Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanni Nicola Roviello | -- | 2244 | 2023-03-08 18:27:12 | | | |
| 2 | Catherine Yang | -13 word(s) | 2231 | 2023-03-09 01:39:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Roviello, G.N.; Pedraza-Chaverri, J. Mitochondrial Therapy in HPV-Related Cancers. Encyclopedia. Available online: https://encyclopedia.pub/entry/41997 (accessed on 07 February 2026).
Cruz-Gregorio A, Aranda-Rivera AK, Roviello GN, Pedraza-Chaverri J. Mitochondrial Therapy in HPV-Related Cancers. Encyclopedia. Available at: https://encyclopedia.pub/entry/41997. Accessed February 07, 2026.
Cruz-Gregorio, Alfredo, Ana Karina Aranda-Rivera, Giovanni N. Roviello, José Pedraza-Chaverri. "Mitochondrial Therapy in HPV-Related Cancers" Encyclopedia, https://encyclopedia.pub/entry/41997 (accessed February 07, 2026).
Cruz-Gregorio, A., Aranda-Rivera, A.K., Roviello, G.N., & Pedraza-Chaverri, J. (2023, March 08). Mitochondrial Therapy in HPV-Related Cancers. In Encyclopedia. https://encyclopedia.pub/entry/41997
Cruz-Gregorio, Alfredo, et al. "Mitochondrial Therapy in HPV-Related Cancers." Encyclopedia. Web. 08 March, 2023.
Copy Citation
The mitochondria are organelles targeted by the human papillomavirus (HPV), and HPV-related cancers depend on the host’s mitochondria for their development and progression. On the other hand, the mitochondria are also important during pharmacological treatment, such as chemotherapy, since they are key organelles for the increase in reactive oxygen species (ROS), which significantly increases cell death due to the presence of oxidative stress (OS). In this way, the mitochondria in HPV infection and in the development of HPV-related cancer could be targeted to reduce or eliminate HPV infections or HPV-related cancers.
HPV infection
HPV-related cancer
mitochondria
oxidative stress
mitochondria therapy
1. Introduction
According to the World Health Organization (WHO), cervical cancer is the fourth leading cause of cancer death in women worldwide [1]. It has been established that persistent infection with high-risk human papillomavirus (HR-HPV) constitutes a key risk factor for the development of cervical cancer [2]. HR-HPV is also related to the induction of mucosal squamous epithelial malignancies of the penis, vulva, vagina, and oropharynx [3]. Although there are more than 200 types of HPVs, only HR-HPVs are able to induce cancer. The rest of the HPVs are low-risk HPV (LR-HPV), which are associated with skin warts and papillomatosis [4]. Among the HR-HPVs, viral types HR-HPV-16 and -18 are the most persistent [5]. These viruses are not enveloped viruses, with a capsid of approximately 55 nm [6]. The HPV capsid consists of the structural proteins L1 and L2 that house the viral genome [7], which is formed by a double-stranded circular deoxyribonucleic acid (DNA) of around 8,000 base pairs (bp) [8]. For study purposes, the HPV genome has been divided into three regions: (1) the early region (E: early) that encodes the genes involved in the replication of the viral genome and its maintenance (E1–E8); (2) the late region (L: late) that encodes the genes involved in the structural proteins of the capsid, namely L1 and L2; and (3) the long control region (LCR) that contains regulatory sites for transcription and replication of the HPV genome [9]. In cervical cancer, HPV’s life cycle begins when HPV infects the cells of the basal layer of the squamous epithelium of the cervix. HPV typically reaches these cells through wounds present in the epithelial layer, allowing the HPV L1 protein (Figure 1) to bind to the heparan sulfate proteoglycan receptors, which initiates the infection. It should be noted that the L1 of HR-HPV Type 16 forms a pentamer that is able to interact with the heparin oligosaccharides through the basic Lys-54, Lys-59, Lys-278, Lys-356, and Lys-361 and the polar residues Asn-57, Gln-194, and Thr-358 [10].
