
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea Cattaneo | -- | 2843 | 2023-03-09 15:26:26 | | | |
| 2 | Lindsay Dong | Meta information modification | 2843 | 2023-03-13 01:50:50 | | |
Video Upload Options
Objective During the induction of gaseous anaesthesia, waste anaesthetic gases (WAGs) can be released into workplace air. Occupational exposure to high levels of halogenated WAGs may lead to adverse health effects; hence, it is important to measure WAGs concentration levels to perform risk assessment and for health protection purposes.
1. Introduction
2. Occupational Exposure to Halogenated Anaesthetic Gases in Hospitals
2.1. Halogenated WAGs and Concentrations Time Trends
In addition to being well-known chemical risk factors, halogenated WAGs are key and current stressors of climate change. The fact that desflurane, which has the highest global warming potential among WAGs, is less used than isoflurane and sevoflurane is in line with climate-smart anaesthesia care procedures. The reason behind the increasing use of sevoflurane should be sought in the different pharmacokinetic and toxicodynamic properties of halogenated anaesthetic gases. While patients recovering from anaesthesia feel less confused when desflurane is used, sevoflurane is useful when rapid inhaled induction of anaesthesia is needed [27]. Sevoflurane is also characterized by a low solubility in blood, allowing more precision in the control of anaesthesia and a rapid induction to awakening, an advantage in paediatric anaesthesia and general recovery from anaesthesia [28][29].
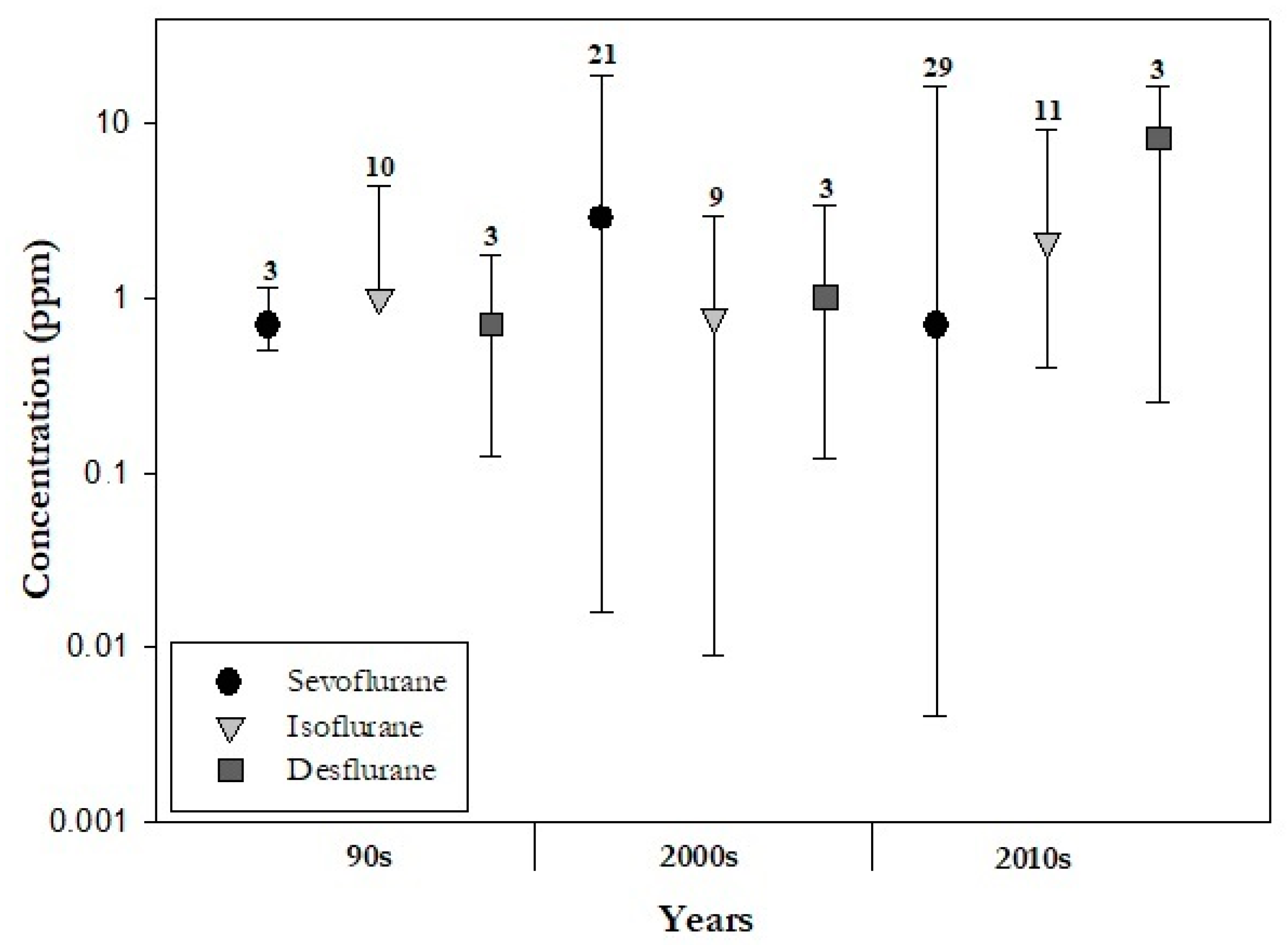
