Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Marta Gonçalves | -- | 2580 | 2023-03-08 11:34:18 | | | |
| 2 | Catherine Yang | -7 word(s) | 2573 | 2023-03-09 01:53:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lomartire, S.; Gonçalves, A.M.M. Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts. Encyclopedia. Available online: https://encyclopedia.pub/entry/41980 (accessed on 07 February 2026).
Lomartire S, Gonçalves AMM. Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts. Encyclopedia. Available at: https://encyclopedia.pub/entry/41980. Accessed February 07, 2026.
Lomartire, Silvia, Ana M. M. Gonçalves. "Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts" Encyclopedia, https://encyclopedia.pub/entry/41980 (accessed February 07, 2026).
Lomartire, S., & Gonçalves, A.M.M. (2023, March 08). Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts. In Encyclopedia. https://encyclopedia.pub/entry/41980
Lomartire, Silvia and Ana M. M. Gonçalves. "Antimicrobial Activity from Rhodophyta and Chlorophyta Extracts." Encyclopedia. Web. 08 March, 2023.
Copy Citation
Macroalgae are aquatic photosynthetic organisms (mainly marine) belonging to the domain Eukarya. Macroalgae are mainly divided into three groups: red algae (Rhodophyta) and green algae (Chlorophyta), which are classified in kingdom Plantae, and brown algae (Ochrophyta, class Phaeophyceae), belonging to kingdom Chromista. Therefore, as terrestrial plants, macroalgae possess interesting biological activities that could be involved in the development of natural and innovative antibiotics. Macroalgae’s biological activities can vary among phyla.
bioactive compounds
Rhodophyta
Chlorophyta
seaweeds
terrestrial plants
1. Rhodophyta
Two different extracts of Gracilaria corticata and Gracilaria edulis (methanolic and dimethyl sulfoxide (DMSO) extracts), were investigated against pathogenic bacteria such as E. coli, Bacillus subtilis, B. cereus, S. aureus, Photobacterium sp., and Pseudomonas fluorescens [1]. All tested extracts exhibited antimicrobial activity against these pathogenic bacteria (Table 1), and GC-MS analysis has revealed the presence of numerous bioactive metabolites such as sulphurous acid, 2-ethylhexyl isohexyl ester, eugenol, benzene, and phthalic acid in both red macroalgae. Jasna et al. [2] reported high concentrations of eugenol in the clove extract, which proves its potential for antibacterial and antioxidant properties. The antibacterial mechanism of action of eugenol consists of the disruption of the cell structure by the incorporation within the lipopolysaccharides layer of the bacteria’s cell membrane, which leads to the intracellular components’ release and the death of the bacteria [3]. It may be possible that the same mechanism of actions happened with the G. corticata and G. edulis extracts.
S. aureus and E. coli growth reductions have been shown by testing these pathogenic bacteria against Grateloupia turuturu ethanolic and polysaccharide extracts. The results show that both extracts revealed antibacterial activity, with polysaccharides exhibiting higher antimicrobial activity. The FTIR-ATR analysis made it possible to characterize G. turuturu polysaccharides, concluding that they are composed by a hybrid kappa/iota carrageenan (Figure 1) with traces of agar, in both phases of the life cycle. This suggests that these compounds may be responsible for this activity; therefore, this red alga may be of pharmaceutical interest, since it was possible to observe, both in the ethanolic extracts and polysaccharides extracts, the ability to inhibit the growth of two different bacterial strains [4].
