
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro Giordano | -- | 1374 | 2023-03-09 09:41:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 1374 | 2023-03-10 10:07:51 | | |
Video Upload Options
The implant of the first IPP in 1973, performed by Branteley Scott was a turning point in the history of penile prosthesis, revolutionizing the treatment of erectile dysfunction (ED). Since then, the idea of an inflatable device has not changed much. However, the innovations in design, materials, surgical techniques, and perioperative management led to a more natural, durable, and reliable device featuring fewer complications and greater patient satisfaction. Currently, IPP is associated with high patient satisfaction and excellent long-term outcomes, remaining the gold standard for men with refractory ED.
1. The Advent of Penile Prosthesis Implants
2. Penile Prosthesis
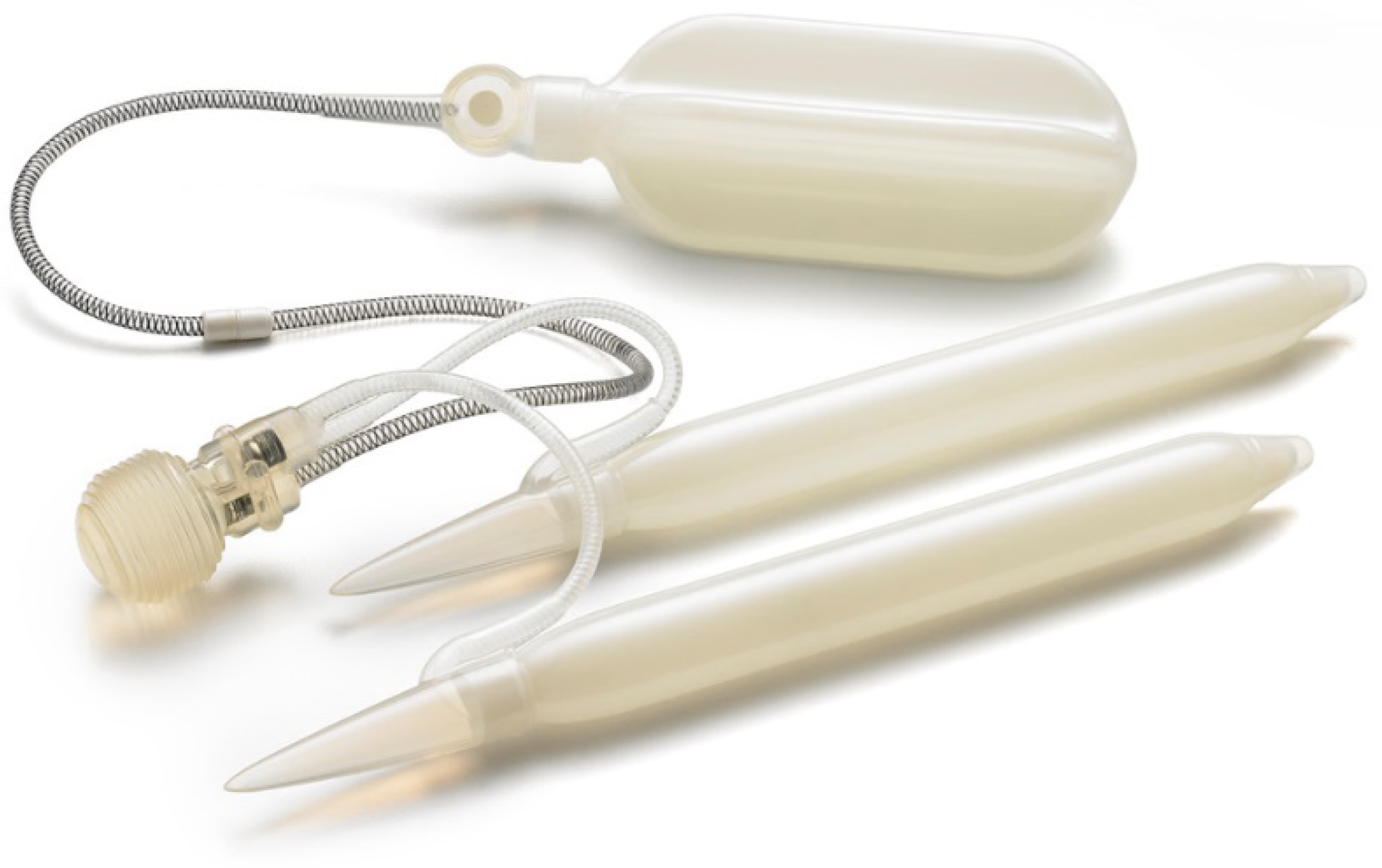

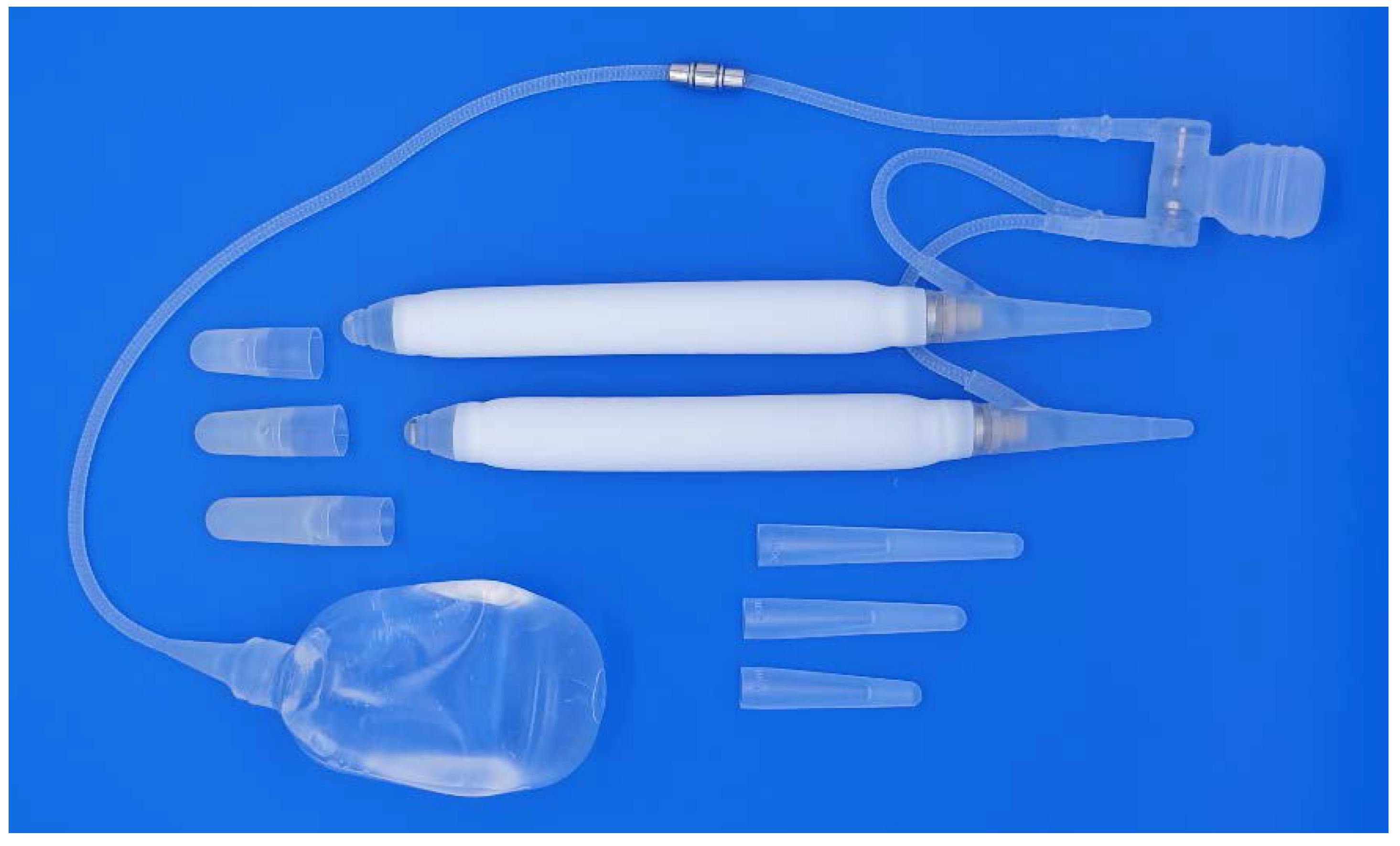
2.1. Infection Rate and Antibiotic Prophylaxis
Infection is the most significant complication following penile prosthesis implantation leading to postoperative morbidity, increasing health care costs, and psychological stress for the patient. Over 80% of post-surgical infections are caused by gram-positive bacteria such as Staphylococcus epidermidis, with the remaining usually caused by gram-negative bacteria such as Escherichia coli, Serratia, and