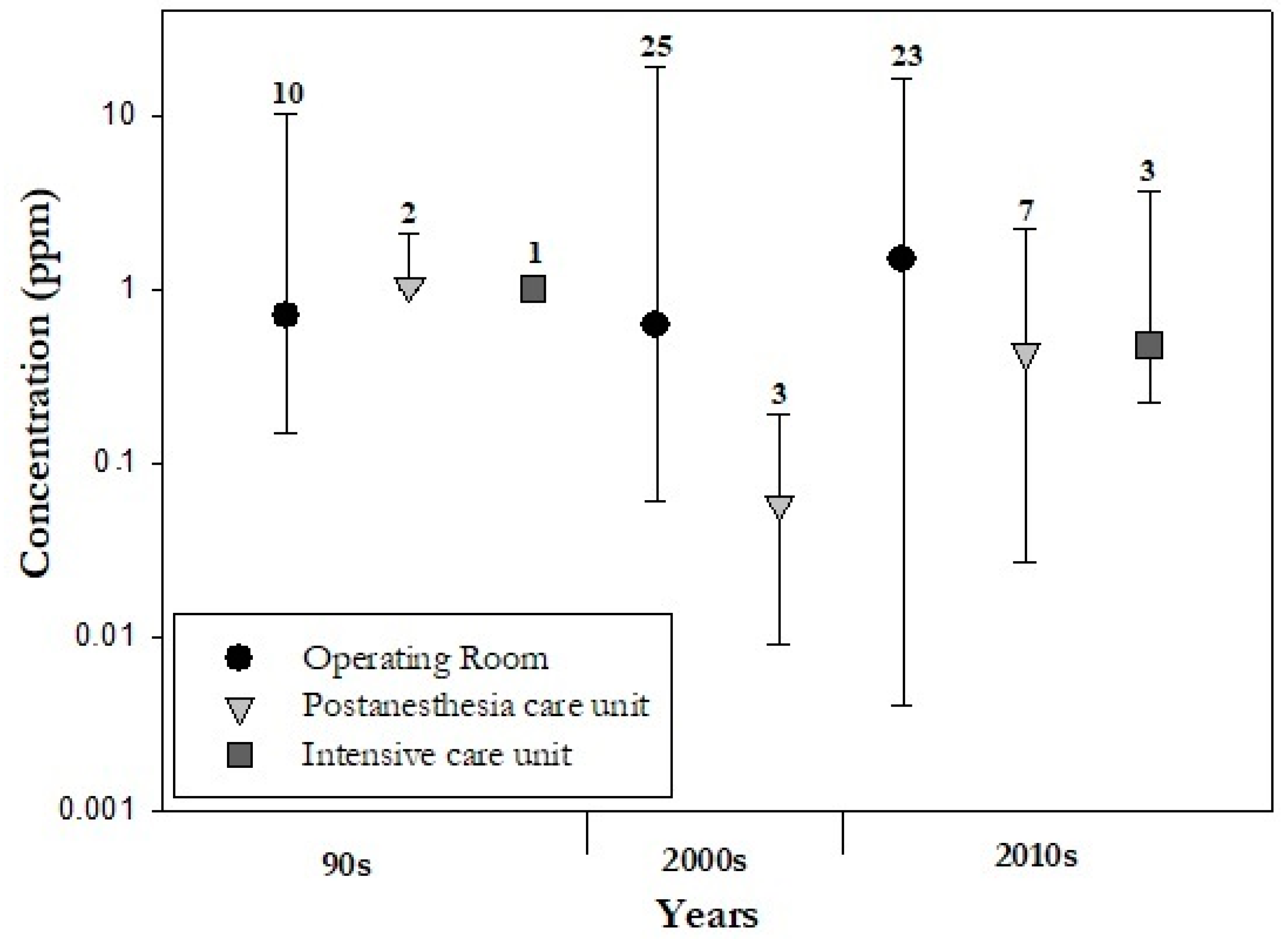
2.1.2. Air contamination by WAGs in Different Hospital Areas
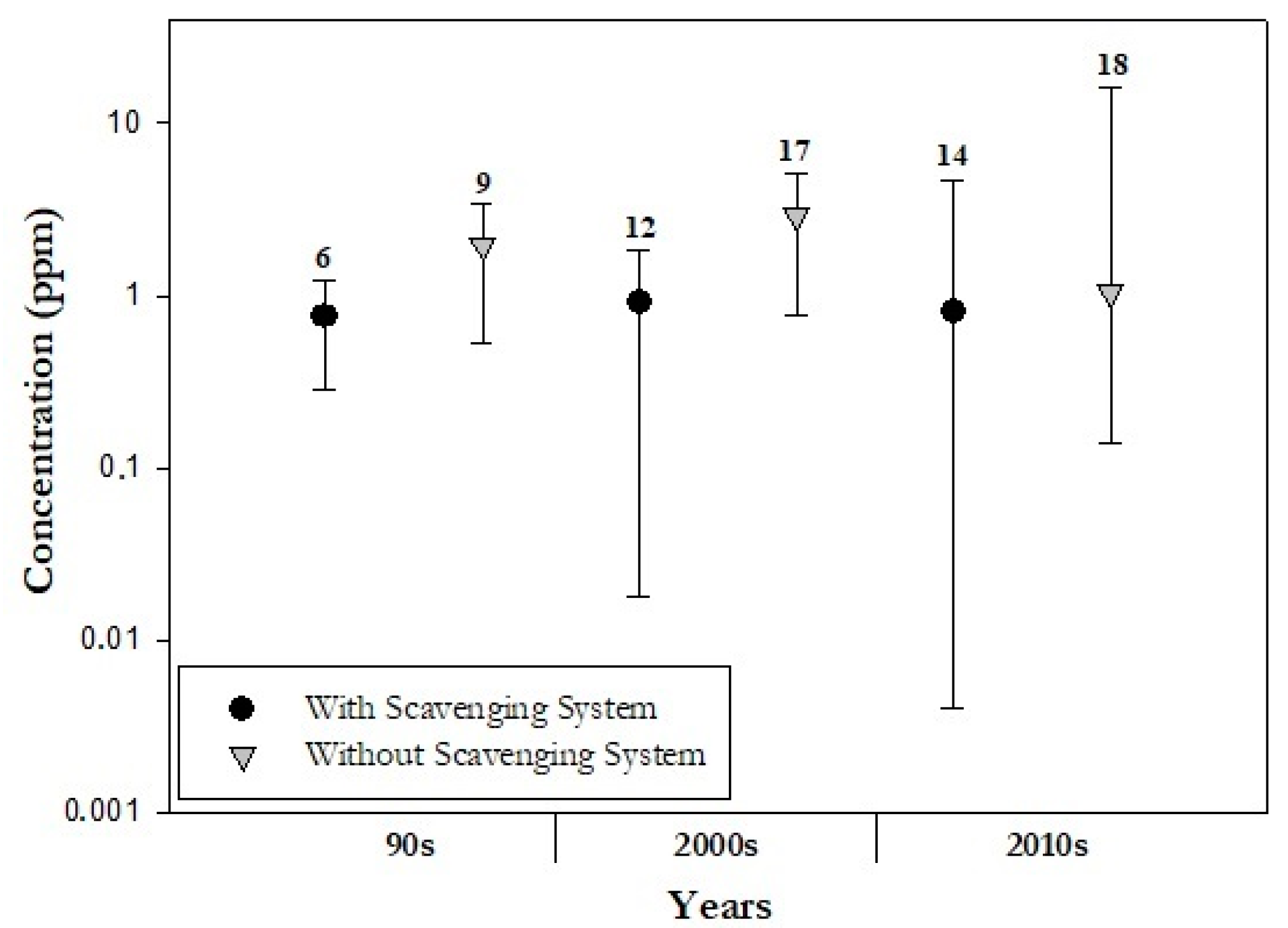
2.1.2. Mitigation of WAGs emissions
2.2. Types and Evolution in Monitoring Techniques
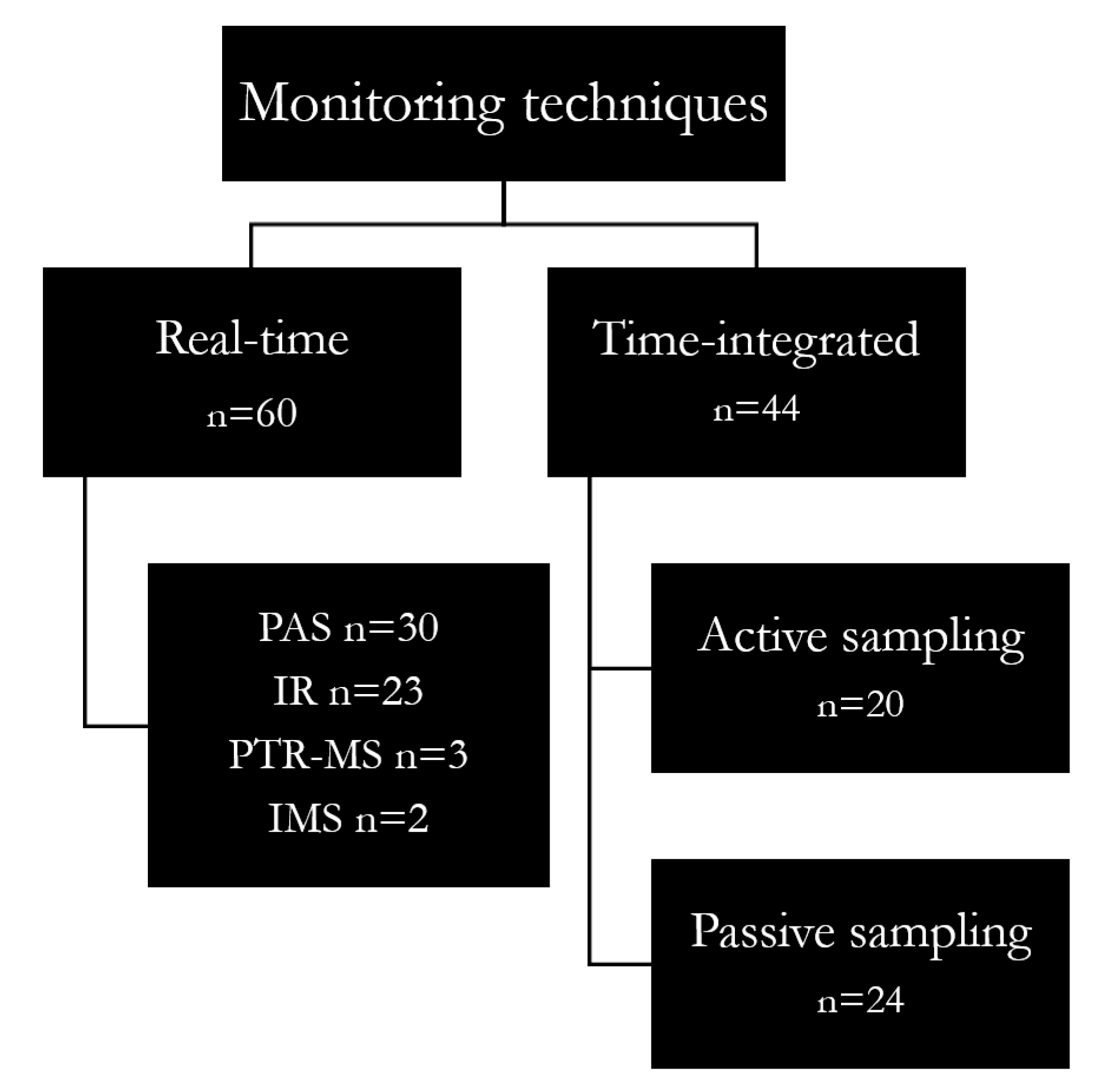
2.2.1. Real-Time Monitoring
2.2.2. Time-Integrated Sampling
2.2.3. Real-Time vs. Time-Integrated Monitoring
3. Conclusions
References
- Vutskits, L.; Xie, Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat. Rev. Neurosci. 2016, 32, 667–675.