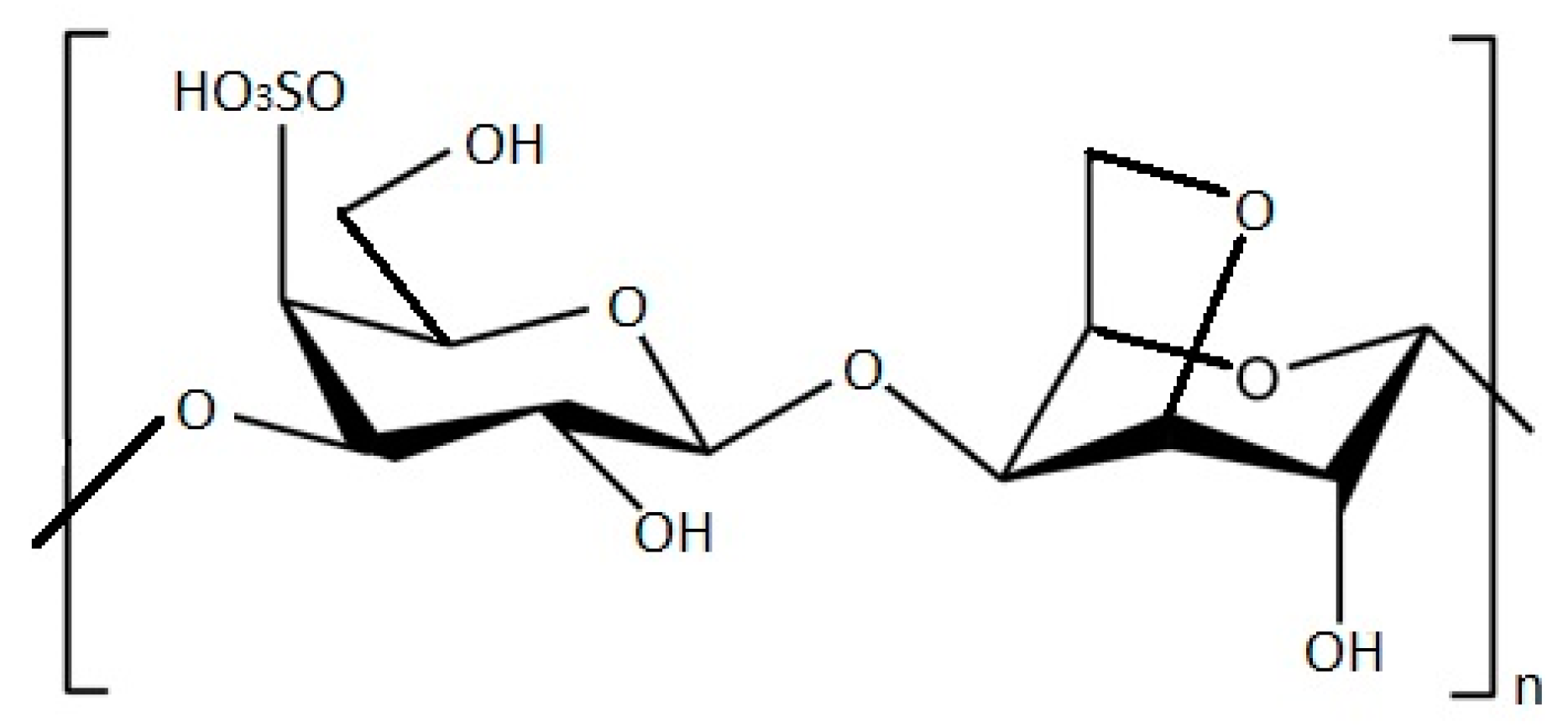
Figure 1. Structure of k-carrageenan molecule.
Methanolic extracts of G. edulis and Hypnea valentiae were tested against human bacterial pathogens Klebsiella oxytoca, E. coli, S. aureus, P. aeruginosa, B. subtilis, Serratia sp., and Salmonella sp. The G. edulis polyphenol compound displayed a maximum of 23 mm of inhibition zone against B. subtilis and the H. valentiae polyphenol compound displayed a maximum of 17 mm of inhibition zone against K. oxytoca (Table 1). Polyphenols from red algae carry potential assets, as they may have a strong pharmaceutical value in the future. Biochemical analysis revealed the presence of flavonoids, saponins, tannin, and steroids in both red algae, while only G. edulis revealed phenolics and alkaloids [5].
The objective of the study of Freitas et al. [6] is to evaluate the antioxidant and antimicrobial activity of twelve red seaweed species commonly found on Portuguese shores, namely Porphyra umbilicalis, Ceramium ciliatum, Osmundea pinnatifida, Chondrus crispus, Sphaerococcus coronopifolius, Plocamium cartilagineum, Corallina officinalis, Ellisolandia elongata, Amphiroa rigida, Jania rubens, Mesophyllum lichenoides, and Liagora viscida. All of them possess interesting antimicrobial properties, and in Table 1 are reported the MIC and inhibition zone diameter of the edible seaweeds P. umbilicalis, O. pinnatifida, and C. crispus.
P. umbilicalis presents, by far, the highest phenol content when compared to the other studied algae, as well as a high scavenging ability. These results likely indicate strong antioxidant activity, as it is known that seaweeds are able to develop antioxidant shielding mechanisms and strategies to withstand highly oxidative environments [6].
The study of Bhuyar et al. [7] demonstrates that different extracts (water and ethanol) of red alga K. alvarezii were more efficient against B. cereus but not against E. coli, as disc diffusion assay results indicated.
Among edible seaweeds, Pyropia orbicularis [8] and Asparagopsis taxiformis both inhibited S. aureus and E. coli, with Klebseilla sp., K. pneumoniae, Pseudomonas fluorescens, Vibrio proteolyticus, and Streptococcus sp. Bacillus subtilis demonstrating a high inhibition zone [9] (Table 1).
