and Treatment, issued by China National Health Commission). For this purpose, based on extensive experimental and clinical studies, it appears that inhalation of a mixture of 66.6% H2 and 33.3% oxygen significantly reduces the rate of deterioration of respiratory lung function in a new coronavirus infection, as well as the development of emphysema and inflammatory reactions in lung tissue in various acute and chronic diseases [31].
3. Routes of Introducing Molecular Hydrogen into the Body
Currently, the range of routes of introducing H2 into the body is extremely wide (Figure 2). First, it is important to emphasize that these methods differ not only in the convenience of application for a specific pathology (e.g., in the case of the treatment of dermatological diseases, hydrogen baths may be the preferred option), but also in the pharmacokinetics of the molecule, which alters its pharmacological activity [32].
Historically, the first way of introducing hydrogen is the use of hyperbaric chambers with an atmosphere rich in hydrogen gas [26]. Despite the encouraging results obtained in the experiments by Dole et al. (1975), work using such a technology was not continued, which may be due to the difficulties of its practical implementation [26].
The most common options for molecular hydrogen therapy are the inhalation of H2-containing gas mixtures of various compositions, the use of hydrogen-saturated water, and the infusion/injection of a sodium chloride solution saturated with H2 (Figure 2) [12]. Each of these pathways has its own characteristics, advantages, and disadvantages, as well as possible different molecular mechanisms of action.
Most studies aimed at evaluating the effectiveness of the use of H2 have used hydrogen-saturated solutions (i.e., hydrogen water, hydrogen saline, etc.). However, studies on the inhalation of hydrogen, especially in clinical use, are increasing [11][17][30][31]. Inhalation of H2 is a fairly simple way of exposure for both laboratory animals and humans. Indeed, this was the method employed by Ohsawa et al. using the rat model of ischemia–reperfusion [30]. In addition, an important advantage of the technology is the possibility of the strict dosing of hydrogen by regulating the exposure time and concentration of H2 in the gas mixture [32][33]. On the other hand, molecular hydrogen is a combustible and explosive gas in the case of its reaction with oxygen. It is assumed that the risk of such a negative effect is quite high when the concentration of H2 in the gas mixture is higher than 4% [17][32][33]. Nevertheless, in some cases, gas mixtures with a high hydrogen content are used, but with special safety requirements. For example, 66.67% H2 and 33.33% O2 is used in the treatment of patients with COVID-19, which is the same hydrogen therapy protocol that has been introduced in China [34]. The effectiveness of H2 inhalation in chronic obstructive pulmonary disease [35][36] and severe bronchial asthma [37] has also been reported. The expediency of such an approach is associated with the variable dose dependence of the antioxidant and anti-inflammatory properties of H2 [32][38].
Considering the physicochemical characteristics and the extremely small size and molecular weight of H2, hydrogen inhalation has ample opportunities for systemic action. Since it easily diffuses through the walls of the alveoli, hydrogen diffuses into the blood plasma and is transported to various organs and tissues. Experimental studies by Cole et al. (2021) have shown that in healthy animals, inhalation of a gas mixture containing 2.4% hydrogen continuously for 72 h does not cause any changes in physiological parameters [39]. At the same time, sanogenetic effects on various organs and tissues have been demonstrated in numerous studies [31][32][33][34][35][36][37].
The most convenient way of introducing molecular hydrogen in clinical practice is drinking water saturated with molecular hydrogen; this option eliminates the explosion and fire hazard of the therapy and ensures its portability, opening up the possibility for the widespread use of H2-containing water. However, this path also has disadvantages associated with low gas solubility [32]. It is known that the saturation of dissolved hydrogen is 0.78 mM (1.57 mg/L) at normal atmospheric pressure and room temperature [40]. This circumstance may be significant since it does not always allow achieving the necessary dose of the molecule to ensure a full clinical effect. In addition, when using this route, it should be considered that the prepared hydrogen water should be applied immediately, since it has a very short period of maintaining the concentration of H2. Moreover, upon ingestion of hydrogen water, a significant amount (>90%) is lost via normal expiration [41]. This at least indicates the high uptake of H2 to pass through the gastrointestinal tract and into the venous system where it reaches the lungs and is exhaled. At the same time, the distribution of hydrogen in various tissues and organs after drinking hydrogen water is not the same. In particular, the penetration of H2 into brain cells when using the route of administration under consideration is minimal [42], which may be of fundamental importance for determining indications for its clinical use.