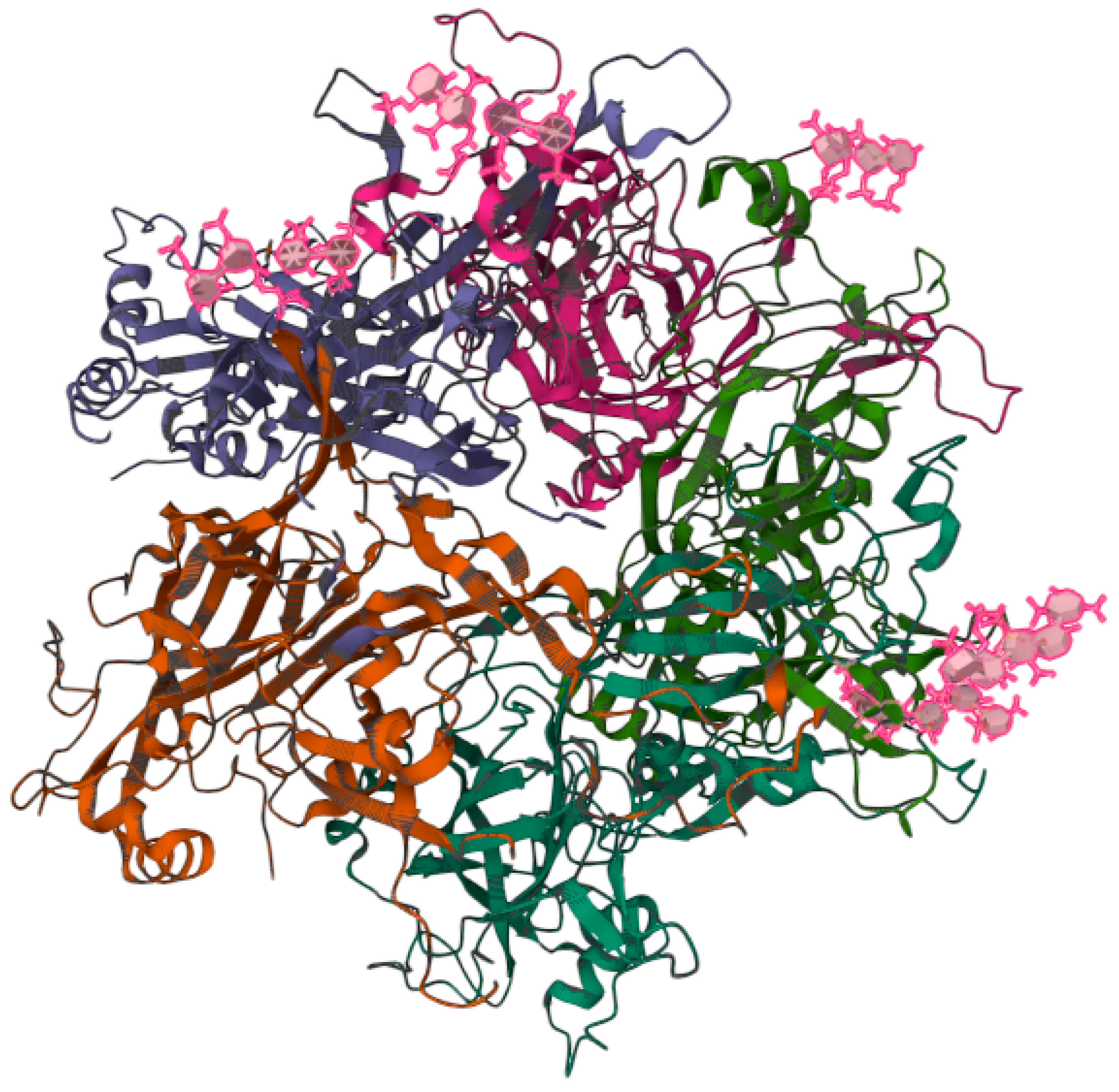
Figure 1. Three-dimensional structure of the heparin-bound pentamer of the L1 protein from human papillomavirus (HPV) Type 16. The image is publicly accessible at the link https://www.rcsb.org/3d-view/5W1O/1 (accessed on 6 February 2023) and corresponds to the structure with PDB ID 5W1O [10]. The viral capsid forms electrostatic and polar interactions with the anionic heparin fragments (pink) through the basic Lys-54, Lys-59, Lys-278, Lys-356, and Lys-361 and the polar residues Asn-57, Gln-194, and Thr-358. The interactions between HPV and the heparan sulfate oligosaccharide are of crucial importance, as they initiate the infection [10].