The in vitro activity of the Gelidium sp. flour extract was evaluated against the most common pathogenic and spoilage bacteria. From the results, it emerged that P. fluorescens and Pseudomonas putida exhibited resistance to components of algal flour extract. Only B. subtilis and Salmonella enterica were inhibited by the lowest MIC. The highest level of inhibition was observed both for Gram-negatives such as Enterobacteriaceae (E. coli, Enterobacter aerogenes, and K. pneumoniae) and proteobacteria (Vibrio alginolyticus).
Due to the simplicity of the extraction methodology and the abundancy of Gelidium sp., further research is envisaged to optimize the extraction of the used compounds and to analyze the molecules involved in antimicrobial action [10].
Red algae are the main producers of halogenated compounds, which exhibited diverse biological activities including antibacterial, antifungal, anti-inflammatory, insecticidal, and carcinogenic effects. Along with several interesting amino acid, acetate, and nucleic acid derivatives, red algae also synthesize terpenoid, polyether, and acetogenin compounds [11][12]. For example, the halogenated sesquiterpene alcohol, elatol, is commonly found in Laurencia sp., and known for its potent antibacterial activity. The compound was isolated for the first time in Laurencia microcladia, collected in the Southern Brazilian coast, and tests showed the antiherbivore and antimicrobial activity of elatol [13].
Table 1. Antimicrobial activity of Rhodophyta species (“nd” = not determined; “–“ = no antimicrobial activity revealed).
| Rhodophyta | Extract Type | Microbes | Minimum Inhibitory Concentration (MIC) | Inhibition Zone Diameter (mm) | Reference |
|---|---|---|---|---|---|
| Asparagopsistaxiformis | Methanolic extract | Staphylococcus aureus | 0.5 mg/mL | >15 | [9] |
| Serratia sp. | 0.5 mg/mL | - | |||
| Klebseilla sp. | 0.5 mg/mL | >1 | |||
| Salmonella sp. | 0.5 mg/mL | - | |||
| Escherichia coli | 0.5 mg/mL | >10 | |||
| Klebseilla pneumonia | 0.5 mg/mL | >1 | |||
| Pseudomonas aeruginosa | 0.5 mg/mL | - | |||
| Pseudomonas fluorescens | 0.5 mg/mL | >10 | |||
| Vibrio proteolyticus | 0.5 mg/mL | >1 | |||
| Streptococcus sp. | 0.5 mg/mL | 10 | |||
| Bacillussubtilis | 0.5 mg/mL | 10 | |||
| Chondrus crispus | Bacillus subtilis | 12.5 mg/mL | - | [6] | |
| Gelidium sp. | Water extract | Salmonella enterica | 12.5 mg/mL | >10 | [10] |
| Klebsiella pneumoniae | 50 mg/mL | >11 | |||
| Listeria monocytogenes | 50 mg/mL | 11 | |||
| Enterobacter aerogenes | 25 mg/mL | >11 | |||
| Proteus mirabilis | 50 mg/mL | >11 | |||
| Vibrio parahaemolyticus |
nd | >11 | |||
| Vibrio alginolyticus | nd | 13 | |||
| Bacillus licheniformis | 25 mg/mL | 11 | |||
| Bacillus cereus | 0.625 mg/mL | >11 | |||
| Bacillus subtilis | 3.125 mg/mL | >10 | |||
| Escherichia coli | 50 mg/mL | >13 | |||
| Pseudomonas putida | - | - | |||
| Pseudomonas fluorescens | - | - | |||
| Gracilaria corticata | Methanolic extract | Escherichia coli | 100 µg/mL | 7 ± 0.01 | [1] |
| Photobacterium sp. | 100 µg/mL | 6 ± 0.04 | |||
| Pseudomonas fluorescens | 100 µg/mL | 8 ± 0.1 | |||
| Staphylococcus aureus | 100 µg/mL | 4 ± 0.10 | |||
| Bacillus subtilis | 100 µg/mL | 8 ± 0.01 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 5 ± 0.10 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.30 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 6 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 5 ± 0.12 | |||
| Gracilaria edulis | Methanolic extract | Escherichia coli | 100 µg/mL | 3 ± 0.01 | [1] |
| Photobacterium sp. | 100 µg/mL | 1 ± 0.00 | |||
| Pseudomonas fluorescens | 100 µg/mL | 3 ± 0.05 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.05 | |||
| Bacillus subtilis | 100 µg/mL | 3 ± 0.03 | |||
| Dimethyl sulfoxide (DMSO) extract | Escherichia coli | 100 µg/mL | 4.5 ± 0.01 | ||
| Photobacterium sp. | 100 µg/mL | 4 ± 0.01 | |||
| Pseudomonas fluorescens | 100 µg/mL | 4 ± 0.10 | |||
| Staphylococcus aureus | 100 µg/mL | 3 ± 0.00 | |||
| Gracilaria edulis | Methanolic extracts | Klebsiella oxytoca | 0.3 mg/mL | 21 | [5] |
| Escherichia coli | 0.3 mg/mL | 19 | |||
| Staphylococcus aureus | 0.3 mg/mL | 18 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 16 | |||