These above reasons necessitated the search for alternative ways of delivering H2 to tissues, including the creation of nanocomposites with a delayed release of gas [43]. It was assumed that these nanocomposites would be included in the oral tablet form, which would ensure maximum compliance for patients. Such targeted H2 therapy can be carried out using hybrid palladium nanocrystals. This approach was tested in experimental conditions in a model of oncopathology, which allowed not only the confirmation of its anticarcinogenic activity, but also its protection of unchanged cells against hyperthermia [44], as well as the manifestation of oxidative stress and damage induced by ischemia and reperfusion [43][45]. The possibility of using various elements as a basis for hydrogen-releasing nanocomposites (in particular, silicon particles [43]) should be emphasized.
A technology fundamentally similar to the creation of nanocrystals as carriers of H2 is the use of microbubble systems (Figure 2). In recent years, it has been suggested that a specific delivery of hydrogen using microbubbles provides maximum bioavailability and a minimization of “transport losses” of the molecule [46]. An important advantage of this pathway is the possibility of introducing significantly higher amounts of gas compared to the intake of water that has been saturated with H2. The effectiveness of the method was demonstrated in a model of ischemic myocardial injury in rats [46].
The third main route of introducing H2 into the body is the use of injections and infusions of an H2-saturated isotonic saline solution [17][32][33]. The specified path also has advantages and disadvantages. For one, this path allows the dosing of the injected amount of hydrogen with high accuracy, the use of different concentrations, an increase in the bioavailability of the agent to the target organ, and, if necessary, a carrying out of the topical effects on strictly defined areas of tissues (e.g., surface localization or areas of catheterization and injection). At the same time, injections of hydrogen solutions pose a certain risk of invasiveness and, consequently, infection, while also requiring the involvement of experienced medical personnel for manipulation. The administration of these solutions is mainly performed intravenously (in patients) or intraperitoneally (in experimental studies using laboratory animals) [17]. The clinical potential of this route of administration is not fully developed, as evidenced the rich experience of intravenous ozone therapy, also based on the effects of a medical gas [47][48][49].
Currently, the literature describes alternative routes of using molecular hydrogen, which use both its systemic and local action (Figure 2). In particular, an example of the effective administration of H2 in dermatology and cosmetology is the use of hydrogen baths for psoriasis [50] and liposuction [51]. A variant of H2 therapy close to this technology is the use of eye drops saturated with the gas in question for the treatment of ischemic lesions of the iris of the eye, as well as the suppression of apoptosis [52]. Stimulation of the endogenous synthesis of H2 by symbiotic microflora is of particular interest. It has been shown that oral administration of lactulose increases the synthesis of H2 by bacteria of the gastrointestinal tract [53]. Moreover, a hydrogen breathing test has been proposed to determine the state of the intestinal microflora by its ability to generate molecular hydrogen [54]. It is important to emphasize that hydrogen is synthesized by microorganisms together with other gases (for example, methane) [55]. At the same time, the amount of hydrogen produced is the result of the balance of activity of the H2-producing (hydrogenogenic) and H2-utilizing (hydrogenotrophic) intestinal microbes [56].
A few more exclusive H2 pathways should also be noted, including probe feeding with the inclusion of a solution saturated with H2 [57], introduction during hemodialysis [58], local treatment of the skin surface [59], the addition of hydrogen to the preservation medium for transplanted organs to prevent cold damage [60], and the washing of various body cavities.