2. Mitochondrial Therapy in HPV-Related Cancers
HPV-related cancers display several degrees of alteration in the mitochondrial metabolism; however, all are dependent on the metabolism of the mitochondria, both for completing their energy metabolism and for avoiding cell death [11][12][13][14]. Because of these roles, the mitochondria are an attractive target for HPV-related cancer therapy, even at early stages such as during HPV infection. For instance, Zhain et al. [15] have shown that alterations in the mitochondrial DNA (mtDNA) are present in the framework of HPV infection. This group reported that the C150T polymorphism present in the mtDNA D-loop was related to HPV infection in patients with cervical cancer. Thus, people that have this polymorphism are more prone to HPV infections. Finding this polymorphism in the population gives an advantage against possible HPV infections, since people can be alerted about their propensity to be infected by HPV and develop HPV-related cancers. However, apart from diagnostics, the mitochondria may also be a therapeutic target in cancer. It was shown that in models of cancer such as HeLa, the development of cancer cell is highly glucose-dependent, and the use of rotenone (Figure 2), a strong inhibitor of Complex I ETS, induces the production of ROS, arrests growth, and leads to mitochondrial apoptosis [16]. Moreover, the cycle of HeLa cells was arrested at the G0/G1 phase, which was accompanied by the release of cyt c and the second activator of caspase (Smac)/direct IAP binding protein with low pI (DIABLO) from the mitochondria to the cytosol, triggering the activation of procaspase-9 and -3 and the induction of cleaved PARP under treatment with triphenyl tin (TPT)-benzimidazolethiol [17].
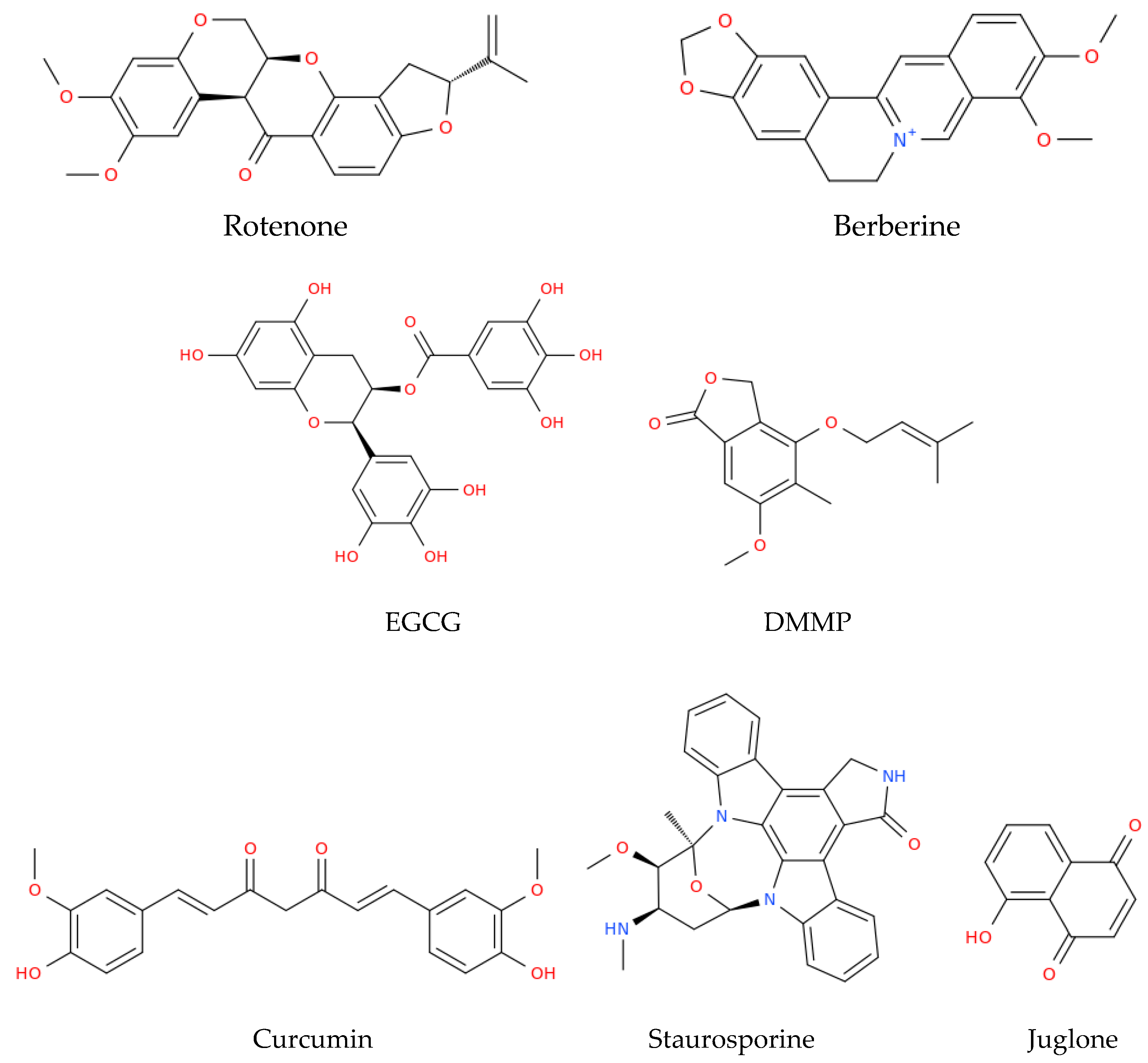
Figure 2. Structural representation of some of the main natural compounds that are able to target the mitochondria. EGCG, epigallocatechin gallate; DMMP, 4-(3′,3′-dimethylallyloxy)-5-methyl-6-methoxy-phthalide.
The latter compound was used to achieve the downregulation of E6 from HPV, which restored the expression of p53, inducing mitochondrial apoptosis [17] (Table 1). The cyano-derivative of 11-keto-β-boswellic acid, butyl 2-cyano-3, 11-dioxours-1,12-dien-24-oate (BCDD), also reduced the expression of E6 in the nucleus of HPV-18 HeLa cells, which promoted the