| Bacillus subtilis | 0.3 mg/mL | 23 | |||
| Serratia sp. | 0.3 mg/mL | 20 | |||
| Salmonella sp. | 0.3 mg/mL | 22 | |||
| Grateloupia turuturu | Ethanolic extract | Staphylococcus aureus | 10 mg/mL | - | [4] |
| Escherichia coli | 10 mg/mL | - | |||
| Polysaccharides (carrageenan) | Staphylococcus aureus | 7.5 mg/mL | - | ||
| Escherichia coli | 7.5 mg/mL | - | |||
| Hypnea valentiae | Methanolic extract | Klebsiella oxytoca | 0.3 mg/mL | 17 | [5] |
| Escherichia coli | 0.3 mg/mL | 12 | |||
| Staphylococcus aureus | 0.3 mg/mL | 14 | |||
| Pseudomonas aeruginosa | 0.3 mg/mL | 11 | |||
| Bacillus subtilis | 0.3 mg/mL | 15 | |||
| Serratia sp. | 0.3 mg/mL | 13 | |||
| Salmonella sp. | 0.3 mg/mL | 16 | |||
| Kappaphycus alvarezii | Ethanolic extract | Escherichia coli | - | - | [7] |
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Hot water extract | Escherichia coli | - | - | ||
| Bacillus cereus | 0.5 mg/mL | <10 | |||
| Osmundea pinnatifida | Bacillus subtilis | 1.56 mg/mL | - | [6] | |
| Porphyra umbilicalis | Aqueous extract | Bacillus subtilis | 3.13 mg/mL | - | [6] |
| Pyropiaorbicularis | Methanolic extract | Staphylococcus aureus | 250 mg/mL | nd | [8] |
| Escherichia coli | 500 mg/mL |
2. Chlorophyta
Caulerpa racemosa and Caulerpa lentillifera, also known as “sea grapes”, are green seaweeds commonly found in different parts of the world. They are widely used as whole food, but they also possess interesting therapeutic properties. It has been investigated whether C. racemosa and C. lentillifera from Malaysia have antibacterial properties. Crude extracts from seaweed were obtained using chloroform, methanol, and water. The authors measured the total phenolic and flavonoid contents. Both seaweed extracts displayed antibacterial abilities against neuropathogenic E. coli K1 and methicillin-resistant S. aureus (MRSA). The results show that the C. racemosa chloroform extract had the highest total phenolic content and the strongest antibacterial effect against MRSA, but it did not demonstrate similar promising results against E. coli K1. The chloroform extract of C. lentillifera gives a moderate antibacterial effect on MRSA but poorly on E. coli K1. In both species, the methanol extracts only show a moderate antibacterial effect against both MRSA and E. coli K1. A positive correlation has been revealed between the TPC and antibacterial activity, suggesting that the antimicrobial action may be due to the presence of phenolics. Both the C. racemosa and C. lentillifera water extracts promote the growth of the bacteria. According to the study, C. racemosa chloroform extracts mostly contain polyunsaturated and monounsaturated fatty acids, terpenes, and alkaloids. As a result, C. racemosa has the potential to be an excellent source of new antibacterial compounds. Still, the mechanisms of action of these compounds are unclear; therefore, further studies are needed along with the improvement of isolation and purification techniques of bioactive compounds [14].
C. racemosa, along with Ulva intestinalis, have also been investigated to test their antibacterial activity against Vibrio fluvialis. It appears that both exhibit antibacterial activity against V. fluvialis bacteria, with C. racemosa exhibiting a higher activity [15].
Nagappan and Vairappan [16] evaluated the antibacterial properties of the green seaweed C. lentillifera and C. racemosa methanolic extracts against E. coli, S. aureus, Streptococcus sp., Salmonella sp., and S. aureus. The higher MIC has been determined for C. racemosa species against Streptococcus sp.
Ravikumar et al. [17] investigated Caulerpa cuppressoides, Enteromorpha intestinalis, and Ulva lactuca antimicrobial activity. Tests were carried out considering different extraction solvents, such as benzene, butanol, propanol, acetone, and water. The results reveal high inhibitory activities against S. aureus, P. aeruginosa for C. cupressoides propanol extracts, while for Streptococcus pyogens and E. coli with acetone extracts. E. intestinalis sees the highest antimicrobial activity for K. pneumoniae from water extracts, while U. lactuca exhibited a higher antimicrobial activity for butanol extracts against S. aureus and acetone extracts tested for P. aeruginosa.