- Herzog-Niescery, J.; Seipp, H.M.; Weber, T.P.; Bellgardt, M. Inhaled anesthetic agent sedation in the ICU and trace gas concentrations: A review. J. Clin. Monit. Comput. 2018, 32, 667–675.
- Özelsel, T.J.P.; Kim, S.; Buro, K.; Tsui, B. Elevated waste anaesthetic gas concentration in the paediatric postanaesthesia care unit. Turk Anesteziyoloji ve Reanimasyon Dern. Derg. 2018, 46, 362–366.
- Gustorff, B.; Lorenzl, N.; Aram, L.; Krenn, C.G.; Jobst, B.P.; Hoerauf, K.H. Environmental monitoring of sevoflurane and nitrous oxide using the cuffed oropharyngeal airway. Anesth. Analg. 2002, 94, 1244–1248.
- Li, S.-H.; Li, S.-N.; Shih, H.-Y.; Yi, H.-D.; Chiang, C.-Y. Personnel Exposure to Waste Sevoflurane and Nitrous Oxide during General Anesthesia with Cuffed Endotracheal Tube. Acta Anaesthesiol. Sin. 2002, 40, 185–190.
- Saber, A.T.; Hougaard, K.S. Isoflurane, Sevoflurane and Desflurane: The Nordic Expert Group for Criteria Documentation of Health Risks from Chemicals; University of Gothenburg: Gothenburg, Sweden, 2009; Volume 43, ISBN 9789185971169.
- Byhahn, C.; Heller, K.; Lischke, V.; Westphal, K. Surgeon’s Occupational Exposure to Nitrous Oxide and Sevoflurane during Pediatric Surgery. World J. Surg 2001, 25, 1109–1112.
- Herzog-Niescery, J.; Vogelsang, H.; Bellgardt, M.; Botteck, N.M.; Seipp, H.-M.M.; Bartz, H.; Weber, T.P.; Gude, P. The child’s behavior during inhalational induction and its impact on the anesthesiologist’s sevoflurane exposure. Paediatr. Anaesth. 2017, 27, 1247–1252.
- Hoerauf, K.H.; Wallner, T.; Akça, O.; Taslimi, R.; Sessler, D.I. Exposure to sevoflurane and nitrous oxide during four different methods of anesthetic induction. Anesth. Analg. 1999, 88, 925–929.
- Hoerauf, K.H.; Funk, W.; Harth, M.; Hobbhahn, J. Occupational exposure to sevoflurane, halothane and nitrous oxide during paediatric anaesthesia. Waste gas exposure during paediatric anaesthesia. Anaesthesia 1997, 52, 215–219.
- Lucio, L.M.C.; Braz, M.G.; Nascimento Junior, P.D.; Braz, J.R.C.; Braz, L.G. Occupational hazards, DNA damage, and oxidative stress on exposure to waste anesthetic gases. Braz. J. Anesthesiol. 2018, 68, 33–41.
- Zaffina, S.; Camisa, V.; Poscia, A.; Tucci, M.G.; Montaldi, V.; Cerabona, V.; Wahocka, M.; Moscato, U. Esposizione professionale al Sevoflurano nelle sale operatorie di pertinenza pediatrica: Il contributo del monitoraggio ambientale con tecnica multi-punto nella valutazione del rischio. G. Ital. Med. Lav. 2012, 24, 266–268.
- Oliveira, C.R.D. Occupational exposure to anesthetic gases residue. Rev. Bras. Anestesiol. 2009, 59, 110–124.
- CCOHS Waste Anesthetic Gases, Hazards of. Ccohs.Ca. 2007. Available online: http://www.ccohs.ca/oshanswers/chemicals/waste_anesthetic.html (accessed on 25 November 2022).
- Borm, P.J.A.; Kant, I.; Houben, G.; van Rijssen-Moll, M.; Henderson, P.T. Monitoring of Nitrous Oxide in Operating Rooms:Identification of Sources and Estimation of Occupational Exposure. J. Occup. Environ. Med. 1990, 32, 1112–1116.
- Da Costa, M.G.; Kalmar, A.F.; Struys, M.M.R.F. Inhaled Anesthetics: Environmental Role, Occupational Risk, and Clinical Use. J. Clin. Med. 2021, 10, 1306.
- Kanmura, Y. Causes of nitrous oxide contamination in operating rooms. Anesthesiology 1999, 90, 693–696.