accumulation of active p53 cells, followed by the inhibition of anti-apoptotic Bcl-2, the augmentation of Drp-1, and disruption of mitochondrial functions, ultimately causing mitochondrial apoptosis [18]. HeLa cells also underwent mitochondrial apoptosis when these cells were treated with berberine, a natural alkaloid derived from the medicinal plant Berberis vulgaris, which is endowed with different biological properties [19]. The treatment with berberine decreased the levels of E6 and E7, increasing the levels of p53 and pRB; this reduced the cells’ viability through the loss of ΔΨm, the activation of caspase-3, and the induction of cleaved PARP [20]. Moreover, in experiments conducted on HeLa cells, some polyphenols present in green and black tea, such as epigallocatechin gallate (EGCG) and theaflavins (TF), respectively, induced the production of ROS, the release of cyt c, and cleavage of caspase-9 and -3, triggering intrinsic apoptosis [21]. Moreover, 4-(3′,3′-dimethylallyloxy)-5-methyl-6-methoxy-phthalide (DMMP), an agent from the endophytic fungus Pestalotiopsis photiniae, also induced mitochondrial apoptosis in HeLa cells [22]. This potential antitumor compound arrested the cell cycle in the G1 phase, induced the loss of ΔΨm, enhanced p53 levels, and increased the mRNA expression of pro-apoptotic Bcl-2 family genes (PUMA, NOXA, Bax, Bad, and Bim) in order to promote mitochondrial apoptosis [22]. Curcumin, a phytopolylphenol isolated from Curcuma longa, also induced intrinsic apoptosis in cervical cancer cell lines through the release of cyt c and the activation of caspase-3 and -9 [23]. In line with the latter drug, some compounds, such as atovaquone, have also shown anticancer properties in cervical cancer cell lines and in vivo models. In particular, this antiprotozoal drug induced intrinsic apoptosis by inhibiting mitochondrial complex III both in vitro, in SiHa cells, and in vivo, in a cervical cancer xenograft mouse model [24]. On the other hand, the use of drugs such as staurosporine, a potent inhibitor of multiple protein kinases, decreased the expression of E6 and E7 oncoprotein in Caski and HeLa cervical tumor cells. This compound induced an increase in p53, the release of cyt c into the cytosol, and the activation of caspases-9 and -3, leading to PARP cleavage and apoptosis related to the mitochondria [25].