Ulva fasciata, U. lactuca, Cladophora vagabunda, Caulerpa taxifolia, Chaetomorpha anteninna, and Chaetomorpha linum crude extracts were tested against E. coli clinical and laboratory strains, namely E. coli NCTC 10418, E. coli ATCC 25923, Proteus vulgaris, P. mirabilis, P. aeruginosa, P. putida, Salmonella typhi clinical strain, Salmonella typhi NCTC 8385, Serratia macerans, as well as K. pneumoniae, S. aureus ATCC 25922, B. subtilis, Streptococcus pneumoniae, Enterobacter faecalis, and Mycobacterium aurum.
The highest activity against the bacterial strain was detected in the diethyl acetate extract of C. antennina against S. aureus laboratory strain, while the highest inhibitory zone against the Gram-negative bacterial species was observed in the dichloromethane/methanol extract of C. taxifolia against E. coli strains. This alga presents the highest inhibitory activities among all the tested algae that shows MIC and minimum zone of inhibition performed with the disc diffusion method. U. fasciata exhibited broad-spectrum antibacterial activity [18]. The effects of the methanol extracts of Ulva sp. against the following multidrug-resistant bacteria isolated from patients in Saudi Arabia and Malaysia were tested. The lowest MIC was for the 0.5 µg/mL extract of Ulva sp. against S. agalactiae (group B), whereas S. saprophyticus exhibited more resistance to Ulva sp. with an MIC of 16 µg/mL. This investigation conducted by Al-Zahrani et al. [19] is an example of the potential for obtaining new sources of antimicrobial agents to develop new therapeutically interesting molecules from easy cultivable seaweeds.
The results of Srikonga et al. [20], which evaluated the effects of the green seaweed U. intestinalis methanolic, ethanolic, dichloromethane, and hexane extracts, demonstrated antimicrobial activity against Gram-positive bacteria. The methanolic extracts exhibited activity against B. cereus, MRSA, and S. aureus, while the ethanolic and dichloromethane extracts affected only L. monocytogenes. All these four microbes are affected by the hexane extract. For these species, the authors calculated the MIC. The extract of Enteromorpha sp. had been tested by Swathi et al. [21] against P. aeruginosa, S. aureus, and E. coli to analyze its antibacterial activity by the disc diffusion method. At the concentrations of 150 g/mL and 200 g/mL, it produced a zone of clearance with diameters of 11 ± 0.2 mm and 13 ± 0.2 mm, respectively, against P. aeruginosa. It exhibited inhibitory zones of 10 ± 0.2 mm, 16 ± 0.2 mm, and 18 ± 0.2 mm at the concentrations of 100 g/mL, 150 g/mL, and 200 g/mL, respectively, for S. aureus and 11 ± 0.2 mm, 15 ± 0.2 mm, and 18 ± 0.2 mm, respectively, against E. coli. This demonstrated the excellent antioxidant and antibacterial properties of Enteromorpha sp. The bioactive compounds present in the green seaweed extract of Enteromorpha compressa were tested for its antimicrobial activity against human pathogens such as Klebsiella sp., Salmonella sp., S. aureus, and Proteus sp. Salmonella sp. was found to be more susceptible to E. compressa ethanolic extracts compared with the effect against other tested bacteria [22]. Phytochemical analysis confirmed the presence of phenols, alkaloids, flavonoids, steroids, and terpenoids that may be responsible for the antibacterial activity.
Cadar et al. [23] investigated the extracts of U. lactuca to determine total polyphenols content and antibacterial activity against S. aureus, Staphylococcus epidermidis, P. aeruginosa, and E. coli. Ampicillin was used as a standard drug and control. The chloroform extract demonstrated the largest inhibitory zone against S. aureus, comparable to that of conventional ampicillin. The extract in n-hexane displayed the biggest inhibitory zone against Staphylococcus epidermides and P. aeruginosa, comparable to the ampicillin control. The extracts in n-hexane and chloroform produced the largest areas of inhibition in the case of E. coli; however, they present low values compared to the ampicillin standard. The authors deduced from the tests that ampicillin-like antibacterial activity was present in n-hexane and chloroform extracts. Due to the presence of known bioactive chemical components that promote this property, U. lactuca validates its potential for antimicrobial properties [23].
The Codium species have received the least attention from exploring the biological activities of Chlorophyceae members for potential biomedical applications. C. intricatum methanol extract was tested for antibacterial activity against a variety of bacterial infections. It displayed a broad spectrum of inhibitory effects against MRSA and modest action against B. cereus and L. monocytogenes in a research conducted by Arguelles et al. [24].