As mentioned previously, different routes of administration differ not only in their proximity to the point of exposure, but also in their pharmacokinetics [32]. In particular, it was found that the concentration of hydrogen in the blood increases rapidly after inhalation, but 3 min after cessation, it decreases to 1/40 of the peak value [61]. At the same time, the amount of hydrogen in arterial blood during inhalation always exceeds that in venous blood, which may indicate the diffusion of the gas into the tissues [61]. It is also shown that the peak values during inhalation of H2 and the intake of hydrogen water are achieved at the same time (e.g., 10–30 min depending on concentration/dose). However, the duration that H2 remains in the body before returning to baseline lasts longer than 30 min [62]. It should be noted that the effect on molecular cascades (e.g., on the expression of NF-KB and other regulatory proteins in liver tissue) is more pronounced for hydrogen water, and the combination of its intake with H2 inhalation enhances the effect [62]. Interestingly, the tissue concentration of H2 was significantly higher and persisted for a longer time after inhalation compared to drinking hydrogen water [42]. Naturally, with intravenous administration of isotonic solutions saturated with hydrogen, the peak breath concentration of the molecule is reached as quickly as possible, within 1 min [10].
Thus, at present, there is a wide range of routes of introducing molecular hydrogen into the body, differing not only in the topical and physico–chemical parameters, but also in the pharmacokinetics of the action of the molecule.
References
- Zafonte, R.S.; Wang, L.; Arbelaez, C.A.; Dennison, R.; Teng, Y.D. Medical Gas Therapy for Tissue, Organ, and CNS Protection: A Systematic Review of Effects, Mechanisms, and Challenges. Adv. Sci. 2022, 9, 2104136.
- Teng, Y.D.; Wang, L.; Kabatas, S.; Ulrich, H.; Zafonte, R.D. Cancer Stem Cells or Tumor Survival Cells? Stem Cells Dev. 2018, 27, 1466–1478.
- Nazarov, E.I.; Khlusov, I.A.; Noda, M. Homeostatic and endocrine responses as the basis for systemic therapy with medical gases: Ozone, xenon and molecular hydrogen. Med. Gas Res. 2021, 11, 174–186.
- Vanin, A.F. Physico-Chemistry of Dinitrosyl Iron Complexes as a Determinant of Their Biological Activity. Int. J. Mol. Sci. 2021, 22, 10356.
- Olas, B. Gasomediators (·NO, CO, and H₂S) and their role in hemostasis and thrombosis. Clin. Chim. Acta 2015, 445, 115–121.
- Dickinson, R.; Franks, N.P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit. Care 2010, 14, 229.
- Deng, R.M.; Li, H.Y.; Li, X.; Shen, H.T.; Wu, D.G.; Wang, Z.; Chen, G. Neuroprotective effect of helium after neonatal hypoxic ischemia: A narrative review. Med. Gas Res. 2021, 11, 121–123.
- Winkler, D.A.; Thornton, A.; Farjot, G.; Katz, I. The diverse biological properties of the chemically inert noble gases. Pharmacol. Ther. 2016, 160, 44–64.
- Martusevich, A.; Surovegina, A.; Popovicheva, A.; Didenko, N.; Artamonov, M.; Nazarov, V. Some Beneficial Effects of Inert Gases on Blood Oxidative Metabolism: In Vivo Study. BioMed Res. Int. 2022, 2022, 5857979.
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen–comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 1–21.
- Shen, M.; Zhang, H.; Yu, C.; Wang, F.; Sun, X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med. Gas Res. 2014, 4, 17.
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F. A “philosophical molecule,” hydrogen may overcome senescence and intractable diseases. Med. Gas Res. 2020, 10, 47–49.
- Tao, G.; Song, G.; Qin, S. Molecular hydrogen: Current knowledge on mechanism in alleviating free radical damage and diseases. Acta Biochim. Biophys. Sin. 2019, 51, 1189–1197.
- Wang, L.; Zhao, C.; Wu, S.; Xiao, G.; Zhuge, X.; Lei, P.; Xie, K. Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing miR-21 expression. Shock 2018, 50, 308–315.
- Wu, J.; Wang, R.; Yang, D.; Tang, W.; Chen, Z.; Sun, Q.; Liu, L.; Zang, R. Hydrogen postconditioning promotes survival of rat retinal ganglion cells against ischemia/reperfusion injury through the PI3K. Akt pathway. Biochem. Biophys. Res. Commun. 2018, 495, 2462–2468.