Table 1. Treatments (compounds or preparations) evaluated for targeting the mitochondria in HPV-related cancers.
| Mitochondria Targeting Treatment | Mitochondrial Proteins Involved | Regulation of HPV Oncoproteins | Effects | Ref. | |
|---|---|---|---|---|---|
| HeLa cells | Rotenone | ↑Bax | Not studied | ↑ROS, ↓ATP, cell cycle arrest, ↑caspase-3, resulting in mitochondrial apoptosis | [16] |
| HeLa cells | TPT-benzimidazolethiol | ↑Bax mRNA Cyt c and Smac/DIABLO release |
↓E6 oncoprotein | ↑p53, ↑caspase-3 and -9, G0/G1 cell cycle arrest, resulting in mitochondrial apoptosis | [17] |
| HeLa cells | Cyano derivative of 11-keto-β-boswellic acid, BCDD | ↑Drp1 ↑Bax ↓Bcl2 ↑Cyt c release |
↓E6 mRNA | ↑p53, resulting in mitochondrial apoptosis | [18] |
| SiHa and HeLa cells | Berberine | ↓ ΔΨm | ↓E6 and E7 oncoproteins | ↑Caspase-3, resulting in mitochondrial apoptosis and ↓cell viability |
[19] |
| HeLa cells | EGCG and TF | Cyt c release | Not studied | ↑ROS, ↑caspase-3 and -9, resulting in mitochondrial apoptosis | [21] |
| HeLa cells | DMMP | ↑PUMA, ↑NOXA, ↑Bax, ↑Bad, ↑Bim, ↓ ΔΨm | Not studied | Cell cycle arrest in G1, mitochondrial apoptosis | [22] |
| HeLa, SiHa cells and a xenograft mouse model | Curcumin | ↑Caspase-3 and -9 ↑Bax Cyt c release |
Not studied | Mitochondrial apoptosis | [23] |
| SiHa cells and a xenograft mouse model | Atovaquone | Inhibits Complex III, inhibits mitochondrial respiration | Not studied | ↓Cell viability | [24] |
| Caski and HeLa cells | Staurosporine | ↑ Cyt c release | ↓E6 and E7 oncoproteins | ↑p53, ↑caspase-3 and -9, resulting in mitochondrial apoptosis | [25] |
| CaSki cell lines | Juglone | ↑Bax ↓Bcl2 ↑ Cyt c release |
Not studied | Cell cycle arrest in G2/M, ↑caspase-3, resulting in mitochondrial apoptosis | [26] |
| ME-180 and SiHa cell lines | Chloroform extract of Rasagenthi mezhugu | ↓ ΔΨm | Not studied | Mitochondrial apoptosis | [27] |
| HeLa, and CaSki cell lines | LEF of Phyllanthus amarus | ↑Bax ↓Bcl2 ↓ ΔΨm |
Not studied | ↑ROS, ↓E6, ↑p53, resulting in mitochondrial apoptosis | [28] |
| HeLa and CaSki cells | Lipid derived from Pinellia pedatisecta | ↑ Bax | ↓E6 mRNA | ↑p53, ↑caspase-3, resulting in mitochondrial apoptosis | [29] |
| HNSCC HPV(+) cells | Fenretinide | ↑NOXA | Not studied | Mitochondrial apoptosis | [30] |
| HNSCC HPV(+) cells | 4-HPR | ↑Caspase-3 |
Not studied | ↑ROS, MTP, resulting in mitochondrial apoptosis | [31] |
Abbreviations: Bax, Bcl-2-associated X protein; ROS, reactive oxygen species; ATP, adenosine triphosphate; TPT, triphenyl tin; Smac, second mitochondria derived activator of caspase; DIABLO, direct IAP binding protein with low pI; Drp1, dynamin-related protein 1; Bax, Bcl-2-associated X protein; Bcl2, B cell lymphoma; ΔΨm, mitochondrial membrane potential; LEF, lignan enriched fraction; EGCG, (−)-epigallocatechin gallate; TF, theaflavins; DMMP, 4-(3′,3′-dimethylallyloxy)-5-methyl-6-methoxy-phthalide; PUMA, p53 upregulated modulator of apoptosis; 4-HPR, N-(4-hydroxyphenyl)retinamide; MTP, mitochondrial membrane permeability transition; mRNA, messenger RNA; BCDD, butyl 2-cyano-3, 11-dioxours-1,12-dien-24-oate; cyt c, cytochrome c; HNSCC, head and neck squamous cell carcinoma. ↑: increase, ↓: decrease.