Proteus mirabilis. More recently, infection sources have shifted to a larger proportion of gram-negative bacteria and fungi [19]. Prosthetic materials attract bacterial seeding during the time of surgery both through direct inoculation and hematogenous or lymphatic spread [20]. Once colonized, bacteria initiate the formation of a glycocalyx biofilm, a multi-layered bacterial microenvironment that prevents antibiotics from getting inside and that often determines the need for device explant.
Several cautions have been adopted to decrease the risk of infection: treating urinary or other site infections before surgery, preoperative night cleaning, preoperative washing with an antiseptic solution, intraoperative antimicrobial washing, and preventing unnecessary traffic into the operation room and “no-touch” technique [21].
Antibiotic and hydrophilic coatings of prostheses have been developed to reduce the risk of infection. The antibiotic administration to the patient before and after the surgery is equally important.
2.2. Patients Satisfaction and Reliability
Besides functional and surgical outcomes, patient satisfaction was a frequent research subject. Patient satisfaction rates are generally very high, in most cases above 80%. The differences observed are mainly related to the brand of penile prosthesis and the device implanted, inflatable or malleable. Several studies on patient satisfaction with malleable prostheses were conducted: the general satisfaction rates in retrospective surveys range from 69% to 86.6% [22][23][24][25].
When the implant of a two-piece IPP (Ambicor®) is indicated, the general patient satisfaction rate is high and varies from 80–96.4% across the studies [26][27][28][29].
References
- BOGORAS N Uber die volle plastische wiederherstellung eines zum Koitus fahigen Penis (Peni plastica totalis). Cent. Chir. 1936, 22, 1271–1276.
- Frumkin, A.P. Reconstruction of the male genitalia. Am Rev Sov. Med 1944, 2, 1944–1945.
- Bergman, R.T.; Howard, A.H.; Barnes, R.W. Plastic reconstruction of the penis. J. Urol. 1948, 59, 1174–1186.
- LOEFFLER, R.A.; SAYEGH, E.S. Perforated acrylic implants in management of organic impotence. J. Urol. 1960, 84, 559–561.
- Lash, H. Silicone implant for impotence. J. Urol. 1968, 100, 709–710.
- Carrion, H.; Martinez, D.; Parker, J.; Hakky, T.; Bickell, M.; Boyle, A.; Weigand, L.; Carrion, R. A History of the Penile Implant to 1974. Sex. Med. Rev. 2016, 4, 285–293.
- Small, M.P.; Carrion, H.M.; Gordon, J.A. Small-Carrion penile prosthesis. New implant for management of impotence. Urology 1975, 5, 479–486.
- Small, M.P. Small-Carrion penile prosthesis: A report on 160 cases and review of the literature. J. Urol. 1978, 119, 365–368.
- Brantley Scott, F.; Bradley, W.E.; Timm, G.W. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology 1973, 2, 80–82.