- Hirano, S.-I.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. Int. J. Mol. Sci. 2021, 22, 4566.
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomed. Pharmacother. 2020, 130, 110589.
- Runtuwene, J.; Amitani, H.; Amitani, M.; Asakawa, A.; Cheng, K.C.; Inui, K. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ 2015, 3, e859.
- Hirano, S.-I.; Yamamoto, H.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Novel Antitumor Agent: Possible Mechanisms Underlying Gene Expression. Int. J. Mol. Sci. 2021, 22, 8724.
- Wang, D.; Wang, L.; Zhang, Y.; Zhao, Y.; Chen, G. Hydrogen gas inhibits lung cancer progression through targeting SMC. Biomed. Pharmacol. 2018, 104, 788–797.
- Boyle, R. Tracts written by the honourable Robert Boyle Containing New Experiments Touching the Relation Betwixt Flame And Air: And About Explosions: An Hydrostatical Discourse Occasion’d by Some Objections of Dr. Henry More Against Some Explications of New Experiments Made by the Author of these Tracts: To Which is Annex’t, an Hydrostatical Letter, Dilucidating an Experiment about a Way of Weighing Water in Water; Printed for Richard Davis, Book-Seller in Oxon. 1672. Available online: https://quod.lib.umich.edu/e/eebo2/A29057.0001.001/1:21.1?rgn=div2;view=fulltext (accessed on 22 January 2023).
- Cavendish, H. XIX. Three papers, containing experiments on factitious air. Phil. Trans. R. Soc. 1766, 56, 141–184.
- Beddoes, T. A Letter to Erasmus Darwin, M.D. On A New Method of Treating Pulmonary Consumption, and Some Other Diseases Hitherto Found Incurable; Bulgin & Rosser: Bristol, UK, 1793.
- Beddoes, T. Considerations on the Medicinal Use, and on the Production of Factitious Airs. Ann. Med. 1796, 1, 245–265.
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127.
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154.
- Lanphier, E.H. Human respiration under increased pressures. Symp. Soc. Exp. Biol. 1972, 26, 379–394.
- van Haaster, D.J.; Hagedoorn, P.L.; Jongejan, J.A.; Hagen, W.R. On the relationship between affinity for molecular hydrogen and the physiological directionality of hydrogenases. Biochem. Soc. Trans. 2005, 33 Pt 1, 12–14.
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed Hydrogen-Saturated Water for Drinking Use Elicits an Antioxidative Effect: A Feeding Test with Rats. Biosci. Biotechnol. Biochem. 2005, 69, 1985–1987.
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694.
- Guan, W.J.; Chen, R.C.; Zhong, N.S. Strategies for the prevention and management of coronavirus disease 2019. Eur. Respir. J. 2020, 55, 2000597.
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507.
- Fu, Z.; Zhang, J. Molecular hydrogen is a promising therapeutic agent for pulmonary disease. J. Zhejiang Univ. Sci. B 2022, 23, 102–122.
- Guan, W.J.; Wei, C.H.; Chen, A.L.; Sun, X.C.; Guo, G.Y.; Zou, X.; Shi, J.D.; Lai, P.Z.; Zheng, Z.G.; Zhong, N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448–3452.
- Liu, X.; Ma, C.; Wang, X.; Wang, W.; Li, Z.; Wang, X.; Wang, P.; Sun, W.; Xue, B. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1309–1324.
- Zheng, Z.G.; Sun, W.Z.; Hu, J.Y.; Jie, Z.J.; Xu, J.F.; Cao, J.; Song, Y.L.; Wang, C.H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149.
- Huang, P.; Wei, S.; Huang, W.; Wu, P.; Chen, S.; Tao, A.; Wang, H.; Liang, Z.; Chen, R.; Yan, J.; et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int. Immunopharmacol. 2019, 74, 105646.
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076.
- Cole, A.R.; Sperotto, F.; DiNardo, J.A.; Carlisle, S.; Rivkin, M.J.; Sleeper, L.A.; Kheir, J.N. Safety of prolonged inhalation of hydrogen gas in air in healthy adults. Crit. Care Explor. 2021, 3, e543.