Juglone, an antioxidant extracted from the roots, leaves, nut hulls, wood, and bark of Juglans mandshurica, was able to decrease the levels of Bcl2 and increased Bax, causing the release of cyt c from the mitochondria, inducing mitochondrial apoptosis and cell cycle arrest at the G2/M phase in CaSki cell lines [26]. Similar effects were observed with a chloroform extract of Rasagenthi Mezhugu (RM), a formulation from traditional Asian medicine which caused the loss of ΔΨm and the accumulation of apoptotic bodies, indicating the induction of apoptosis [27]. A mitochondrial role of Phyllanthus amarus was also observed in HeLa, and CaSki cell lines. In these cells, the lignan-enriched fraction (LEF) triggered mitochondrial apoptosis mediated by the activation of p53, which induced an increase in Bax and a decrease in Bcl2. LEF also generated the production of ROS, which induced DNA damage and a decrease in ΔΨm. In addition, E6 decreased with treatment with LEF, suggesting that this product contains compounds with an affinity for this oncoprotein. Supporting the latter finding, the principal lignan of Phyllanthus amarus, extracted from the chloroform phase, showed an irreversible affinity for E6 according to an in silico analysis [28], which indicated that the inhibition of E6 is crucial for allowing apoptosis in cervical cancer cells. On the other hand, a lipid derived from Pinellia pedatisecta decreases the mRNA expression of E6 in HR-HPV-positive cervical cancer cell lines such as CaSki and HeLa cells, which was associated with an increase in both messenger RNA (mRNA) and protein levels of p53, Bax, and caspase-3, inducing mitochondrial apoptosis [29]. BCDD also downregulates the E6 mRNA of HR-HPV-18 from HeLa cells, inducing intrinsic apoptosis by restoring p53 and inducing the release of cyt c [18]. In other models of cancer, such as HNSCC HPV(+) or squamous cell carcinoma (SCC), it has been demonstrated that the mitochondria can be targeted to potentially reduce or eliminate these cancers. For instance, it has been found that in HNSCC HPV(+) cells, fenretinide (a retinoid derivative and an inducer of endoplasmic reticulum stress) induced the expression of the pro-apoptotic protein NOXA and led to mitochondrial apoptosis [30]. Moreover, in SCC, specifically in HPV-immortalized/v-Ha-Ras tumorigenic keratinocytes, the synthetic retinoid N-(4-hydroxyphenyl)retinamide (4-HPR) induced the production of ROS, mitochondrial membrane permeability transition (MPT), and apoptosis [31]. Thus, the different compounds reviewed above induce events such as the production of ROS, the activation of proapoptotic proteins, the deactivation of antiapoptotic proteins, the loss of Δψm, the release of cyt c, the activation of caspases, and, in general, lead to the activation of the different mitochondrial apoptosis pathways. This makes these compounds potential cancer drugs that target the mitochondria. However, further investigations are needed, as most of the studies have been conducted in cell models, while animal models would strengthen the present evidence of activity and would attract new experimental efforts in order to assay them regarding HPV-related therapy. HPV-related cancers, such as HNSCC HPV(+), could be targets for new biotherapies such as mitochondrial transplantation, in which functional mitochondria are transplanted into tumors with mitochondrial dysfunctions, activating mitochondrial apoptosis and having an antitumoral effect [32]. In HNSCC HPV(+) cancers, HPV proteins such as E6 induce mitochondrial dysfunction and may also decrease the mitochondrial apoptosis associated with the release of cytochrome c due to p53 decrease [11].