However, in other studies, such as Koz et al.’s [25] investigation, the antibacterial activity of hexane, methanol, and dichloromethane of Codium fragile extracts against several pathogenic bacteria were tested. All three extracts of C. fragile demonstrated a similar weak antimicrobial activity on B. subtilis, MRSA, E. aerogenes, and E. coli compared with the standard antibiotic tobramycin.
The antibacterial activity of methanolic extracts of C. bursa, C. tomentosum, C. dichotomum, and C. fragile were tested against S. aureus, E. coli, K. pneumoniae, and E. faecalis. All Codium extracts exhibited high inhibition against S. aureus, except for C. bursa, for which no antibacterial activity was observed [26]. However, antimicrobial activity studies of Codium sp. are limited and need to be further developed.
Ulvan is a water-soluble sulphated polysaccharide (Figure 2) derived from marine green seaweed, which exhibits a wide range of physiological and biological activities such as anticancer [27], anticoagulant [28], antioxidant, antifungal, and antitumor activities [29][30]. Ulvan essentially contains rhamnose, xylose, glucuronic acid, iduronic acid, and sulphate groups [29][31] and its structure and properties can vary depending on algae species, place of cultivation, and method of extraction [32][33][34][35][36]. The study of Van Tran et al. [37] showed the antibacterial activity of ulvan extracted from U. reticulata against E. coli, P. aeruginosa, and Enterobacter cloacae. The highest inhibition activity was shown in E. cloacae, followed by E. coli, and the lowest inhibitor activity was in P. aeruginosa [37].
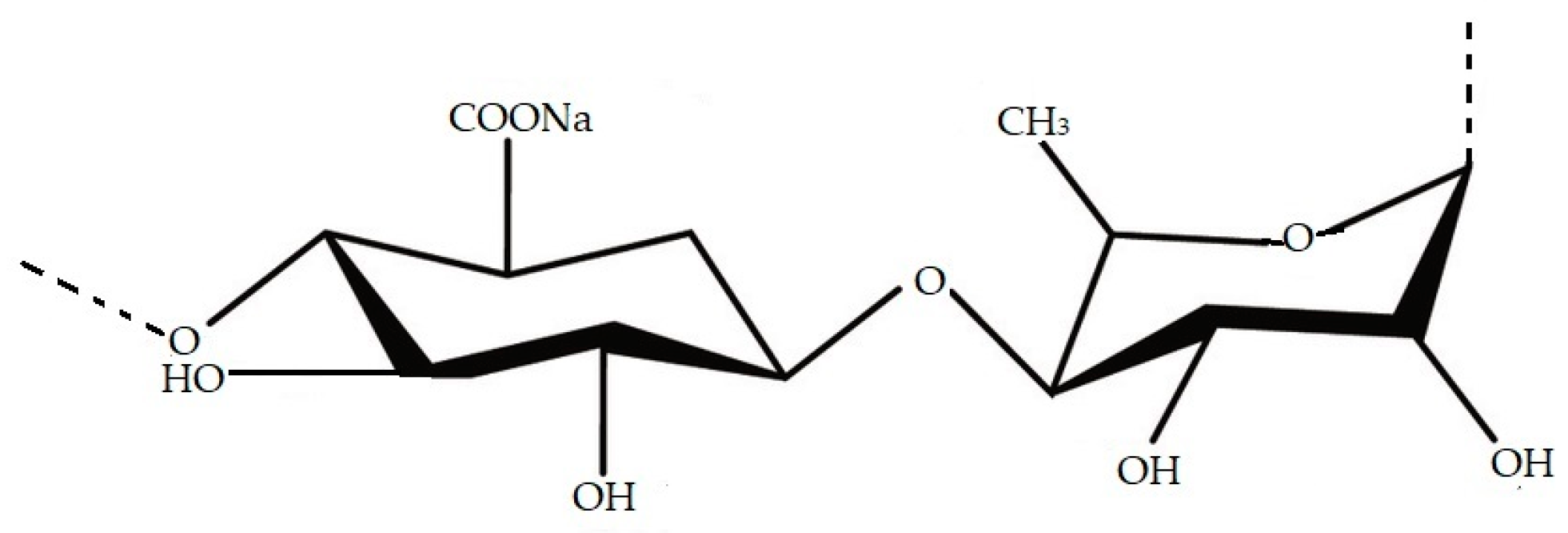
Figure 2. Structure of ulvan molecule.
References
- Arulkumar, A.; Rosemary, T.; Paramasivam, S. Phytochemical Composition, In Vitro Antioxidant, Antibacterial Potential and GC-MS Analysis of Red Seaweeds (Gracilaria corticata and Gracilaria edulis) from Palk Bay, India. Biocatal. Agric. Biotechnol. 2018, 15, 63–71.