- Krajewski, W.; Kucharska, M.; Wesolowski, W.; Stetkiewicz, J.; Wronska-Nofer, T. Occupational exposure to nitrous oxide—The role of scavenging and ventilation systems in reducing the exposure level in operating rooms. Int. J. Hyg. Environ. Health 2007, 210, 133–138.
- Cheung, S.K.; Özelsel, T.; Rashiq, S.; Tsui, B.C. Postoperative environmental anesthetic vapour concentrations following removal of the airway device in the operating room versus the posthanesthesia care unit. Can. J. Anesth. 2016, 63, 1016–1021.
- Sárkány, P.; Tankó, B.; Simon, É.; Gál, J.; Fülesdi, B.; Molnár, C. Does standing or sitting position of the anesthesiologist in the operating theatre influence sevoflurane exposure during craniotomies? BMC Anesthesiol. 2016, 16, 120.
- Scapellato, M.L.; Carrieri, M.; Maccà, I.; Salamon, F.; Trevisan, A.; Manno, M.; Bartolucci, G.B. Biomonitoring occupational sevoflurane exposure at low levels by urinary sevoflurane and hexafluoroisopropanol. Toxicol. Lett. 2014, 231, 154–160.
- Yılmaz, S.; Çalbayram, N.Ç. Exposure to anesthetic gases among operating room personnel and risk of genotoxicity: A systematic review of the human biomonitoring studies. J. Clin. Anesth. 2016, 35, 326–331.
- Deng, H.-B.; Li, F.-X.; Cai, Y.-H.; Xu, S.-Y. Waste anesthetic gas exposure and strategies for solution. J. Anesth. 2018, 32, 269–282.
- Blokker-Veldhuis, M.J.; Rutten, P.M.M.J.; De Hert, S.G. Occupational exposure to sevoflurane during cardiopulmonary bypass. Perfusion 2011, 26, 383–389.
- Casale, T.; Caciari, T.; Rosati, M.V.; Gioffrè, P.A.; Schifano, M.P.; Capozzella, A.; Pimpinella, B.; Tomei, G.; Tomei, F. Anesthetic gases and occupationally exposed workers. Environ. Toxicol. Pharmacol. 2014, 37, 267–274.
- Herzog-Niescery, J.; Botteck, N.M.; Vogelsang, H.; Gude, P.; Bartz, H.; Weber, T.P.; Seipp, H.M. Occupational chronic sevoflurane exposure in the everyday reality of the anesthesia workplace. Anesth. Analg. 2015, 121, 1519–1528.
- Sloan, M.H.; Conard, P.F.; Karsunky, P.K.; Gross, J.B. Sevoflurane Versus Isoflurane: Induction and Recovery Characeristics with Single-Breath Inhaled Inductions of Anesthesia. Anesth. Analg. 1996, 82, 528–532.
- Behne, M.; Wilke, H.-J.; Harder, S. Clinical Pharmacokinetics of Sevoflurane; Adis International: Auckland, New Zealand, 1999.
- Patel, S.S.; Goa, K.L. Desflurane: A review of its pharmacodynamic and pharmacokinetic properties and its efficacy in general anaesthesia. Drugs 1995, 50, 742–767.
- Hall, J.E.; Henderson, K.A.; Oldham, T.A.; Pugh, S.; Harmer, M. Environmental Monitoring during Gaseous Induction with Sevoflurane. Br. J. Anaesth. 1997, 79, 342–345.
- Henderson, K.A.; Matthews, I.P. An environmental survey of compliance with occupational exposure standards (OES) for anaesthetic gases. Anaesthesia 1999, 54, 941–947.
- Tanser, S.J.; Johnson, A. Evaluation of a new paediatric scavenging valve. Paediatr. Anaesth. 2002, 12, 448–450.
- Velzen, J.; Atkinson, S.; Rowley, E.; Martin, J.L. The Tradition of Anaesthetic Rooms: Best Practice or Patient Risk? Procedia Manuf. 2015, 3, 59–66.
- Elmer, J. An Analytical Comparison of Isoflurane Levels in Veterinary Operating Theaters. 2010. Available online: http://idea.library.drexel.edu/handle/1860/3581 (accessed on 25 November 2022).
- Haisch, C. Photoacoustic spectroscopy for analytical measurements. Meas. Sci. Technol. 2011, 23, 12001.