- Asada, R.; Tazawa, K.; Sato, S.; Miwa, N. Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes related markers. Med. Gas Res. 2020, 10, 114–121.
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv. Exp. Med. Biol. 2012, 737, 245–250.
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485.
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral administration of Si-based agent attenuates oxidative stress and ischemia-reperfusion injury in a rat model: A novel hydrogen administration method. Front. Med. 2020, 7, 95.
- Zhao, P.H.; Jin, Z.K.; Chen, Q.; Meng, J.; Lu, X.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241.
- Kou, Z.; Zhao, P.H.; Wang, Z.H.; Jin, Z.; Chen, L.; Su, B.L.; He, Q. Acid-responsive H2-releasing Fe nanoparticles for safe and effective cancer therapy. J. Mater. Chem. B 2019, 7, 2759–2765.
- He, Y.; Zhang, B.; Chen, Y.; Jin, Q.; Wu, J.; Yan, F. Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl. Mater. Interfaces 2017, 9, 21190–21199.
- Katiukhin, L.N. Influence of the course of treatment by injections of ozonized saline on rheological properties of erythrocytes in patients with complex pathology. Hum. Physiol. 2016, 42, 672–677.
- Martusevich, A.K.; Peretyagin, S.P.; Ruchin, M.V.; Struchkov, A.A. Ozone Therapy in Patients with Burn Disease. J. Biomed. Sci. Eng. 2018, 11, 27–35.
- Martínez-Sánchez, G.; Schwartz, A.; Di Donna, V. Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. Antioxidants 2020, 9, 389.
- Zhu, Q.; Wu, Y.; Li, Y.; Chen, Z.; Wang, L.; Xiong, H.; Dai, E.; Wu, J.; Fan, B.; Ping, L.; et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci. Rep. 2018, 8, 8051.
- Asada, R.; Saitoh, Y.; Miwa, N. Effects of hydrogen-rich water bath on visceral fat and skin blotch, with boiling-resistant hydrogen bubbles. Med. Gas Res. 2019, 9, 68–73.
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2010, 51, 487–492.
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic. Biol. Med. 2013, 65, 731–741.
- Zhang, M.; Xu, Y.; Zhang, J.; Sun, Z.; Ban, Y.; Wang, B.; Hou, X.; Cai, Y.; Li, J.; Wang, M.; et al. Application of methane and hydrogen-based breath test in the study of gestational diabetes mellitus and intestinal microbes. Diabetes Res. Clin. Pract. 2021, 176, 108818.
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol. Motil. 2012, 24, 185-e92.
- Ge, L.; Qi, J.; Shao, B.; Ruan, Z.; Ren, Y.; Sui, S.; Wu, X.; Sun, X.; Liu, S.; Li, S.; et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022, 14, 2013764.
- Li, Q.; Kato, S.; Matsuoka, D.; Tanaka, H.; Miwa, N. Hydrogen water intake via tube- feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med. Gas Res. 2013, 3, 20.
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia-reperfusion injury by protecting mitochondrial function in rats. J. Surg. Res. 2014, 192, 564–572.
- Ostojic, S.M. Molecular Hydrogen in Sports Medicine: New Therapeutic Perspectives. Int. J. Sports Med. 2015, 36, 273–279.
- Noda, K.; Shigemura, N.; Tanaka, Y.; Kawamura, T.; Hyun Lim, S.; Kokubo, K. A novel method of preserving cardiac grafts using a hydrogen-rich water bath. J. Heart Lung Transpl. 2013, 32, 241–250.
- Sano, M.; Ichihara, G.; Katsumata, Y.; Hiraide, T.; Hirai, A.; Momoi, M. Pharmacokinetics of a single inhalation of hydrogen gas in pigs. PLoS ONE 2020, 15, e0234626.
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Iwamoto, T. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell Biochem. 2015, 403, 231–241.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Revisions:
2 times
(View History)
Update Date:
10 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