- Ivanovic, J.; Dimitrijevic-brankovic, S.; Misic, D.; Ristic, M.; Zizovic, I. Evaluation and Improvement of Antioxidant and Antibacterial Activities of Supercritical Extracts from Clove Buds. J. Funct. Foods 2012, 5, 416–423.
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115.
- Cardoso, I.; Cotas, J.; Rodrigues, A.; Ferreira, D.; Osório, N.; Pereira, L. Extraction and Analysis of Compounds with Antibacterial Potential from the Red Alga Grateloupia turuturu. J. Mar. Sci. Eng. 2019, 7, 220.
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In Vitro Antioxidant Study of Polyphenol from Red Seaweeds Dichotomously Branched Gracilaria edulis and Robust Sea Moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411.
- Freitas, M.V.; Inácio, L.G.; Ruas, A.; Silva, I.A.; Mouga, T.; Pereira, L.; Afonso, C. Antioxidant and Antimicrobial Properties of Selected Red Seaweeds from Central Portugal. Appl. Sci. 2022, 13, 157.
- Bhuyar, P.; Rahim, M.H.; Sundararaju, S.; Maniam, G.P.; Govindan, N. Antioxidant and Antibacterial Activity of Red Seaweed: Kappaphycus alvarezii against Pathogenic Bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58.
- García, V.; Uribe, E.; Vega-Gálvez, A.; Delporte, C.; Valenzuela-Barra, G.; López, J.; Pastén, A. Health-Promoting Activities of Edible Seaweed Extracts from Chilean Coasts: Assessment of Antioxidant, Anti-Diabetic, Anti-Inflammatory and Antimicrobial Potential. Rev. Chil. Nutr. 2020, 47, 792–800.
- Saim, S.; Sahnouni, F.; Bouhadi, D.; Kharbouche, S. The Antimicrobial Activity of Two Marine Red Algae Collected from Algerian West Coast. Trends Pharmacol. Sci. 2021, 7, 233–242.
- Miranda, J.M.; Trigo, M.; Barros-Velázquez, J.; Aubourg, S.P. Antimicrobial Activity of Red Alga Flour (Gelidium sp.) and Its Effect on Quality Retention of Scomber scombrus during Refrigerated Storage. Foods 2022, 11, 904.
- Maschek, J.A.; Baker, B.J. The Chemistry of Algal Secondary Metabolism. In Algal Chemical Ecology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–20. ISBN 9783540741800.
- Ayyad, S.-E.N.; Al-Footy, K.O.; Alarif, W.M.; Sobahi, T.R.; Bassaif, S.A.; Makki, M.S.; Asiri, A.M.; Al Halawani, A.Y.; Bandria, A.F.; Bandria, F.A.A. Bioactive C15 Acetogenins from the Red Alga Laurencia obtusa. Chem. Pharm. Bull. 2011, 59, 1294–1298.
- dos Santos Oliviera, A.; Veiga-santos, P.; Prado, B.; Filho, D.; Sudatti, D.B.; Pereira, R.C.; Nakamura, C.V.; De Londrina, U.E.; Celso, R.; Cid, G.; et al. Effect of Elatol, Isolated from Red Seaweed Laurencia dendroidea, on Leishmania amazonensis. Mar. Drugs 2010, 8, 2733–2743.
- Yap, W.-F.; Tay, V.; Tan, S.-H.; Yow, Y.-Y.; Chew, J. Decoding Antioxidant and Antibacterial Potentials of Malaysian Green Seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152.
- Aftabuddin, S.; Akter, S.; Hossen, S.; Rahman, M.A. Antioxidant, Antibacterial and Cytotoxic Activity of Caulerpa racemosa (Forsskål) J. Agardh and Ulva (Enteromorpha) intestinalis L. Bangladesh J. Sci. Ind. Res. 2020, 55, 237–244.
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027.
- Ravikumar, S.; Anburajan, L.; Ramanathan, G.; Kaliaperumal, N. Screening of Seaweed Extracts against Antibiotic Resistant Post Operative Infectious Pathogens. Seaweed Res. Util. 2002, 24, 95–99.
- Agbaje-Daniels, F.; Adeleye, A.; Nwankwo, D.; Adeniyi, B.; Seku, F.; Beukes, D. Antibacterial Activities of Selected Green Seaweeds from West African Coast. EC Pharmacol. Toxicol. 2020, 4, 84–92.
- Al-Zahrani, A.; Al-Haj, N.; Omer, H.; Al-Judaibi, A. Impact of Extracts of Marine Macroalgae on Multidrug-Resistant Bacteria. J. Microbiol. Res. 2014, 4, 18–24.