3. Conclusion
In summary, transplantation of functional mitochondria could increase the intrinsic mechanisms of apoptosis due to the presence of mitochondria with an apoptotic system under adequate conditions to induce cell death. Moreover, this therapy could work adequately in HPV-related cervical cancer, where the remaining p53 that is not degraded by E6 could be sufficient to induce mitochondrial apoptosis through the newly transplanted healthy mitochondria. However, this is something that has still to be studied and deserves further urgent exploration, as mitochondria transplants could be an efficacious therapy against HPV-related cancers.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Egawa, N.; Doorbar, J. The Low-Risk Papillomaviruses. Virus Res. 2017, 231, 119–127.
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 890–907.
- de Villiers, E.-M. Cross-Roads in the Classification of Papillomaviruses. Virology 2013, 445, 2–10.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human Papillomavirus (HPV) Infection; International Agency for Research on Cancer: Lyon, France, 2007.
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine 2012, 30, F55–F70.
- Zheng, Z.-M.; Baker, C.C. Papillomavirus genome structure, expression, and post-transcriptional regulation. Front. Biosci. 2006, 11, 2286–2302.
- Dasgupta, J.; Bienkowska-Haba, M.; Ortega, M.E.; Patel, H.D.; Bodevin, S.; Spillmann, D.; Bishop, B.; Sapp, M.; Chen, X.S. Structural Basis of Oligosaccharide Receptor Recognition by Human Papillomavirus. J. Biol. Chem. 2011, 286, 2617–2624.
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Coronado-Martínez, I.; Pedraza-Chaverri, J.; Lizano, M. E6 Oncoproteins from High-Risk Human Papillomavirus Induce Mitochondrial Metabolism in a Head and Neck Squamous Cell Carcinoma Model. Biomolecules 2019, 9, 351.
- Sun, W.; Qin, X.; Zhou, J.; Xu, M.; Lyu, Z.; Li, X.; Zhang, K.; Dai, M.; Li, N.; Hang, D. Mitochondrial DNA copy number in cervical exfoliated cells and risk of cervical cancer among HPV-positive women. BMC Womens Health. 2020, 20, 139.
- Guo, Y.; Meng, X.; Ma, J.; Zheng, Y.; Wang, Q.; Wang, Y.; Shang, H. Human Papillomavirus 16 E6 Contributes HIF-1α Induced Warburg Effect by Attenuating the VHL-HIF-1α Interaction. Int. J. Mol. Sci. 2014, 15, 7974–7986.
- Fan, R.; Hou, W.-J.; Zhao, Y.-J.; Liu, S.-L.; Qiu, X.-S.; Wang, E.-H.; Wu, G.-P. Overexpression of HPV16 E6/E7 Mediated HIF-1α Upregulation of GLUT1 Expression in Lung Cancer Cells. Tumour Biol. 2016, 37, 4655–4663.
- Zhai, K.; Chang, L.; Zhang, Q.; Liu, B.; Wu, Y. Mitochondrial C150T Polymorphism Increases the Risk of Cervical Cancer and HPV Infection. Mitochondrion 2011, 11, 559–563.
- Agarwal, N.R.; Maurya, N.; Pawar, J.S.; Ghosh, I. A Combined Approach against Tumorigenesis Using Glucose Deprivation and Mitochondrial Complex 1 Inhibition by Rotenone. Cell Biol. Int. 2016, 40, 821–831.
- Höti, N.; Ma, J.; Tabassum, S.; Wang, Y.; Wu, M. Triphenyl Tin Benzimidazolethiol, a Novel Antitumor Agent, Induces Mitochondrial-Mediated Apoptosis in Human Cervical Cancer Cells via Suppression of HPV-18 Encoded E6. J. Biochem. 2003, 134, 521–528.
- Khan, S.; Chib, R.; Shah, B.A.; Wani, Z.A.; Dhar, N.; Mondhe, D.M.; Lattoo, S.; Jain, S.K.; Taneja, S.C.; Singh, J. A Cyano Analogue of Boswellic Acid Induces Crosstalk between P53/PUMA/Bax and Telomerase That Stages the Human Papillomavirus Type 18 Positive HeLa Cells to Apoptotic Death. Eur. J. Pharmacol. 2011, 660, 241–248.
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Huy, P.D.Q.; Li, M.S.; Florio, D.; Broersen, K.; De Pandis, M.F.; Roviello, G.N. Plant Isoquinoline Alkaloids as Potential Neurodrugs: A Comparative Study of the Effects of BenzoPhenanthridine and Berberine-Based Compounds on β-Amyloid Aggregation. Chem. Biol. Interact. 2021, 334, 109300.