- Christensen, J. Technical Review—Optical filters and their Use with the Type 1302 & Type 1306 Photoacoustic Gas Monitors. Tech. Rev. 1990, 2, 1–20.
- Herzog-Niescery, J.; Gude, P.; Gahlen, F.; Seipp, H.-M.; Bartz, H.; Botteck, N.M.; Bellgardt, M.; Dazert, S.; Weber, T.P.; Vogelsang, H. Surgeons’ exposure to sevoflurane during paediatric adenoidectomy: A comparison of three airway devices. Anaesthesia 2016, 71, 915–920.
- Palzer, S. Photoacoustic-Based Gas Sensing: A Review. Sensors 2020, 20, 2745.
- Hsu, C.-P.S. Infrared Spectroscopy. Handb. Instrum. Tech. Anal. Chem. 1997, 50, 466–476.
- Hansen, J.; Schaal, N.; Juarez, T.; Woodlee, C. Nitrous Oxide Exposure Among Dental Personnel and Comparison of Active and Passive Sampling Techniques. Ann. Work Expo. Health 2019, 63, 337–348.
- Zabiegała, B.; Kot-Wasik, A.; Urbanowicz, M.; Namieśnik, J. Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal. Bioanal. Chem. 2010, 396, 273–296.
- Jafari, A.; Bargeshadi, R.; Jafari, F.; Mohebbi, I.; Hajaghazadeh, M. Environmental and biological measurements of isoflurane and sevoflurane in operating room personnel. Int. Arch. Occup. Environ. Health 2018, 91, 349–359.
- Imbriani, M.; Ghittori, S.; Pezzagno, G. The Biological Monitoring of Inhalation Anaesthetics. G. Ital. Med. Lav. Ergon. 1998, 20, 44–49.
- Cope, K.A.; Merritt, W.T.; Krenzischek, D.A.; Schaefer, J.; Bukowski, J.; Foster, W.M.; Bernacki, E.; Dorman, T.; Risby, T.H. Phase II collaborative pilot study: Preliminary analysis of central neural effects from exposure to volatile anesthetics in the PACU. J. Perianesthesia Nurs. 2002, 17, 240–250.
- Prado, C.; Periago, F.; Ibarra, I.; Tortosa, J. Evaluation of isoflurane in air by thermal desorption-gas chromatography. J. Chromatogr. A 1993, 657, 131–137.
- Sessler, D.I.; Badgwell, J.M. Exposure of postoperative nurses to exhaled anesthetic gases. Anesth. Analg. 1998, 87, 1083–1088.
- Herzog-Niescery, J.; Steffens, T.; Bellgardt, M.; Breuer-Kaiser, A.; Gude, P.; Vogelsang, H.; Weber, T.P.; Seipp, H.M. Photoacoustic gas monitoring for anesthetic gas pollution measurements and its cross-sensitivity to alcoholic disinfectants. BMC Anesthesiol. 2019, 19, 148.
- Kaljurand, M.; Gorbatšova, J.; Mazina-Šinkar, J. A gas chromatograph for citizen science. Microchem. J. 2021, 165, 106195.
- Ritzu, S.; Boccalon, P.; Sanchez, M.A.; Arcangeli, G.; Capelli, V. Esposizione a gas anestetici: Risultati di 13 anni di monitoraggio ambientale e biologico in un’ azienda ospedaliera. G. Ital. Med. Lav. 2007, 29, 411.
- Virgili, A.; Scapellato, M.L.; Maccà, I.; Perini, M.; Carrieri, M.; Gori, G.; Saia, B.; Bartolucci, G.B. Esposizione professionale a gas anestetici in alcuni ospedali del Veneto. G. Ital. Med. Lav. 2002, 24, 447–450.
- Sackey, P.V.; Martling, C.R.; Nise, G.; Radell, P.J. Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the Anesthetic Conserving Device. Crit. Care Med. 2005, 33, 585–590.
- Fanti, G.; Borghi, F.; Spinazzè, A.; Rovelli, S.; Campagnolo, D.; Keller, M.; Cattaneo, A.; Cauda, E.; Cavallo, D.M. Features and practicability of the next-generation sensors and monitors for exposure assessment to airborne pollutants: A systematic review. Sensors 2021, 21, 4513.
- EN 482:2021; Workplace Exposure—Procedures for the Determination of the Concentration of Chemical Agents—Basic Performance Requirements. CEN-European Committee for Standardization: Brussels, Belgium, 2021; pp. 20–21.