- Srikong, W.; Bovornreungroj, N.; Mittraparparthorn, P.; Bovornreungroj, P. Antibacterial and Antioxidant Activities of Differential Solvent Extractions from the Green Seaweed Ulva intestinalis. ScienceAsia 2017, 43, 88–95.
- Swathi, N.; Kumar, A.G.; Parthasarathy, V.; Sankarganesh, P. Isolation of Enteromorpha Species and Analyzing Its Crude Extract for the Determination of in Vitro Antioxidant and Antibacterial Activities. Biomass Convers. Biorefinery 2022, 3, 1–10.
- Priya, N.; Kokila, M.; Janani, J. Comparative Phytochemical Studies and Antibacterial Activity of Green and Brown Seaweeds Extract of Enteromorpha compressa and Padina pavonica. Int. J. Res. Eng. Sci. Manag. 2021, 4, 136–139.
- Cadar, E.; Negreanu-Pirjol, T.; Negreanu-Pirjo, B.-S. Antioxidant and Antibacterial Potential of Ulva lactuca Species from Romanian Black Sea Coast. Eur. J. Nat. Sci. Med. 2022, 8705, 26–38.
- Arguelles, E.D.L.R. Evaluation of Nutritional Composition and In Vitro Antioxidant and Antibacterial Activities of Codium intricatum Okamura from Ilocos Norte (Philippines). Jordan J. Biol. Sci. 2020, 13, 375–382.
- Koz, F.F.Y.; Yavasoglu, N.U.; Demirel, Z.; Sukatar, A.; Ozdemir, G. Antimicrobial Activities of Some Macroalgal Essential Oil and Extracts from Antioxidant and Antimicrobial Activities of Codium fragile (Suringar) Hariot (Chlorophyta) Essential Oil and Extracts. Asian J. Chem. 2009, 21, 1197–1209.
- Ibtissam, C.; Hassane, R.; Jose, M.; Francisco, D.S.; Antonio, G.V.; Hassan, B.; Mohamed, K. Screening of Antibacterial Activity in Marine Green and Brown Macroalgae from the Coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262.
- Kaeffer, B.; Benard, C.; Lahaye, M.; Herve, M.B.; Cherbut, C. Biological Properties of Ulvan, a New Source of Green Seaweed Sulfated Polysaccharides, on Cultured Normal and Cancerous Colonic Epithelial Cells. Planta Med. 1999, 65, 527–531.
- Mao, W.; Zang, X.; Li, Y.; Zhang, H. Sulfated Polysaccharides from Marine Green Algae Ulva conglobata and Their Anticoagulant Activity. J. Appl. Phycol. 2006, 18, 9–14.
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223.
- Alves, A.; Sousa, R.A.; Reis, R.L. In Vitro Cytotoxicity Assessment of Ulvan, a Polysaccharide Extracted from Green Algae. Phyther. Res. 2013, 27, 1143–1148.
- Quemener, B.; Lahaye, M.; Bobin-Dubigeon, C. Sugar Determination in Ulvans by a Chemical-Enzymatic Method Coupled to High Performance Anion Exchange Chromatography. J. Appl. Phycol. 1997, 9, 179–188.
- Alves, A.; Sousa, R.A.; Reis, R.L. A Practical Perspective on Ulvan Extracted from Green Algae. J. Appl. Phycol. 2013, 25, 407–424.
- Ray, B.; Lahaye, M. Cell-Wall Polysaccharides from the Marine Green Alga Ulva “rigida” (Ulvales, Chlorophyta). Extraction and Chemical Composition. Carbohydr. Res. 1995, 274, 251–261.
- Lahaye, M.; Inizan, F.; Vigouroux, J. Carbohydrate Polymers NMR Analysis of the Chemical Structure of Ulvan and of Ulvan-Boron Complex Formation. Carbohydr. Polym. 1998, 36, 239–249.
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Food Hydrocolloids Effect of Extraction Conditions on the Yield and Purity of Ulvan Extracted from Ulva lactuca. Food Hydrocoll. 2020, 31, 375–382.
- Thanh, T.T.T.; Quach, T.M.T.; Nguyen, T.N.; Vu Luong, D.; Bui, M.L.; Tran, T.T. Van Structure and Cytotoxic Activity of Ulvan Extracted from Green Seaweed Ulva lactuca. Int. J. Biol. Macromol. 2016, 93, 695–702.
- Van Tran, T.T.; Truong, H.B.; Ha, N.; Tran, V.; Thu, T.M. Structure, Conformation in Aqueous Solution and Antimicrobial Activity of Ulvan Extracted from Green Seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
09 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