- Jonas, U.; Jacobi, G.H. Silicone-silver penile prosthesis: Description, operative approach and results. J. Urol. 1980, 123, 865–867.
- Sadeghi-Nejad, H. Penile prosthesis surgery: A review of prosthetic devices and associated complications. J. Sex. Med. 2007, 4, 296–309.
- Rodriguez, K.M.; Pastuszak, A.W. A history of penile implants. Transl. Androl. Urol. 2017, 6, S851–S857.
- Milbank, A.J.; Montague, D.K.; Angermeier, K.W.; Lakin, M.M.; Worley, S.E. Mechanical failure of the American Medical Systems Ultrex inflatable penile prosthesis: Before and after 1993 structural modification. J. Urol. 2002, 167, 2502–2506.
- Carson, C.C. Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J. Urol. 2004, 171, 1611–1614.
- Wolter, C.E.; Hellstrom, W.J.G. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J. Sex. Med. 2004, 1, 221–224.
- Hakky, T.; Lentz, A.; Sadeghi-Nejad, H.; Khera, M. The Evolution of the Inflatable Penile Prosthesis Reservoir and Surgical Placement. J. Sex. Med. 2015, 12, 464–467.
- Infla10® NB Inflatable Penile Prosthesis. Designed for Compromised Anatomies. Available online: https://www.rigicon.com/infla10-nb/ (accessed on 27 October 2022).
- ZSI 475 FTM. Available online: https://zephyr-surgical-implants.webflow.io/products/zsi-475-ftm/zsi-475-ftm (accessed on 27 October 2022).
- Wein, A.J.; Kavoussi, L.R.; Partin, A.W. Surgery for erectile dysfunction. In Campbell-Walsh Urology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 709–721.
- Carson, C.C.; Robertson, C.N. Late hematogenous infection of penile prostheses. J. Urol. 1988, 139, 50–52.
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile Dysfunction: AUA Guideline. J. Urol. 2018, 200, 633–641.
- Chiang, H.S.; Wu, C.C.; Wen, T.C. 10 years of experience with penile prosthesis implantation in Taiwanese patients. J. Urol. 2000, 163, 476–480.
- Salama, N. Satisfaction with the malleable penile prosthesis among couples from the Middle East--is it different from that reported elsewhere? Int. J. Impot. Res. 2004, 16, 175–180.
- Minervini, A.; Ralph, D.J.; Pryor, J.P. Outcome of penile prosthesis implantation for treating erectile dysfunction: Experience with 504 procedures. BJU Int. 2006, 97, 129–133.
- Falcone, M.; Rolle, L.; Ceruti, C.; Timpano, M.; Sedigh, O.; Preto, M.; Gonella, A.; Frea, B. Prospective analysis of the surgical outcomes and patients’ satisfaction rate after the AMS Spectra penile prosthesis implantation. Urology 2013, 82, 373–376.
- Lux, M.; Reyes-Vallejo, L.; Morgentaler, A.; Levine, L.A. Outcomes and satisfaction rates for the redesigned 2-piece penile prosthesis. J. Urol. 2007, 177, 262–266.
- Gentile, G.; Franceschelli, A.; Massenio, P.; Tuccio, A.; Cocci, A.; Divenuto, L.; Romagnoli, D.; Natali, A.; Vitarelli, A.; Cormio, L.; et al. Patient’s satisfaction after 2-piece inflatable penile prosthesis implantation: An Italian multicentric study. Arch. Ital. di Urol. Androl. organo Uff. Soc. Ital. di Ecogr. Urol. e Nefrol. 2016, 88, 1–3.
- Levine, L.A.; Estrada, C.R.; Morgentaler, A. Mechanical reliability and safety of, and patient satisfaction with the Ambicor inflatable penile prosthesis: Results of a 2 center study. J. Urol. 2001, 166, 932–937.
- Natali, A.; Olianas, R.; Fisch, M. Penile implantation in Europe: Successes and complications with 253 implants in Italy and Germany. J. Sex. Med. 2008, 5, 1503–1512.