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine Modulates AP-1 Activity to Suppress HPV Transcription and Downstream Signaling to Induce Growth Arrest and Apoptosis in Cervical Cancer Cells. Mol. Cancer 2011, 10, 39.
- Singh, M.; Singh, R.; Bhui, K.; Tyagi, S.; Mahmood, Z.; Shukla, Y. Tea Polyphenols Induce Apoptosis Through Mitochondrial Pathway and by Inhibiting Nuclear Factor-ΚB and Akt Activation in Human Cervical Cancer Cells. Oncol. Res. 2011, 19, 245–257.
- Chen, C.; Hu, S.-Y.; Luo, D.-Q.; Zhu, S.-Y.; Zhou, C.-Q. Potential Antitumor Agent from the Endophytic Fungus Pestalotiopsis Photiniae Induces Apoptosis via the Mitochondrial Pathway in HeLa Cells. Oncol. Rep. 2013, 30, 1773–1781.
- Singh, M.; Singh, N. Molecular Mechanism of Curcumin Induced Cytotoxicity in Human Cervical Carcinoma Cells. Mol. Cell Biochem. 2009, 325, 107–119.
- Tian, S.; Chen, H.; Tan, W. Targeting Mitochondrial Respiration as a Therapeutic Strategy for Cervical Cancer. Biochem. Biophys. Res. Commun. 2018, 499, 1019–1024.
- Bernard, B.; Prétet, J.-L.; Charlot, J.-F.; Mougin, C. Human Papillomaviruses Type 16+ and 18+ Cervical Carcinoma Cells Are Sensitive to Staurosporine-Mediated Apoptosis. Biol. Cell 2003, 95, 17–26.
- Zhao, X.; Song, X.; Zhao, J.; Zhu, W.; Hou, J.; Wang, Y.; Zhang, W. Juglone Inhibits Proliferation of HPV-Positive Cervical Cancer Cells Specifically. Biol. Pharm. Bull. 2019, 42, 475–480.
- Riyasdeen, A.; Periasamy, V.S.; Paul, P.; Alshatwi, A.A.; Akbarsha, M.A. Chloroform Extract of Rasagenthi Mezhugu, a Siddha Formulation, as an Evidence-Based Complementary and Alternative Medicine for HPV-Positive Cervical Cancers. Evid. Based Complement. Altern. Med. 2012, 2012, 136527.
- Paul, S.; Patra, D.; Kundu, R. Lignan Enriched Fraction (LRF) of Phyllanthus Amarus Promotes Apoptotic Cell Death in Human Cervical Cancer Cells in Vitro. Sci. Rep. 2019, 9, 14950.
- Li, G.-L.; Jiang, W.; Xia, Q.; Chen, S.-H.; Ge, X.-R.; Gui, S.-Q.; Xu, C.-J. HPV E6 Down-Regulation and Apoptosis Induction of Human Cervical Cancer Cells by a Novel Lipid-Soluble Extract (PE) from Pinellia Pedatisecta Schott in Vitro. J. Ethnopharmacol. 2010, 132, 56–64.
- Britt, E.L.; Raman, S.; Leek, K.; Sheehy, C.H.; Kim, S.W.; Harada, H. Combination of Fenretinide and ABT-263 Induces Apoptosis through NOXA for Head and Neck Squamous Cell Carcinoma Treatment. PLoS ONE 2019, 14, e0219398.
- Bruno, S.; Tenca, C.; Saverino, D.; Ciccone, E.; Grossi, C.E. Apoptosis of Squamous Cells at Different Stages of Carcinogenesis Following 4-HPR Treatment. Carcinogenesis 2002, 23, 447–456.
- Chang, J.-C.; Chang, H.-S.; Wu, Y.-C.; Cheng, W.-L.; Lin, T.-T.; Chang, H.-J.; Kuo, S.-J.; Chen, S.-T.; Liu, C.-S. Mitochondrial Transplantation Regulates Antitumour Activity, Chemoresistance and Mitochondrial Dynamics in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 30.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
975
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
09 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




